Abstract
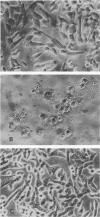
Antibiotic-associated pseudomembranous colitis has been linked with Clostridium difficile toxin. We examined the effect of toxins from four strains of C. difficile isolated from patients with pseudomembranous colitis on colonic adenylate (EC 4.6.1.1) and guanylate cyclase (EC 4.6.1.2) activities. Partially purified toxins had a cytotoxic effect on hamster fibroblasts in culture at a concentration of 10 ng/ml. Likewise, these toxins enhanced colonic guanylate cyclase activity two- to threefold, with the maximal stimulation being at 10 ng/ml. These toxins also enhanced guanylate cyclase activity in ileum, cecum, and duodenum. Both the cytotoxic activity on hamster fibroblasts and the enhancement of hamster guanylate cyclase activity were inhibited by antiserum to C. difficile toxin. These same toxins inhibited adenylate cyclase activity at a 100-ng/ml concentration, but had no effect at 10 ng/ml. They also had no effect at any concentration on colonic Na+-K+ adenosine triphosphatase. To be sure that the findings were not due to a contaminant, a purified C. difficile cytotoxin was used, and the same findings were found with the pure cytotoxin (at a 100-fold-lower concentration). The data suggest that activation of guanylate cyclase may be a factor in the pathogenesis of antimicrobial-associated pseudomembranous colitis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartlett J. G., Chang T. W., Gurwith M., Gorbach S. L., Onderdonk A. B. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N Engl J Med. 1978 Mar 9;298(10):531–534. doi: 10.1056/NEJM197803092981003. [DOI] [PubMed] [Google Scholar]
- Benner E. J., Tellman W. H. Pseudomembraneous colitis as a sequel to oral lincomycin therapy. Am J Gastroenterol. 1970 Jul;54(1):55–58. [PubMed] [Google Scholar]
- Chang T. W., Gorbach S. L., Bartlett J. B. Neutralization of Clostridium difficile toxin by Clostridium sordellii antitoxins. Infect Immun. 1978 Nov;22(2):418–422. doi: 10.1128/iai.22.2.418-422.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claflin A. J., Vesely D. L., Hudson J. L., Bagwell C. B., Lehotay D. C., Lo T. M., Fletcher M. A., Block N. L., Levey G. S. Inhibition of growth and guanylate cyclase activity of an undifferentiated prostate adenocarcinoma by an extract of the balsam pear (Momordica charantia abbreviata). Proc Natl Acad Sci U S A. 1978 Feb;75(2):989–993. doi: 10.1073/pnas.75.2.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L. E., McNeill C. J., Wells R. F. Clindamycin-associated colitis. JAMA. 1973 Mar 19;223(12):1379–1380. [PubMed] [Google Scholar]
- Ehrich M., Van Tassell R. L., Libby J. M., Wilkins T. D. Production of Clostridium difficile antitoxin. Infect Immun. 1980 Jun;28(3):1041–1043. doi: 10.1128/iai.28.3.1041-1043.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fee H. J., Ament M. E., Holmes E. C. Pseudomembranous colitis associated with cephazolin therapy. Am J Surg. 1977 Feb;133(2):247–248. doi: 10.1016/0002-9610(77)90092-7. [DOI] [PubMed] [Google Scholar]
- Field M., Graf L. H., Jr, Laird W. J., Smith P. L. Heat-stable enterotoxin of Escherichia coli: in vitro effects on guanylate cyclase activity, cyclic GMP concentration, and ion transport in small intestine. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2800–2804. doi: 10.1073/pnas.75.6.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frandsen E. K., Krishna G. A simple ultrasensitive method for the assay of cyclic AMP and cyclic GMP in tissues. Life Sci. 1976 Mar 1;18(5):529–541. doi: 10.1016/0024-3205(76)90331-3. [DOI] [PubMed] [Google Scholar]
- George R. H., Symonds J. M., Dimock F., Brown J. D., Arabi Y., Shinagawa N., Keighley M. R., Alexander-Williams J., Burdon D. W. Identification of Clostridium difficile as a cause of pseudomembranous colitis. Br Med J. 1978 Mar 18;1(6114):695–695. doi: 10.1136/bmj.1.6114.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg N. D., Haddox M. K., Nicol S. E., Glass D. B., Sanford C. H., Kuehl F. A., Jr, Estensen R. Biologic regulation through opposing influences of cyclic GMP and cyclic AMP: the Yin Yang hypothesis. Adv Cyclic Nucleotide Res. 1975;5:307–330. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Larson H. E., Price A. B. Pseudomembranous colitis: Presence of clostridial toxin. Lancet. 1977 Dec 24;2(8052-8053):1312–1314. doi: 10.1016/s0140-6736(77)90363-4. [DOI] [PubMed] [Google Scholar]
- Lusk R. H., Fekety R., Silva J., Browne R. A., Ringler D. H., Abrams G. D. Clindamycin-induced enterocolitis in hamsters. J Infect Dis. 1978 Apr;137(4):464–475. doi: 10.1093/infdis/137.4.464. [DOI] [PubMed] [Google Scholar]
- Mittal C. K., Murad F. Activation of guanylate cyclase by superoxide dismutase and hydroxyl radical: a physiological regulator of guanosine 3',5'-monophosphate formation. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4360–4364. doi: 10.1073/pnas.74.10.4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkin G. D., Fekety F. R., Silva J., Jr Antibiotic-induced colitis implication of a toxin neutralised by Clostridium sordellii antitoxin. Lancet. 1977 Nov 26;2(8048):1103–1106. doi: 10.1016/s0140-6736(77)90547-5. [DOI] [PubMed] [Google Scholar]
- Rolfe R. D., Finegold S. M. Purification and characterization of Clostridium difficile toxin. Infect Immun. 1979 Jul;25(1):191–201. doi: 10.1128/iai.25.1.191-201.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapiro R. L., Newman A. Acute enterocolitis. A complication of antibiotic therapy. Radiology. 1973 Aug;108(2):263–268. doi: 10.1148/108.2.263. [DOI] [PubMed] [Google Scholar]
- Sharp G. W., Hynie S. Stimulation of intestinal adenyl cyclase by cholera toxin. Nature. 1971 Jan 22;229(5282):266–269. doi: 10.1038/229266a0. [DOI] [PubMed] [Google Scholar]
- Straub K. D., Carver P. Sanguinarine, inhibitor of Na-K dependent ATP'ase. Biochem Biophys Res Commun. 1975 Feb 17;62(4):913–922. doi: 10.1016/0006-291x(75)90410-6. [DOI] [PubMed] [Google Scholar]
- Tedesco F. J., Barton R. W., Alpers D. H. Clindamycin-associated colitis. A prospective study. Ann Intern Med. 1974 Oct;81(4):429–433. doi: 10.7326/0003-4819-81-4-429. [DOI] [PubMed] [Google Scholar]
- Vesely D. L., Castro A., Levey G. S. Decreased rat hepatic guanylate cyclase activity in streptozotocin-induced diabetes mellitus. Diabetes. 1977 Apr;26(4):308–313. doi: 10.2337/diab.26.4.308. [DOI] [PubMed] [Google Scholar]
- Vesely D. L., Rovere L. E., Levey G. S. Activation of guanylate cyclase by streptozotocin and 1-methyl-1-nitrosourea. Cancer Res. 1977 Jan;37(1):28–31. [PubMed] [Google Scholar]
- Vesely D. L. Testosterone and its precursors and metabolites enhance guanylate cyclase activity. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3491–3494. doi: 10.1073/pnas.76.7.3491. [DOI] [PMC free article] [PubMed] [Google Scholar]