Abstract
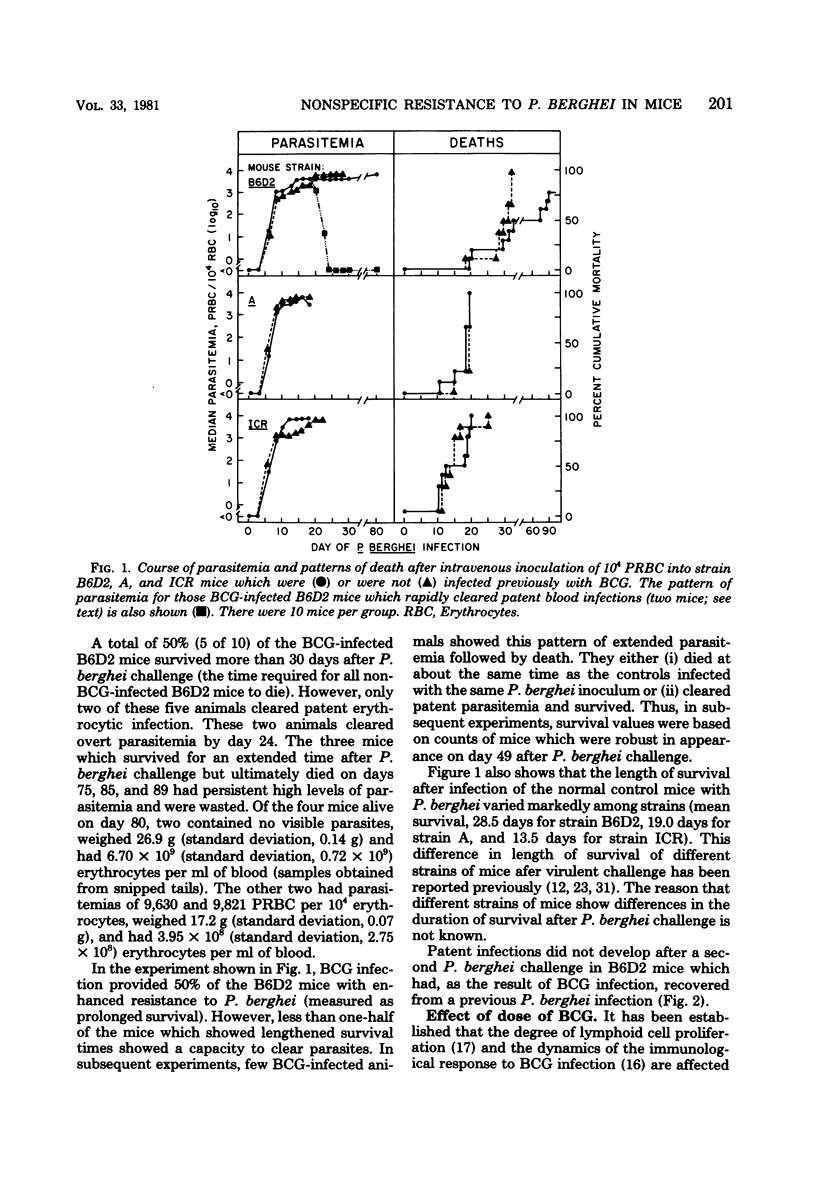
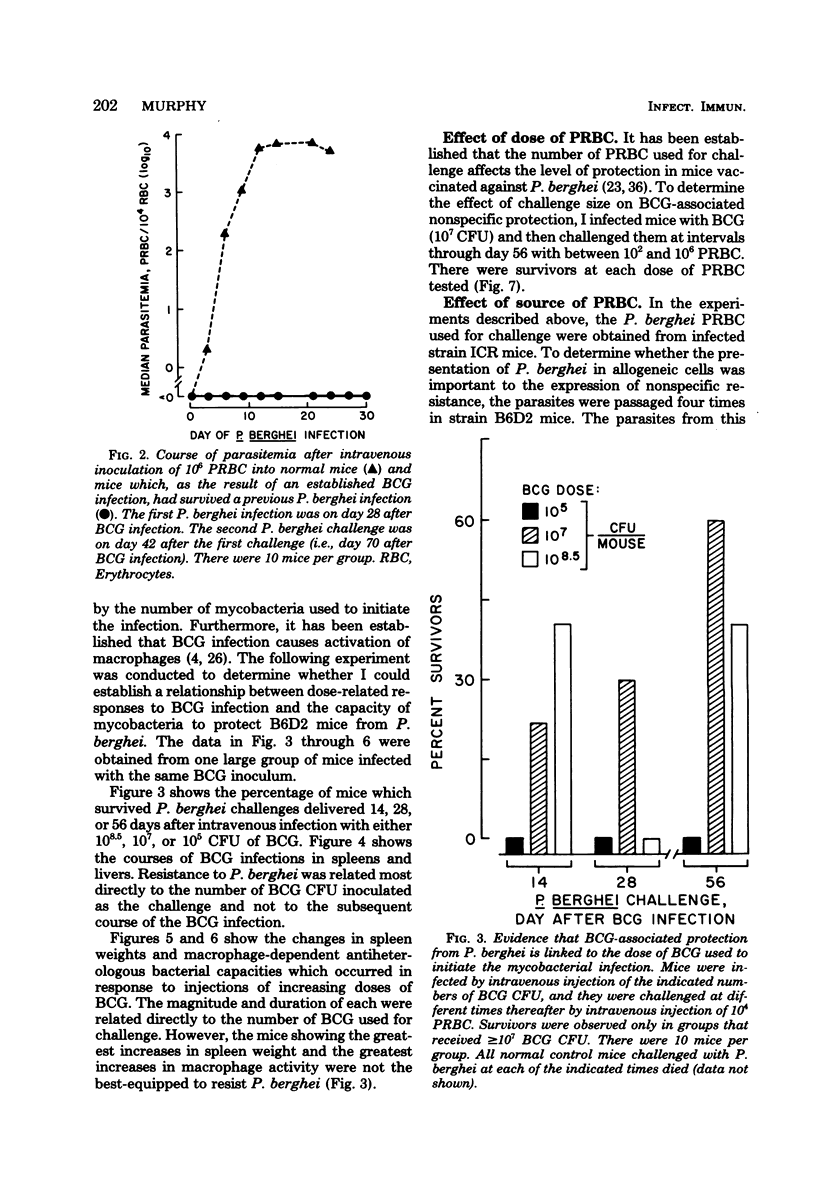
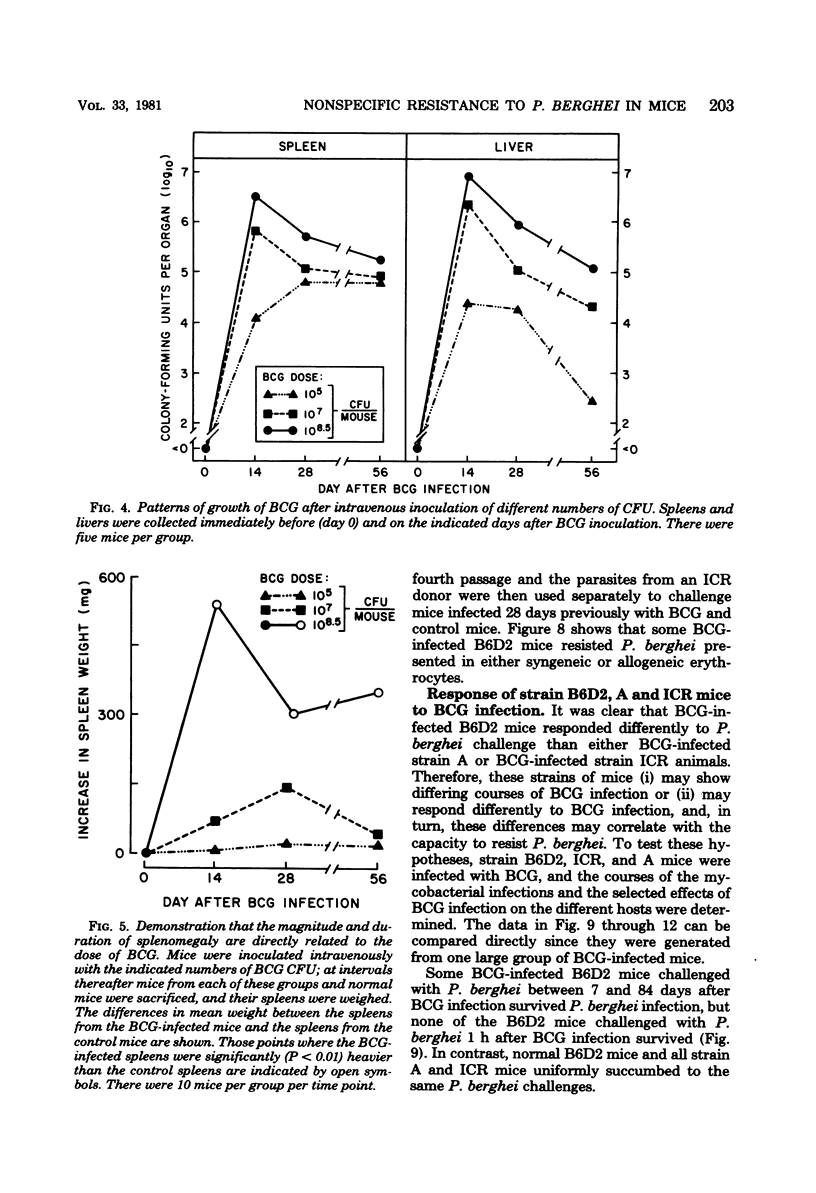
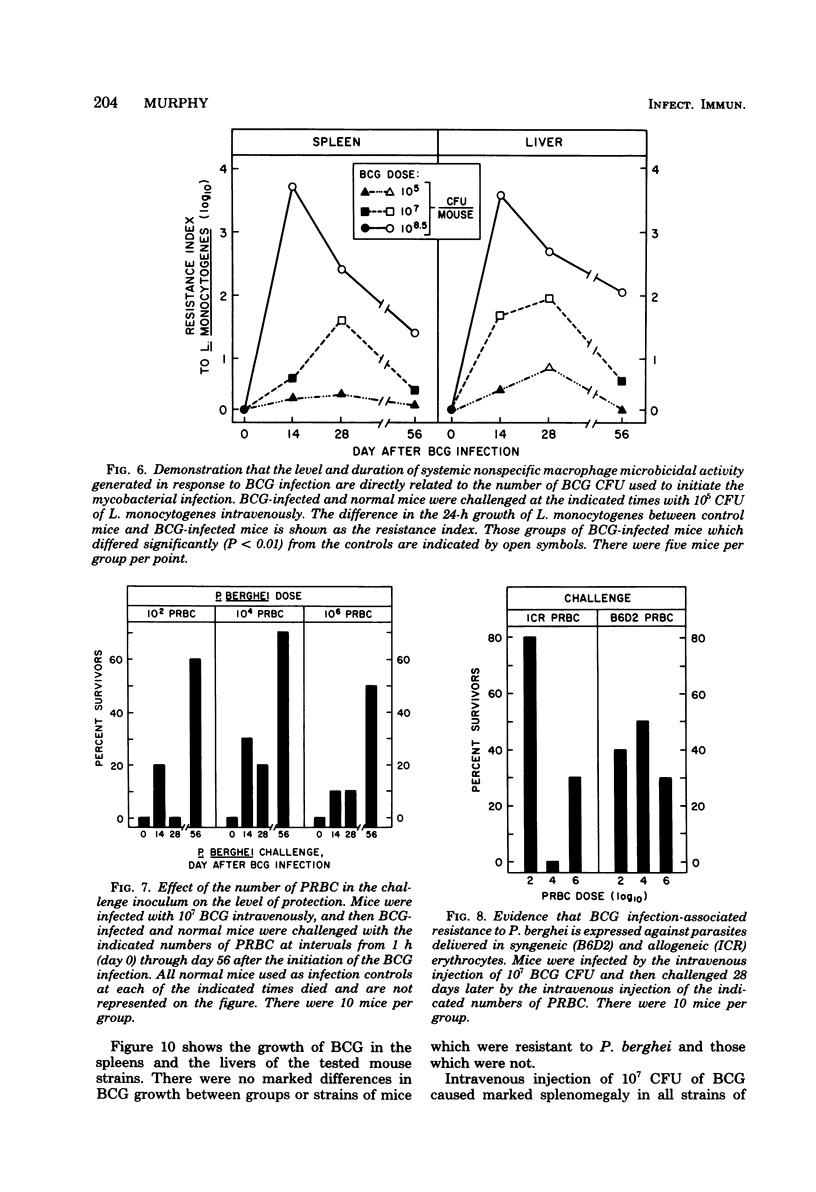
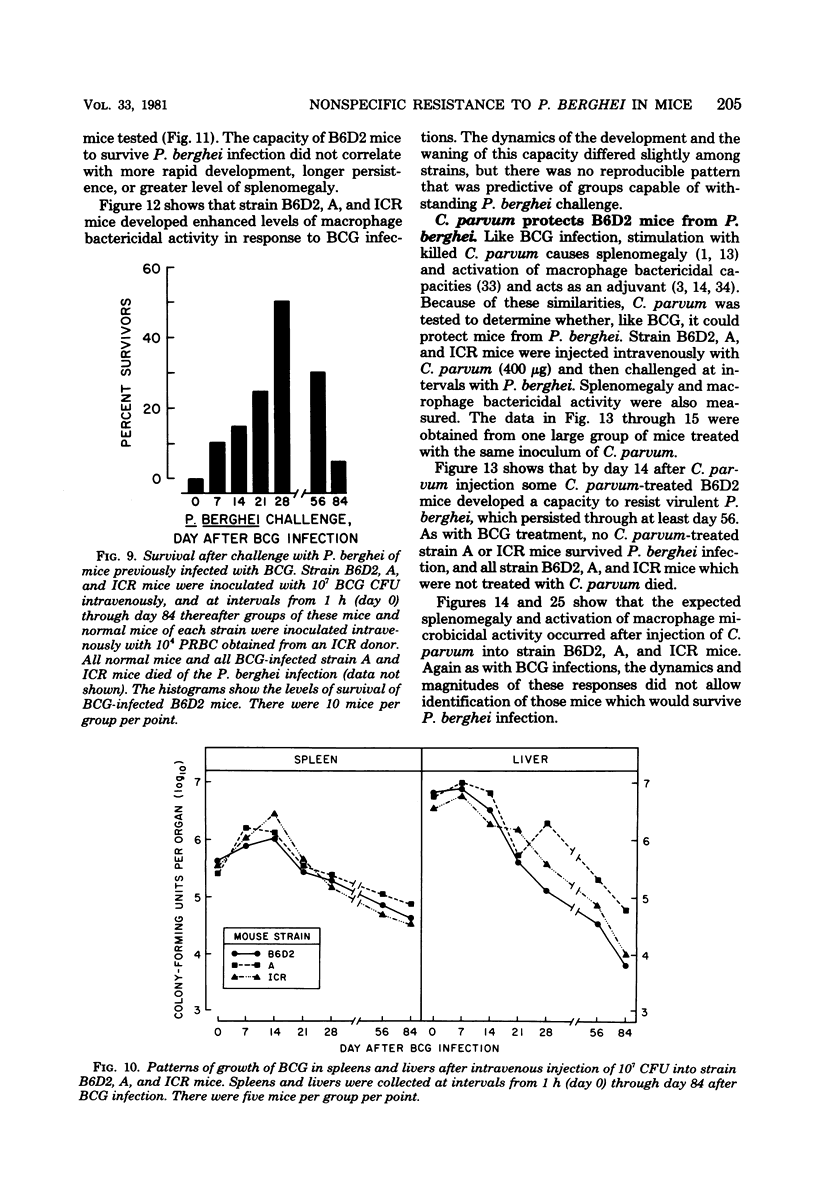
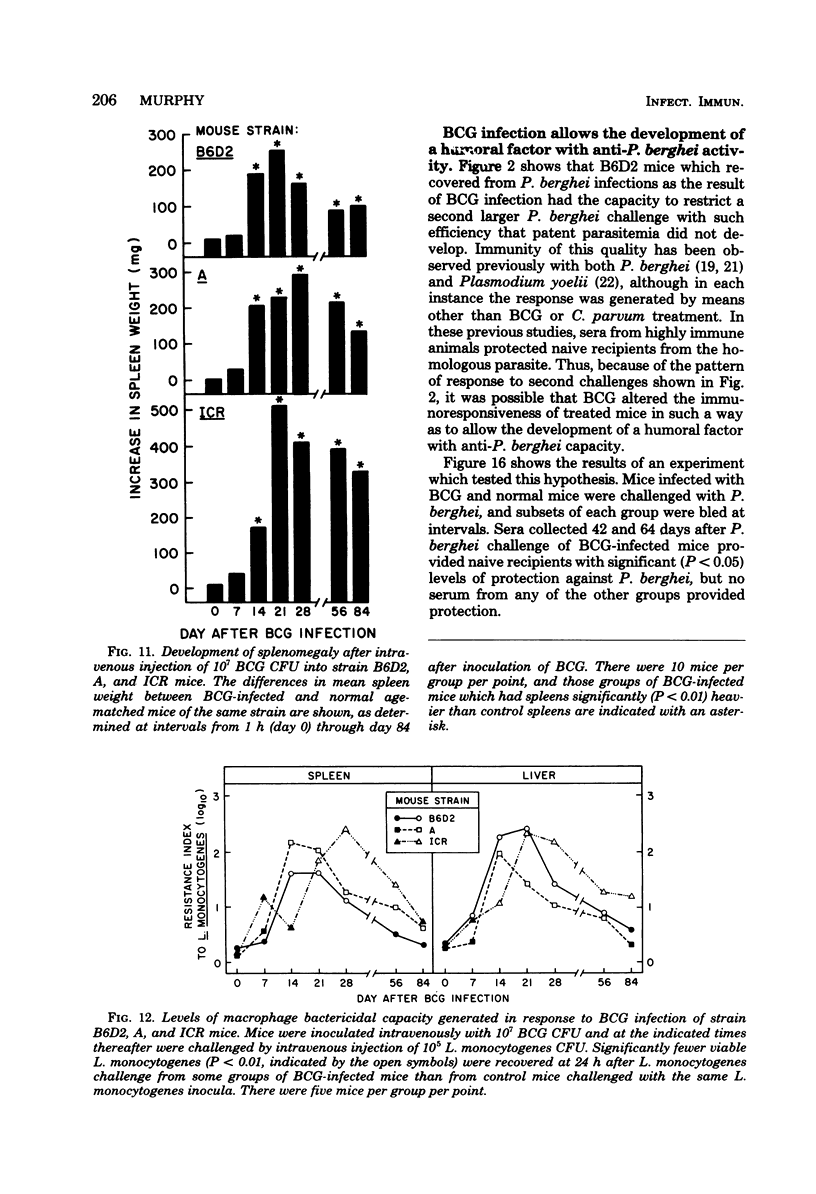
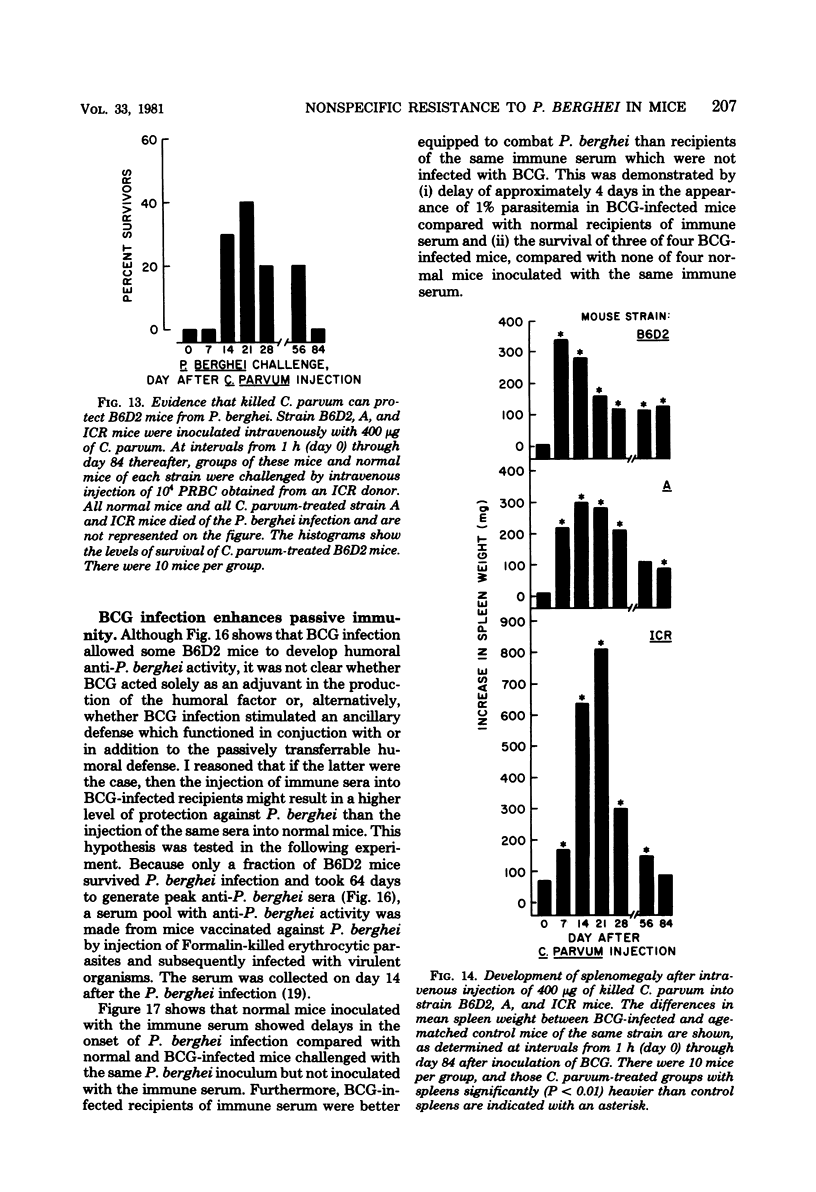
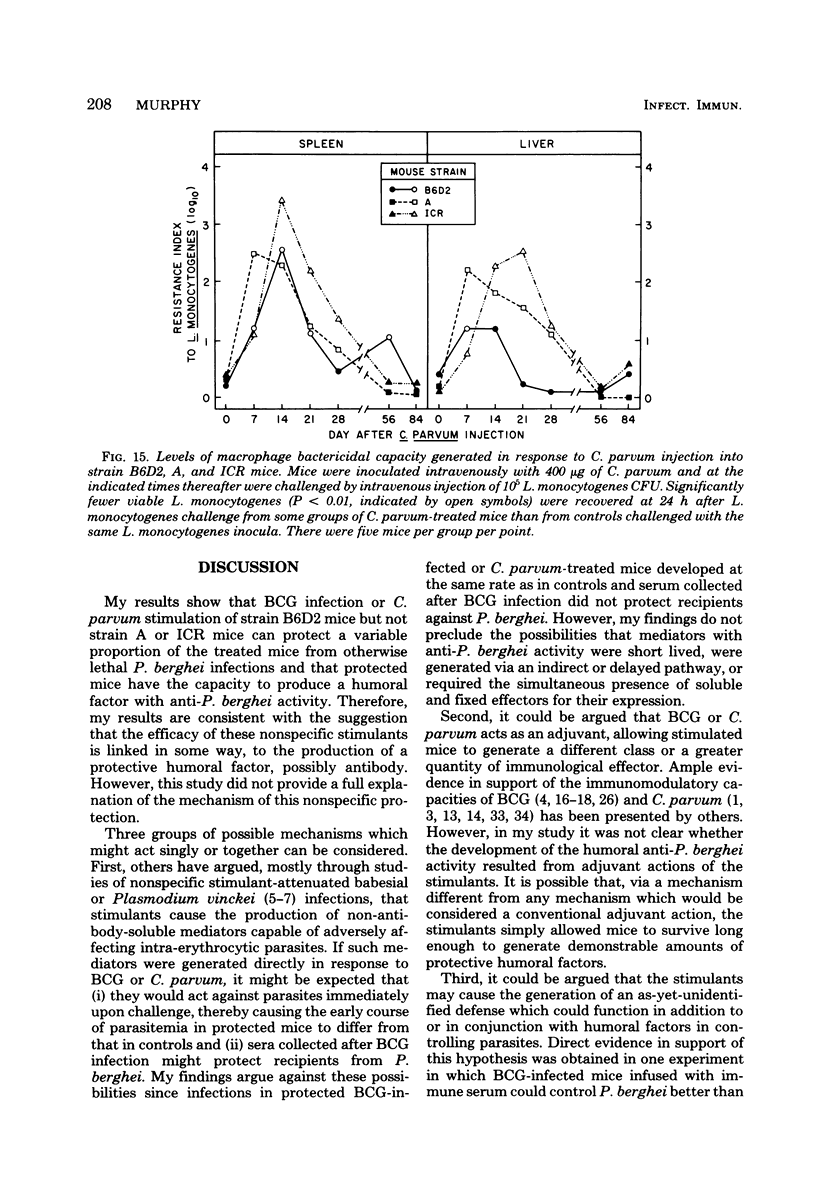
Infection with Mycobacterium bovis (BCG) or injection of killed Corynebacterium parvum protected some strain B6D2 F1 (C57BL/6xDBA/2) mice but did not protect strain ICR or A mice from lethal challenge with Plasmodium berghei strain NYU-2. B6D2 mice were not protected against challenges delivered immediately after intravenous injection of these materials, but rather protection developed by day 7 and persisted through at least day 84. Infections in protected mice progressed to about 10% parasitemia in parallel with infections initiated with the same inoculum in untreated controls. However, infections in most of the protected mice were cleared subsequently, whereas infections in untreated controls were uniformly fatal. A small number of treated mice developed protracted high-level erythrocytic infections, which led to markedly delayed death. BCG-infected mice which survived P. berghei infections had a factor in their sera which protected passively immunized recipients from P. berghei. BCG-infected mice passively immunized with protective serum controlled P. berghei infections better than normal mice given the same amount of the same serum and challenged with the same P. berghei inoculum. The capacity of BCG-infected B6D2 mice to resist P. berghei infection was not directly related to the pattern of growth of BCG, to the degree of splenomegaly, or to the level of activation of macrophages (measured as microbicidal capacity) caused by BCG infection. Therefore, I concluded that (i) BCG infection or injection of killed C. parvum altered the immunological potential of B6D2 mice in such a way as to allow the production of measurable levels of a protective humoral factor in response to infection with P. berghei; (ii) BCG infection caused the generation of a capacity which, when expressed in the presence of immune serum, provided an anti-P. berghei capacity which was superior to that provided by BCG infection alone or immune serum in the absence of BCG infection; and (iii) not all strains of mice could be protected from P. berghei by BCG or C. parvum injection.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adlam C., Broughton E. S., Scott M. T. Enhanced resistance of mice to infection with bacteria following pre-treatment with Corynebacterium parvum. Nat New Biol. 1972 Feb 16;235(59):219–220. doi: 10.1038/newbio235219a0. [DOI] [PubMed] [Google Scholar]
- Barker L. R. Acquired immunity to Plasmodium berghei yoelii in mice. Trans R Soc Trop Med Hyg. 1971;65(5):586–590. doi: 10.1016/0035-9203(71)90040-x. [DOI] [PubMed] [Google Scholar]
- Biozzi G., Stiffel C., Mouton D., Bouthillier Y., Decreusefond C. A kinetic study of antibody producing cells in the spleen of mice immunized intravenously with sheep erythrocytes. Immunology. 1968 Jan;14(1):7–20. [PMC free article] [PubMed] [Google Scholar]
- Blanden R. V., Lefford M. J., Mackaness G. B. The host response to Calmette-Guérin bacillus infection in mice. J Exp Med. 1969 May 1;129(5):1079–1107. doi: 10.1084/jem.129.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN S., McGREGOR I. A., CARRINGTON S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961 Nov 25;192:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Cox F. E., Allison A. C. Protection of mice against Babesia spp. and Plasmodium spp. with killed Corynebacterium parvum. Parasitology. 1977 Feb;74(1):9–18. doi: 10.1017/s003118200004748x. [DOI] [PubMed] [Google Scholar]
- Clark I. A. Resistance to Babesia spp. and Plasmodium sp. in mice pretreated with an extract of Coxiella burnetii. Infect Immun. 1979 May;24(2):319–325. doi: 10.1128/iai.24.2.319-325.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark I. A., Wills E. J., Richmond J. E., Allison A. C. Suppression of babesiosis in BCG-infected mice and its correlation with tumor inhibition. Infect Immun. 1977 Aug;17(2):430–438. doi: 10.1128/iai.17.2.430-438.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diggs C. L., Osler A. G. Humoral immunity in rodent malaria. II. Inhibition of parasitemia by serum antibody. J Immunol. 1969 Feb;102(2):298–305. [PubMed] [Google Scholar]
- Diggs C. L., Osler A. G. Humoral immunity in rodent malaria. III: Studies on the site of antibody action. J Immunol. 1975 Apr;114(4):1243–1247. [PubMed] [Google Scholar]
- GREENBERG J., KENDRICK L. P. Parasitemia and survival in inbred strains of mice infected with Plasmodium berghei. J Parasitol. 1957 Aug;43(4):413–419. [PubMed] [Google Scholar]
- Green T. J., Kreier J. P. Demonstration of the role of cytophilic antibody in resistance to malaria parasites (Plasmodium berghei) in rats. Infect Immun. 1978 Jan;19(1):138–145. doi: 10.1128/iai.19.1.138-145.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALPERN B. N., PREVOT A. R., BIOZZI G., STIFFEL C., MOUTON D., MORARD J. C., BOUTHILLIER Y., DECREUSEFOND C. STIMULATION DE L'ACTIVIT'E PHAGOCYTAIRE DU SYST'EME R'ETICULOENDOTH'ELIAL PROVOQU'EE PAR CORYNEBACTERIUM PARVUM. J Reticuloendothel Soc. 1964 Jan;1:77–96. [PubMed] [Google Scholar]
- Jahiel R. I., Nussenzweig R. S., Vilcek J., Vanderberg J. Protective effect of interferon inducers on Plasmodium berghei malaria. Am J Trop Med Hyg. 1969 Nov;18(6):823–835. doi: 10.4269/ajtmh.1969.18.823. [DOI] [PubMed] [Google Scholar]
- Lefford M. J. Induction and expression of immunity after BCG immunization. Infect Immun. 1977 Dec;18(3):646–653. doi: 10.1128/iai.18.3.646-653.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B., Auclair D. J., Lagrange P. H. Immunopotentiation with BCG. I. Immune response to different strains and preparations. J Natl Cancer Inst. 1973 Nov;51(5):1655–1667. doi: 10.1093/jnci/51.5.1655. [DOI] [PubMed] [Google Scholar]
- Mackaness G. B., Lagrange P. H., Ishibashi T. The modifying effect of BCG on the immunological induction of T cells. J Exp Med. 1974 Jun 1;139(6):1540–1552. doi: 10.1084/jem.139.6.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. R. Host defenses in murine malaria: analysis of the mechanisms of immunity to Plasmodium berghei generated in response to immunization with formalin-killed blood-stage parasites. Infect Immun. 1979 Jun;24(3):707–712. doi: 10.1128/iai.24.3.707-712.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. R. Host defenses in murine malaria: immunological characteristics of a protracted state of immunity to Plasmodium yoelii. Infect Immun. 1980 Jan;27(1):68–74. doi: 10.1128/iai.27.1.68-74.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. R., Lefford M. J. Host defenses in murine malaria: evaluation of the mechanisms of immunity to Plasmodium yoelii infection. Infect Immun. 1979 Feb;23(2):384–391. doi: 10.1128/iai.23.2.384-391.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. R., Lefford M. J. Host defenses in murine malaria: induction of a protracted state of immunity with a formalin-killed Plasmodium berghei blood parasite vaccine. Infect Immun. 1978 Dec;22(3):798–803. doi: 10.1128/iai.22.3.798-803.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. R., Lefford M. J. Host defenses in murine malaria: successful vaccination of mice against Plasmodium berghei by using formolized blood parasites. Am J Trop Med Hyg. 1979 Jan;28(1):4–11. doi: 10.4269/ajtmh.1979.28.4. [DOI] [PubMed] [Google Scholar]
- Newborg M. F., North R. J. On the mechanism of T cell-independent anti-Listeria resistance in nude mice. J Immunol. 1980 Feb;124(2):571–576. [PubMed] [Google Scholar]
- North R. J., Kirstein D. P. T-cell-mediated concomitant immunity to syngeneic tumors. I. Activated macrophages as the expressors of nonspecific immunity to unrelated tumors and bacterial parasites. J Exp Med. 1977 Feb 1;145(2):275–292. doi: 10.1084/jem.145.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. T cell dependence of macrophage activation and mobilization during infection with Mycobacterium tuberculosis. Infect Immun. 1974 Jul;10(1):66–71. doi: 10.1128/iai.10.1.66-71.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. The concept of the activated macrophage. J Immunol. 1978 Sep;121(3):806–809. [PMC free article] [PubMed] [Google Scholar]
- Nussenzweig R. S. Increased nonspecific resstance to malaria produced by administration of killed Corynebacterium parvum. Exp Parasitol. 1967 Oct;21(2):224–231. doi: 10.1016/0014-4894(67)90084-7. [DOI] [PubMed] [Google Scholar]
- Rank R. G., Weidanz W. P., Bondi A. Nonsterilizing immunity in avian malaria: an antibody-independent phenomenon. Proc Soc Exp Biol Med. 1976 Feb;151(2):257–259. doi: 10.3181/00379727-151-39186. [DOI] [PubMed] [Google Scholar]
- Roberts D. W., Weidanz W. P. T-cell immunity to malaria in the B-cell deficient mouse. Am J Trop Med Hyg. 1979 Jan;28(1):1–3. doi: 10.4269/ajtmh.1979.28.1. [DOI] [PubMed] [Google Scholar]
- Seitz H. M. The Plasmodium berghei-infection in isogenic F1 (C57Bl x DBA)-mice. I. The course of the infection and immunization experiments. Tropenmed Parasitol. 1975 Dec;26(4):417–425. [PubMed] [Google Scholar]
- Smrkovski L. L., Strickland G. T. Rodent malaria: BCG-induced protection and immunosuppression. J Immunol. 1978 Oct;121(4):1257–1261. [PubMed] [Google Scholar]
- Tuttle R. L., North R. J. Mchanisms of antitumor action of Corynebacterium parvum: nonspecific tumor cell destruction at site of immunologically mediated sensitivity reaction to C. parvum. J Natl Cancer Inst. 1975 Dec;55(6):1403–1411. doi: 10.1093/jnci/55.6.1403. [DOI] [PubMed] [Google Scholar]
- Warr G. W., Sljivić V. S. Enhancement and depression of the antibody response in mice caused by Corynebacterium parvum. Clin Exp Immunol. 1974 Jul;17(3):519–532. [PMC free article] [PubMed] [Google Scholar]
- Weinbaum F. I., Evans C. B., Tigelaar R. E. Immunity to Plasmodium Berghei yoelii in mice. I. The course of infection in T cell and B cell deficient mice. J Immunol. 1976 Nov;117(5 PT2):1999–2005. [PubMed] [Google Scholar]
- Wellde B. T., Ward R. A., Ueoka R. Aspects of immunity in mice inoculated with irradiated Plasmodium berghei. Mil Med. 1969 Sep;134(10):1153–1164. [PubMed] [Google Scholar]


