Abstract
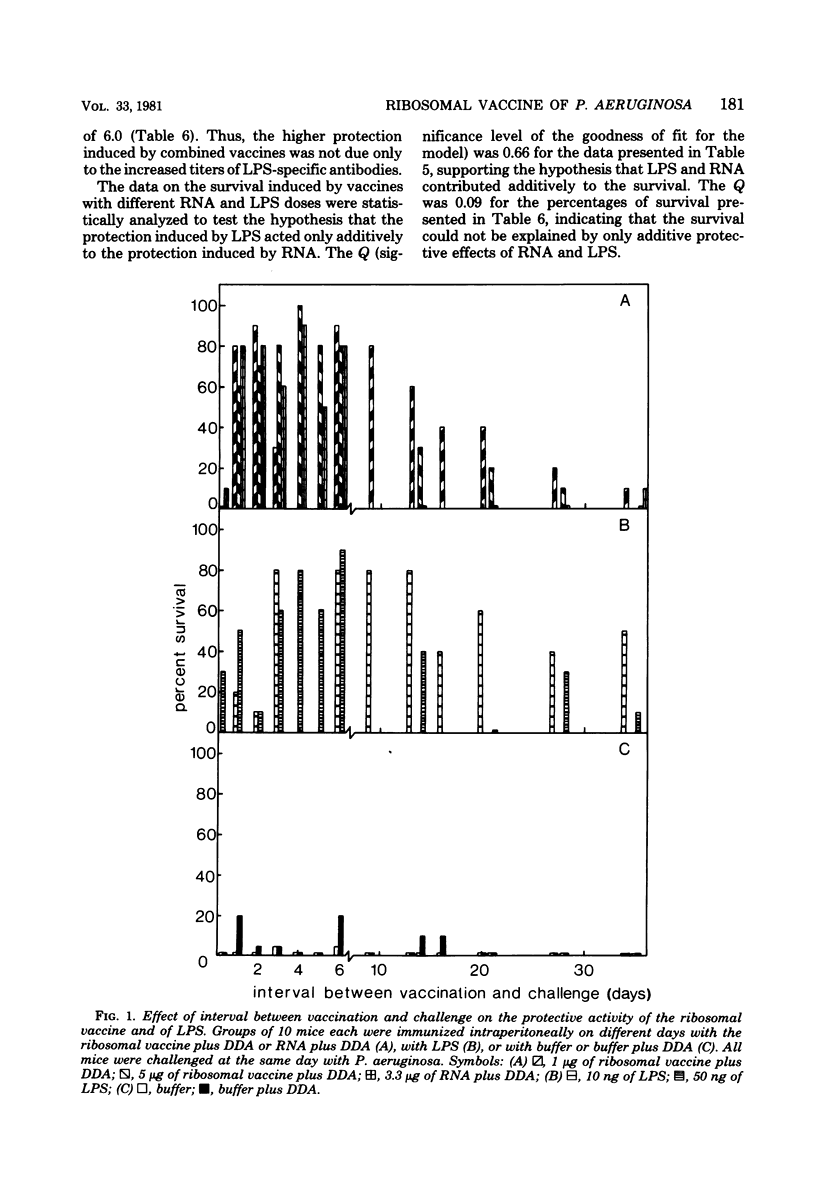
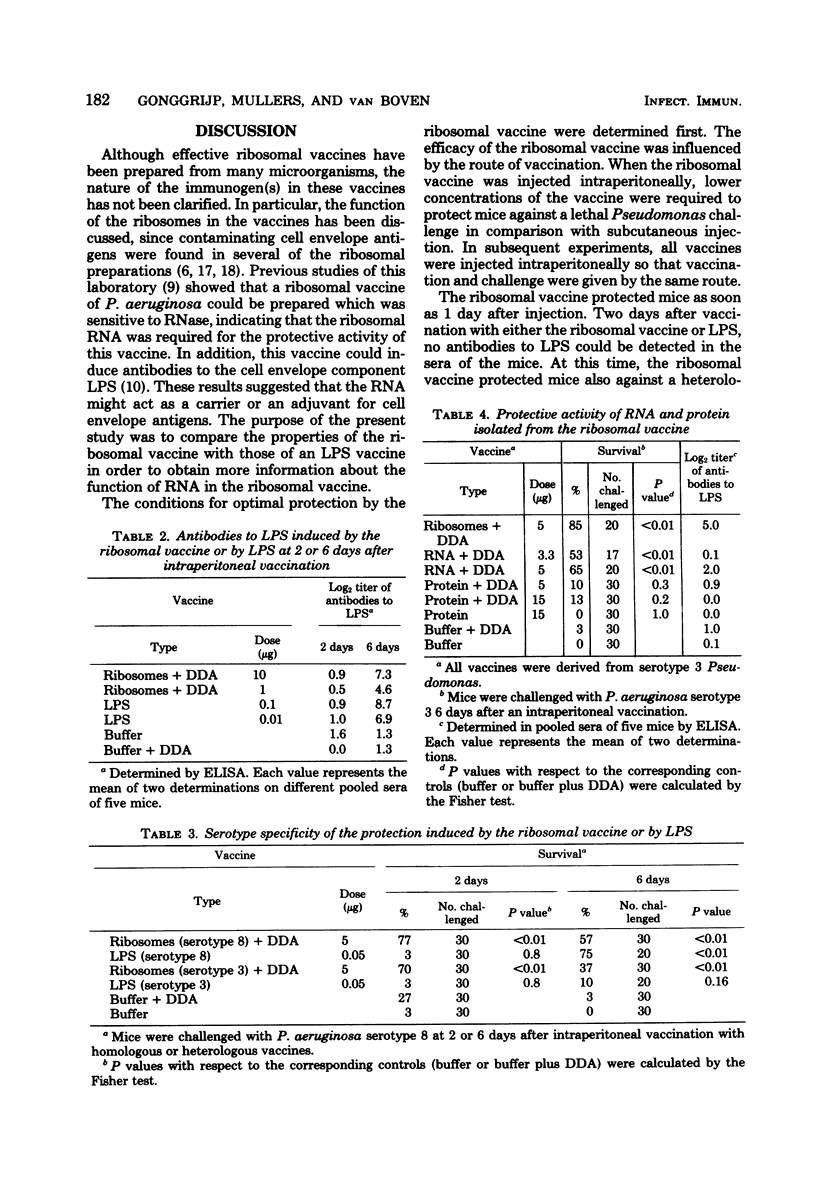
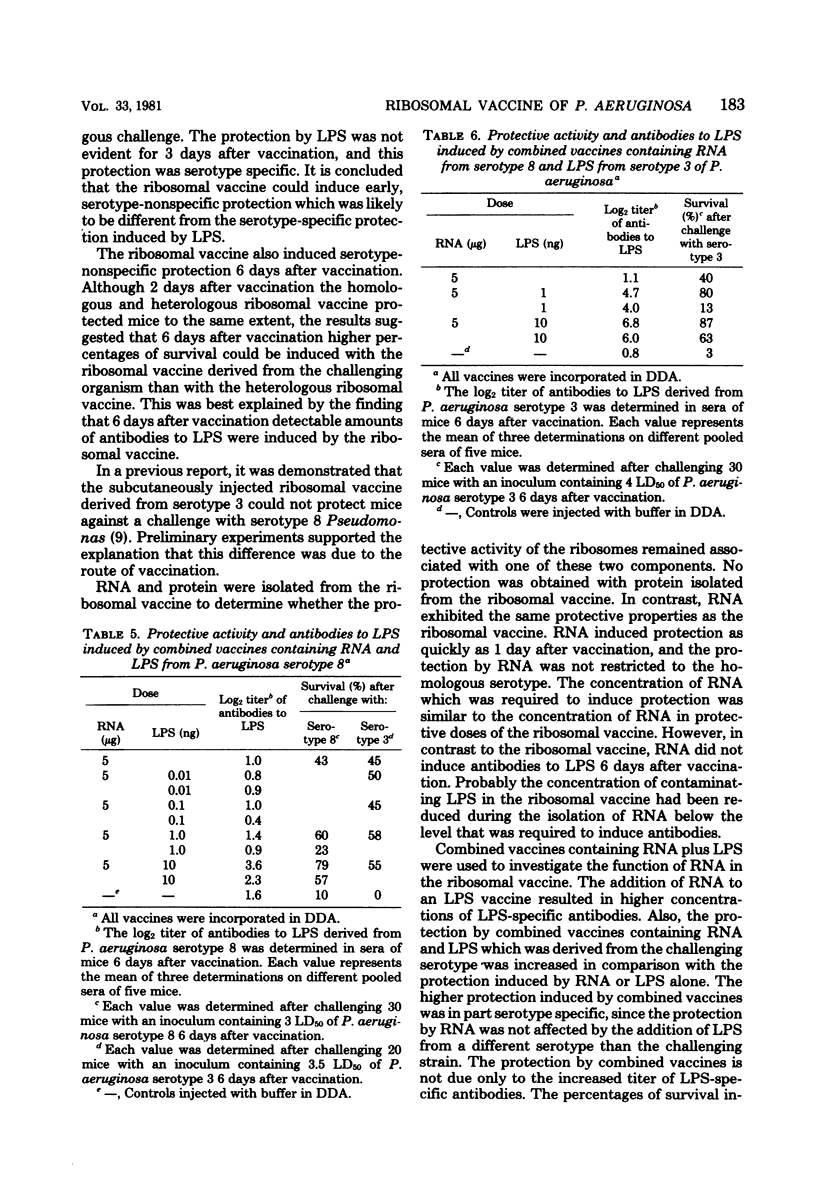
A ribosomal vaccine of Pseudomonas aeruginosa and a vaccine containing purified lipopolysaccharide (LPS) were compared with respect to their capacity to protect mice against a lethal challenge with P. aeruginosa. The route of vaccination appeared to be important for the protective activity of the ribosomal vaccine. Optimal protection was measured if both the immunizing and the challenge injection were given intraperitoneally. The ribosomal vaccine protected mice as early as 1 day after vaccination, and the protection lasted at least 6 days. LPS-specific antibodies were detectable 6 but not 2 days after vaccination. The ribosomal vaccine protected mice also against a heterologous serotype of Pseudomonas. Injection of purified LPS did not protect mice earlier than at day 3, and the protection induced by LPS was serotype specific. Ribonucleic acid (RNA) isolated from the ribosomal vaccine had the same protective properties as the ribosomes. RNA induced serotype-nonspecific protection as quickly as 1 day after injection, and the protection lasted at least 6 days. However, the capacity to induce antibodies to LPS was lost or reduced. It is concluded that the serotype-nonspecific protection induced by RNA and the serotype-specific protection induced by LPS are due to different mechanisms. Experiments with combined vaccines containing RNA and LPS demonstrated that the addition of RNA to LPS resulted in a slight increase in LPS-specific antibodies. The data presented indicate that both the serotype-specific protection induced by LPS and the serotype-nonspecific protection induced by RNA contribute to the protective activity of the ribosomal vaccine.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andron LA I. I., Eigelsbach H. T. Biochemical and immunological properties of ribonucleic acid-rich extracts from Francisella tularensis. Infect Immun. 1975 Jul;12(1):137–142. doi: 10.1128/iai.12.1.137-142.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo F. G., Remington J. S. Protection against Toxoplasma gondii in mice immunized with Toxoplasma cell fractions, RNA and synthetic polyribonucleotides. Immunology. 1974 Oct;27(4):711–721. [PMC free article] [PubMed] [Google Scholar]
- Coppel S., Youmans G. P. Specificity of acquired resistance produced by immunization with mycobacterial cells and mycobacterial fractions. J Bacteriol. 1969 Jan;97(1):114–120. doi: 10.1128/jb.97.1.114-120.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field L. H., Parker C. D., Manclark C. R., Berry L. J. Evaluation of a ribosomal vaccine against pertussis. Infect Immun. 1979 May;24(2):346–351. doi: 10.1128/iai.24.2.346-351.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Gonggrijp R., Mullers W. J., Lemmens P. J., van Boven C. P. Ribonuclease-sensitive ribosomal vaccine of Pseudomonas aeruginosa. Infect Immun. 1980 Jan;27(1):204–210. doi: 10.1128/iai.27.1.204-210.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonggrijp R., Volleberg M. P., Lemmens P. J., van Boven C. P. Evidence for the presence of lipopolysaccharide in a ribonuclease-sensitive ribosomal vaccine of Pseudomonas aeruginosa. Infect Immun. 1981 Mar;31(3):896–905. doi: 10.1128/iai.31.3.896-905.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy S. J., Kurland C. G., Voynow P., Mora G. The ribosomal proteins of Escherichia coli. I. Purification of the 30S ribosomal proteins. Biochemistry. 1969 Jul;8(7):2897–2905. doi: 10.1021/bi00835a031. [DOI] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Horton D., Rodemeyer G., Haskell T. H. Analytical characterization of lipopolysaccharide antigens from seven strains of Pseudomonas aeruginosa. Carbohydr Res. 1977 May;55:35–47. doi: 10.1016/s0008-6215(00)84441-9. [DOI] [PubMed] [Google Scholar]
- Lieberman M. M. Pseudomonas ribosomal vaccines: preparation, properties, and immunogenicity. Infect Immun. 1978 Jul;21(1):76–86. doi: 10.1128/iai.21.1.76-86.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. H., Berry L. J. The use of strain LT2-Ml in identifying the protective antigens in a Salmonella typhimurium-derived ribosomal vaccine. J Reticuloendothel Soc. 1978 Feb;23(2):135–143. [PubMed] [Google Scholar]
- Misfeldt M. L., Johnson W. Identification of protective cell surface proteins in ribosomal fractions from Salmonella typhimurium. Infect Immun. 1979 Jun;24(3):808–816. doi: 10.1128/iai.24.3.808-816.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISSBACH A., HURWITZ J. The formation of 2-keto-3-deoxyheptonic acid in extracts of Escherichia coli B. I. Identification. J Biol Chem. 1959 Apr;234(4):705–709. [PubMed] [Google Scholar]
- Weinstein M. J., Waitz J. A., Came P. E. Induction of resistance to bacterial infections of mice with poly I-poly C. Nature. 1970 Apr 11;226(5241):170–170. doi: 10.1038/226170a0. [DOI] [PubMed] [Google Scholar]
- Youmans A. S., Youmans G. P. Effect of trypsin and ribonuclease on the immunogenic activity of ribosomes and ribonucleic acid isolated from Mycobacterium tuberculosis. J Bacteriol. 1966 Jun;91(6):2146–2154. doi: 10.1128/jb.91.6.2146-2154.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]


