Summary
A cilia-driven leftward flow of extracellular fluid breaks bilateral symmetry in the dorsal midline of the neurula stage vertebrate embryo. The left-specific Nodal signaling cascade in the lateral plate mesoderm (LPM) is key to asymmetric morphogenesis and placement of organs during subsequent development. The nature of the initial asymmetric cue(s) as well as the transfer of information from the midline to the left side has remained elusive. Gap junctional communication has been previously involved in Xenopus left-right (LR) development, however a function at cleavage stages was inferred from inhibitor experiments. Here we show by heptanol-mediated block of connexin function that flow stages during neurulation represent the critical time window. Flow in Xenopus occurs at the gastrocoel roof plate (GRP), a ciliated sheath of cells of mesodermal fate transiently positioned within the dorsal epithelial lining of the forming archenteron. We reasoned that endodermal cells immediately adjacent to the GRP are important for transfer of asymmetry. A systematic screen identified two connexin genes, Cx26 and Cx32, which were co-expressed in these lateral endodermal cells. Gain- and loss-of-function experiments pinpointed Cx26 as the critical connexin for LR development, while Cx32 had no effect on laterality. Importantly, GRP morphology, ciliation and flow were not affected in Cx26 morphants. Our results demonstrate a decisive role of Cx26 in the transfer of laterality cues from the GRP to the left LPM, providing a novel access to the identification of the initial asymmetric signal generated by flow.
Keywords: Cx26, Cx32, Connexin, Left-right asymmetry, Gap junctions, Gap junctional communication, Xenopus laevis
Introduction
Superimposed on an overt bilaterally symmetrical body plan, vertebrates display an invariant asymmetric arrangement of most organs in the chest and abdomen, such as heart, lungs and stomach (situs solitus) (Hamada et al., 2002; Capdevila et al., 2000; Hirokawa et al., 2006). Asymmetric axis specification during embryogenesis comprises at least four distinct steps: (1) breakage of the bilateral symmetry of the early embryo; (2) transfer of asymmetric cues to the left lateral plate mesoderm (LPM); (3) induction of the asymmetric Nodal signaling cascade in the left LPM; and (4) asymmetric morphogenesis and placement of the developing organs. While the last two steps have been studied in great depth in recent years (Männer, 2009; Burn and Hill, 2009; Shen, 2007; Schier, 2003), symmetry breakage has remained controversial (Vandenberg and Levin, 2010; Tabin, 2005; Blum et al., 2009) and little is known about the nature of the initial asymmetric information and its transfer to the LPM (Oki et al., 2007; Marjoram and Wright, 2011).
In fish, amphibian and mammalian embryos a cilia-driven leftward flow of extracellular fluid is required for asymmetric Nodal induction in the left LPM (Hirokawa et al., 2006; Blum et al., 2009). Flow occurs at an epithelium which is found in the dorsal midline of the neurula embryo at the posterior pole of the forming notochord (Blum et al., 2007; Lee and Anderson, 2008). These epithelia, which are present only very transiently during neurulation, vary in size and shape. They are represented by Kupffer's vesicle (KV) in teleost fish, the gastrocoel roof plate (GRP) in amphibians and the posterior notochord (PNC) / node in mammalian embryos (Blum et al., 2009). Loss of flow invariably results in aberrant expression of asymmetric marker genes and misplacement of organs (heterotaxia) or situs inversion (Hamada, 2008).
Whether leftward flow represents the initial step of vertebrate symmetry breakage has remained a matter of debate. Besides a failure to detect cilia and flow in avian embryos (Tabin, 2005), functionally relevant molecular asymmetries already during early cleavage stages have been described in the African clawed frog Xenopus laevis (Levin et al., 2002; Fukumoto et al., 2005; Levin and Mercola, 1999; Levin and Mercola, 1998). An alternative mode of symmetry breakage has been put forward, according to which asymmetric activity of the ion pump ATP4 (formally called gastric H+, K+-ATPase) sets up a voltage gradient which drives small and charged molecules such as the monoamine serotonin through gap junctions (GJ) to accumulate asymmetrically in specific blastomeres at the 64-cell stage (Vandenberg and Levin, 2010; Vandenberg and Levin, 2009; Levin, 2005). This so-called ‘ion-flux’ model has recently been challenged. Serotonin, though required for left-right (LR) asymmetric development, was found to be symmetrically distributed in the early Xenopus embryo. Serotonin signaling was shown to act as a competence factor for Wnt-induced specification of the superficial mesoderm (SM), from which the ciliated GRP develops, where leftward flow in frog occurs (Beyer et al., 2012).
Functional studies, however, have unequivocally involved GJs in LR development in amphibian, avian and mammalian embryos. Heptanol (HepOH) and lindane were used as inhibitors of gap junctional communication (GJC) in frog, chicken and rabbit embryos (Levin and Mercola, 1998; Levin and Mercola, 1999; Feistel and Blum, 2008). In Xenopus, where a dominant-negative connexin (Cx) construct as well as ectopic expression of Cx26, Cx43 and Cx37 additionally confirmed GJC involvement in LR specification, GJs were required up to gastrulation, in accordance with the ‘ion-flux’ model (Levin and Mercola, 1998). In chicken, interference with GJC compromised the earliest molecular asymmetry, i.e. shh expression in the node (Levin and Mercola, 1999), which meanwhile has been shown to result from asymmetric cell migration during gastrulation (Gros et al., 2009). In rabbit, the time window extended through flow stages and it was proposed that flow impacted on the opening status of GJs (Feistel and Blum, 2008). In zebrafish, Cx43.4 was found to be specifically expressed in the developing KV and to be required for KV morphogenesis, specifically for lumen formation (Hatler et al., 2009). In humans involvement of GJC in the determination of organ situs is less clear: an initial report of Cx43 mutations in six patients with visceroatrial heterotaxia (Britz-Cunningham et al., 1995) was challenged by four subsequent studies which failed to reveal Cx43 gene defects in together 93 patients (Casey and Ballabio, 1995; Splitt et al., 1995; Gebbia et al., 1996; Debrus et al., 1997). Although a general role of GJC in laterality determination is thus strongly supported by experimental evidence from several vertebrate model systems, the sensitive step(s), the identity of relevant Cx genes and the mode of action remains elusive.
In the present study we re-evaluated the time window of GJC requirement for LR development in Xenopus, specifically with respect to leftward flow, which had not been described when the initial studies were performed. Injection of HepOH into the gastrocoel demonstrated that GJC was needed throughout flow stages. An in situ screen for Cx genes expressed at the relevant stages and in relevant tissues identified Cx26 and Cx32. Both genes were co-expressed in the endodermal epithelial lining of the gastrocoel adjacent to the GRP. Gain- and loss-of-function experiments demonstrated that only Cx26 was involved in LR development, and that Cx26 was required for asymmetric Nodal cascade induction downstream of flow. These results strongly suggest a role of Cx26 in the transfer of asymmetric cue(s) from the midline to the LPM.
Results
GJC is required during flow stages for asymmetric development
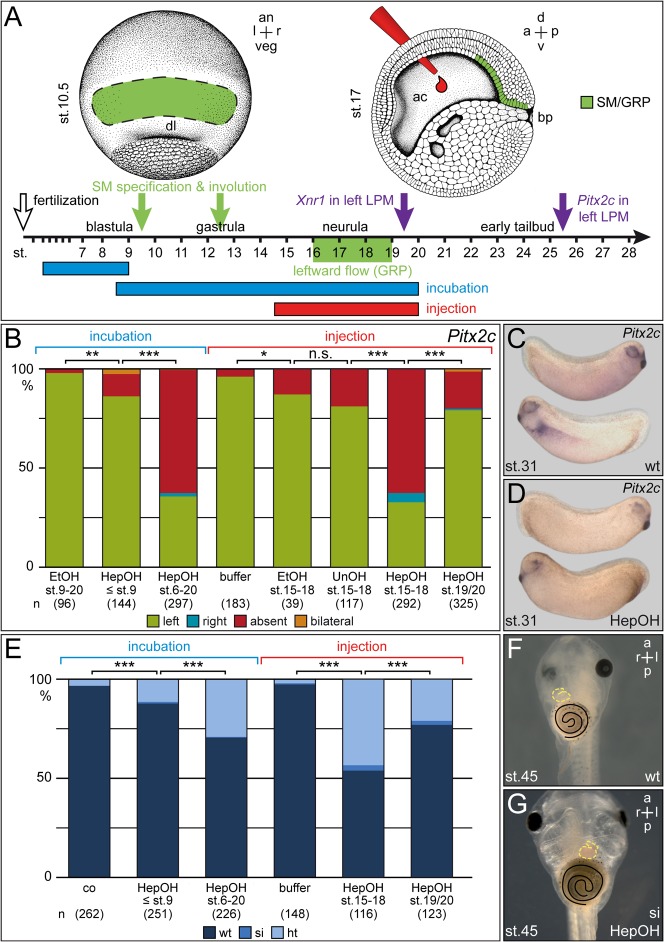
In order to determine the critical time frame for GJC in LR development, incubation and injection experiments were performed. The long-chain alcohol HepOH was used as universal GJ inhibitor. HepOH was proposed to act by squeezing channels closed following membrane insertion (Guan et al., 1997; Juszczak and Swiergiel, 2009). Ethanol (EtOH) was used as control, because of the known impact of EtOH on early Xenopus development (Yelin et al., 2007; Yelin et al., 2005). Incubations at high concentration of EtOH or HepOH (≥0.02%) impaired gastrulation with blastopore closure defects (data not shown) and shortened anterior-posterior (AP) axes (supplementary material Fig. S1). Concentrations of 0.01% were used throughout this study, as dorso-anterior development was unaffected by this treatment (Fig. 1D). In addition, only embryos with a wildtype dorso-anterior index (DAI) of 5 were evaluated for LR defects. Treatment of embryos from early cleavage through mid-blastula (4-cell to stage 9) or from early blastula through neurulation (stage 6–20) resulted in absence of Pitx2c expression from the left LPM (Fig. 1A–D) and in heterotaxia, as assessed by heart looping, gut coiling and the positioning of the gall bladder (Fig. 1E–G). Remarkably, effects were much more pronounced when treatments were performed starting at blastula as opposed to cleavage stages (Fig. 1B,E), in conflict with the ‘ion-flux’ model.
Fig. 1. Neurula stage embryos require GJC for LR development.
(A) Scheme depicting type and time course of treatments. (B–D) HepOH treatments of neurula stage embryos resulted in absence of Pitx2c expression in the left LPM of 2-day tadpoles. (B) Summary of experimental data. (C,D) Representative wildtype (C) and HepOH-treated (D) embryos displaying left-asymmetric (C) or absent (D) expression of Pitx2c in the left LPM. (E–G) Consequences of treatments for situs development in the 3-day tadpole. (E) Summary of results. (F,G) Characteristic untreated (F) and HepOH-incubated (G) tadpoles displaying normal organ situs (F) or situs inversion (G), as determined by the direction of heart looping (outlined by dashed yellow line) and gut coiling (outlined in black). Note that ethanol and undecanol were inefficient. Note also that early treatments before gastrulation (≤ stage 9) and late injections (stage 19/20) were less striking or without effect. a, anterior; an, animal; d, dorsal; EtOH, ethanol; HepOH, HepOH; ht, heterotaxia; n.s., not significant; p, posterior; si, situs inversus; st., stage; UnOH, undecanol; v, ventral; veg, vegetal; wt, wildtype; *, significant (p<0.05); **, highly significant (p<0.01); *** very highly significant (p<0.001). Numbers in brackets represent number of analyzed specimens. Statistical significances were calculated using Pearson's chi square test.
The initial study on GJC in the frog reported that the time frame for interference with LR development closed at stage 12, i.e. late gastrulation (Levin and Mercola, 1998) and thus well before leftward flow sets in at stage 15/16 (early neurula). As stage 12 marks exactly the time point when SM cells have completed involution into the gastrocoel to form the GRP by stage 13 (Shook et al., 2004), we wondered whether ineffectiveness of later treatments were caused by inaccessibility of the relevant tissue (SM/GRP) to the drug. Therefore a small droplet of about 20 nl of pure HepOH was injected directly into the gastrocoel (Fig. 1A). EtOH and undecanol (UnOH), which do not impact on GJC, were used as controls. These experiments (summarized in Fig. 1B) clearly demonstrate that interference with GJC during flow stages (15–18) affect both Pitx2c expression and organ situs to the same extent as incubation from stage 6 onwards. Injection at stage 19/20 was less efficient (Fig. 1B,E), i.e. at a stage when the GRP cells fold off to become integrated into the notochord, hypochord and somites, and when nodal becomes first expressed in the left LPM. Taken together, these experiments show that GJC acts during flow stages, suggesting a role on flow itself or on steps downstream.
Cx26 and Cx32 are co-expressed in the epithelial lining of the gastrocoel
As a first step towards investigating a role for GJC in flow and/or downstream events, we aimed at identifying connexin genes expressed during the relevant stages. A literature and database (http://www.xenbase.org) search was applied to identify candidate genes. Cx43, the gene reported to be involved in human laterality (Britz-Cunningham et al., 1995), was expressed only from tadpole stages onwards (van der Heyden et al., 2001). To re-evaluate expression of this subunit, RT-PCR and whole-mount in situ hybridization (WMISH) of staged embryos was applied. While low-level expression was detectably by RT-PCR at all stages analyzed (data not shown), a localized signal was not seen by WMISH (Landesman et al., 2003) (data not shown). Therefore, Cx43 was not further investigated here. Cx40.4 was described to be active in somitic tissue (De Boer et al., 2005). As the lateral GRP cells are of somitic fate (sGRP) (Shook et al., 2004), we carefully analyzed Cx40.4 expression at neurula stages. No expression was seen in the GRP or adjacent mesendodermal tissues (supplementary material Fig. S2), which is why we did not pursue the analysis of Cx40.4 further. Cx26 and Cx32 were shown to be transcribed during neurulation (de Boer et al., 2006). These two subunits were of particular interest, because they were shown to form heterotypic channels in other contexts, suggesting redundant or complementary functions (Sosinsky, 1995; Barrio et al., 1991; Bevans et al., 1998; Ayad et al., 2006). RT-PCR analysis demonstrated expression of Cx26 from the 4-cell stage through stage 35 (two day tadpole), and of Cx32 from early neurulation (stage 13) onwards (supplementary material Fig. S3).
The spatio-temporal expression patterns of both genes were determined by WMISH of staged embryos. In contrast to the RT-PCR analysis (supplementary material Fig. S3), Cx26 signals were not detected in the 4-cell embryo, suggesting that the RT-PCR results represented low-level ubiquitous expression. Staining was seen at the dorsal lip during gastrulation (stage 10.5; Fig. 2A). Histological sections revealed that the signal was localized to involuting endodermal cells (Fig. 2A′). At stage 17, i.e. when robust leftward flow had developed at the GRP, Cx26 was restricted to the endodermal lining of the gastrocoel with the marked exception of the GRP itself (Fig. 2B,C′,C″). At later time points endodermal expression was seen in the dorsal half (Fig. 2D,D′). Signals were also identified in the pronephros and liver (Fig. 2D′), as previously described (de Boer et al., 2006).
Fig. 2. Cx26 and Cx32 are co-expressed in the epithelial lining of the gastrocoel next to the GRP.
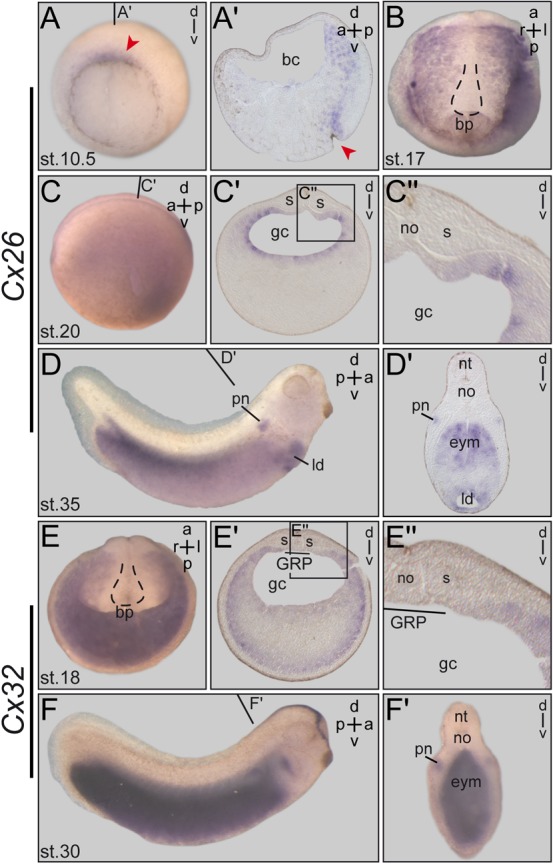
Temporal and spatial expression patterns were determined by WMISH of staged embryos. (A–D) Cx26. (A) In the early gastrula embryo Cx26 expression around the blastopore is enhanced on the dorsal side (arrowhead). (A′) Histological section shows Cx26 staining in the involuting endodermal cells. (B,C) In the stage 17 neurula embryo Cx26 mRNA is found in the endoderm lining the gastrocoel. (B) Dorsal explant. Outline of GRP, which is free of staining, indicated by dashed line. (C) Sectioning (C′) of whole-mount embryo (C) shows Cx26 expression all around the gastrocoel with the marked exception of the GRP, demonstrated in blowup shown in (C″). (D) Cx26 expression at stage 35. Transversal section (D′) shows staining in the pronephros, the liver diverticulum and in the dorsal half of the endoderm. (E,F) Cx32. (E) Expression in posterior half of stage 18 neurula embryo. Section in (E′) demonstrates staining in two open ring-like domains representing the limit of the endoderm, compassing the endodermal yolk cells. Blowup in (E″) shows that the GRP is free of Cx32 expression. (F) Cx32 expression at stage 30. Transversal hemisection in (F′) reveals mRNA staining in the pronephros, hatching gland and throughout the endoderm. a, anterior; bc, blastocoel; bp, blastopore; d, dorsal; eym, endodermal yolk mass; gc, gastrocoel; l, left; ld, liver diverticulum; no, notochord; nt, neural tube; p, posterior; pn, pronephros; r, right;s, somite; st., stage; v, ventral. The position of the GRP is indicated by dashed lines in (B) and (E). Red arrowheads mark the position of the blastopore in (A,A′). Lines in (C,D,F) indicate planes of histological sections in (C′,D′,F′).
Cx32 mRNA was first detected in the endoderm of the neurula embryo (Fig. 2E). Cross sections revealed expression in two ring-like domains, with a stronger outer ring marking the outermost endodermal cells, and a weaker inner ring lining the gastrocoel (Fig. 2E′). The GRP region itself (i.e. tissue of mesodermal fate), however, was excluded (Fig. 2E). Faint staining in endodermal cells in between the two rings was detected as well (Fig. 2E′). Endodermal expression persisted through stage 30, when strong staining throughout the endoderm was seen (Fig. 2F,F′). Additional sites of Cx32 mRNA localization were identified in the hatching gland and the primordium of the stomodeum at stage 24 (supplementary material Fig. S3) and in the pronephros of the stage 30 tadpole (Fig. 2F′). In summary these expression patterns demonstrated co-expression of Cx26 and Cx32 in the epithelial lining of the gastrocoel with the explicit exception of the GRP proper.
Left-sided over-expression of Cx26 disrupts normal LR development
The initial study which involved GJC in LR development used HepOH to block GJC as well as gain-of-function of Cx26, Cx37 and Cx43. Additionally a hybrid constructs was used which presumably acted in a dominant-negative manner (Levin and Mercola, 1998; Paul et al., 1995). In all cases heterotaxia was induced in injected specimens (Levin and Mercola, 1998). As Cx26 was only recently renamed (from formerly Cx29) (de Boer et al., 2006) we wondered whether the two genes identified as candidates in our expression screen would act in the same way upon over- or ectopic expression in the embryo. Fusion constructs were used in order to analyze the potential of Cx26 and Cx32 to form heterotypic channels. C-terminal fusions were cloned, as this type of hybrid protein was previously shown to form functional channels. Cx26 was fused to eGFP, and Cx32 to RFP. RNAs were injected into one animal cell of the 4–8 cell embryo, and localization was assessed at blastula stage. Fig. 3 shows co-localization of both hybrid constructs in cytoplasmic vesicles (likely representing ER vesicles) and at the membrane, suggesting that Cx26 and Cx32 were in principle able to form heteromeric channels during early frog development.
Fig. 3. Cx26 and Cx32 form heteromeric connexons in Xenopus embryos.
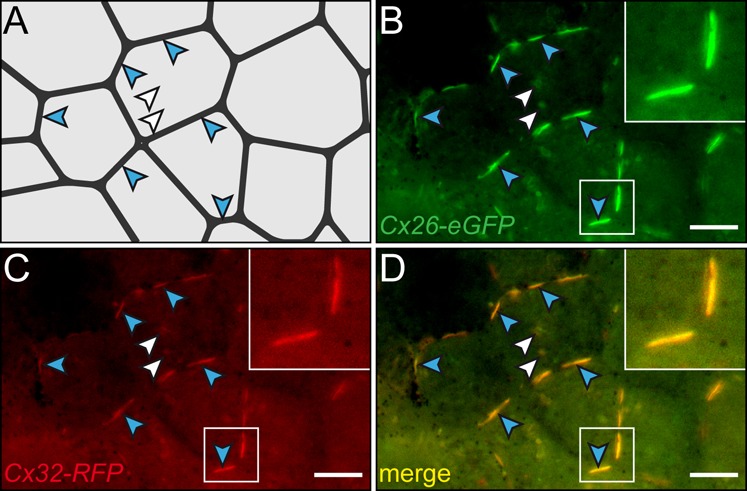
Fusion constructs (Cx26-eGFP and Cx32-RFP) were co-injected into the animal region of single blastomeres at the 4-8 cell stage and cultured through blastula-gastrula stages. Fluorescent fusion proteins were co-expressed in ectodermal cells of the animal cap region. Expression was found in vesicles (white arrowheads) and the plasma membrane (blue arrowheads). (A) Cell boundaries. Positions highlighted in (B–D) are indicated by arrowheads. (B) Cx26-eGFP staining. (C) Cx32-RFP staining. (D) Overlay of frames. Scale bar represents 10 µm.
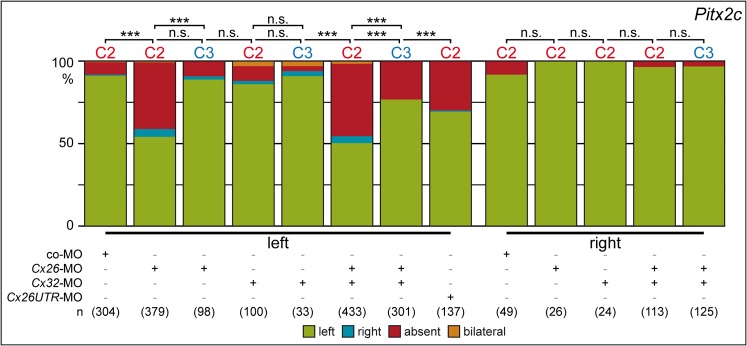
Next we injected expression constructs encoding full-length Cx26 and Cx32 alone or in combination into left or right blastomeres at the 4-cell stage. Based on the expression patterns we wanted to target endodermal cells bordering the GRP. Lineage tracing experiments were performed by co-injecting rhodamine dextran and LacZ at the 4-cell stage. Fig. 4A summarizes the results and shows that injections into the dorsal marginal zone (C1 lineage) targeted the GRP. More lateral injections (C2 lineage) ended up in lateral GRP cells and adjacent endodermal tissue as well as somitic cells and dorsal ectodermal tissue. Labeling of the ventral marginal region (C3 and C4 lineage) stained the lateral plate and epidermis of stage 17 neurula embryos, and only very few laterally located endodermal cells (Vick et al., 2009). To aim at the epithelial lining of the gastrocoel, i.e. to target the tissue where Cx26 and Cx32 are expressed, injections were performed into the C2 lineage. Results are summarized in Fig. 4B. Injections of Cx26 and Cx32 into the right blastomeres did not affect left-asymmetric expression of Pitx2c, no matter whether constructs were applied alone or in combination. Left-sided injections of Cx26, however, disrupted Pitx2c induction, while Cx32 had no effect. Parallel injection of both RNAs did not enhance the effects seen with Cx26 alone. These experiments argue for specificity of effects seen with Cx26 and question a role of Cx32 in LR development.
Fig. 4. Overexpression of Cx26 but not Cx32 impairs LR development.
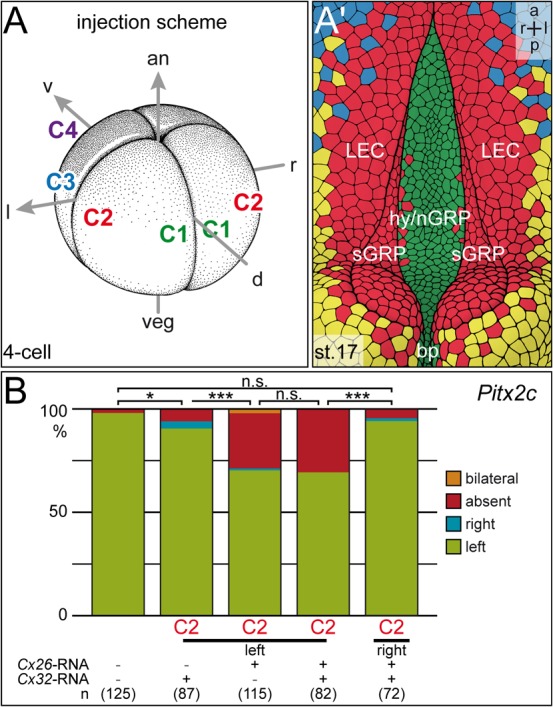
Full-length expression constructs were injected alone or in combination into single left or right blastomeres of the 4-8 cell embryos, and Pitx2c expression was analyzed in the 2-day tadpole. (A,A′) Injection scheme and cell lineage at stage 17. (A) Injections were targeted to the C2 lineage, i.e. cells which during flow stages (st. 17; A′) end up in the marginal cells of the GRP (somitic GRP; sGRP) and the adjacent lateral endodermal crest cells (LEC). (B) Summary of results. Note that right-sided injections did not alter Pitx2c expression. Note also that left-sided Cx26 overexpression prevented Pitx2c induction in the left LPM with very high statistical significance, while Cx32 had no effect on laterality, whether applied alone or in combination with Cx26. Numbers in brackets represent number of embryos analyzed. Statistical significances were calculated using Pearson's chi square test.
Altered Pitx2c expression in Cx26 but not in Cx32 morphants
To assess the endogenous role of candidate connexins, loss-of-function experiments were performed. Antisense morpholino oligonucleotides (MOs) were designed which targeted the translational start site of Cx26 and Cx32. As the gain-of-function experiments described above indicated a left-specific function of Cx26, knockdowns were performed in a side-specific manner. Injections were targeted to either the C2 or the C3 lineage (Fig. 4A). Right-sided MO-injections had no effect, while targeting of Cx26-MO to the left side prevented left-asymmetric induction of Pitx2c in about 80% of cases (Fig. 5). The specificity of the Cx26 knockdown was tested by using a non-overlapping second MO, which targeted the 5′-UTR. Although this MO was slightly less efficient, qualitatively the same result, i.e. predominantly absence of Pitx2c expression in the left LPM, was observed (Fig. 5). Knockdown of Cx32, in contrast, was inefficient (Fig. 5). Co-injection of Cx32-MO and Cx26-MO did not increase the percentage of specimens lacking Pitx2c expression seen in Cx26 morphants alone (Fig. 5). These experiments clearly showed that Cx26 but not Cx32 was required for correct LR development, and that this function was needed on the left but not on the right side of the embryo. In a last set of injections we investigated potential dorso-ventral differences. Although ventral targeting revealed some degree of LR disruption, as assessed by Pitx2c expression, dorsal injections were far more efficient (Fig. 5), with the ventral effects likely being due to incomplete separation of C2 and C3 lineages at the time point of injection (Vick et al., 2009).
Fig. 5. Cx26 but not Cx32 is required for LR development.
Lineage and side-specific knockdown of Cx26 and Cx32 alone or in combination as specified, followed by Pitx2c expression analysis in morphant tadpoles. Injections were targeted to the C2 lineage, i.e. the somitic GRP cells and the adjacent endodermal cells, or to the C3 lineage, i.e. more lateral endodermal cells (Fig. 4A,A′). Note that Cx32 knockdown did not affect Pitx2c expression, and that Cx26 was only required on the left side. Note also two non-overlapping Cx26-MOs, which targeted the 5′-UTR and the start AUG, respectively, gave qualitatively identical results, confirming the specificity of the knockdown. Note further that targeting to the endodermal cells adjacent to the GRP (C2 lineage) resulted in highly significant disruption of Pitx2c expression, while effects were not significant in lateral endodermal cells (C3 lineage). Numbers represent number of embryos analyzed. Statistical significances were calculated using Pearson's chi square test.
GRP ciliation and leftward flow occur normally in Cx26 morphants
Our previous studies on serotonin function (Beyer et al., 2012) and the role of ATP4 in LR development (Walentek et al., 2012) have revealed that aberrant LR marker gene expression can result from defects in the specification of the SM, from reduced cilia length or mispolarization, or from loss of cilia altogether, all of which result in altered flow patterns. Scanning electron microscopy was used to analyze the gross morphology and ciliation of the GRP. As shown in Fig. 6A–D the GRP of morphants displayed wildtype characteristics. As a last control experiment we analyzed flow directly, as impaired cilia motility would have resulted in the observed LR phenotypes as well. Dorsal explants of wildtype (n = 5) or Cx26-MO (n = 8) injected neurula embryos were prepared, fluorescent beads were added and flow was analyzed by time-lapse videography as described (Maisonneuve et al., 2009; Vick et al., 2009). Flow in morphants was indistinguishable from uninjected specimens (data not shown). In summary, our study demonstrates that Cx26 is required in a left-specific manner for LR axis formation in the frog Xenopus, and that the relevant time window for Cx26 action lies downstream of leftward flow at the GRP and upstream of Pitx2c expression in the left LPM.
Fig. 6. GRP morphology and ciliation are unaffected in Cx26 morphants.
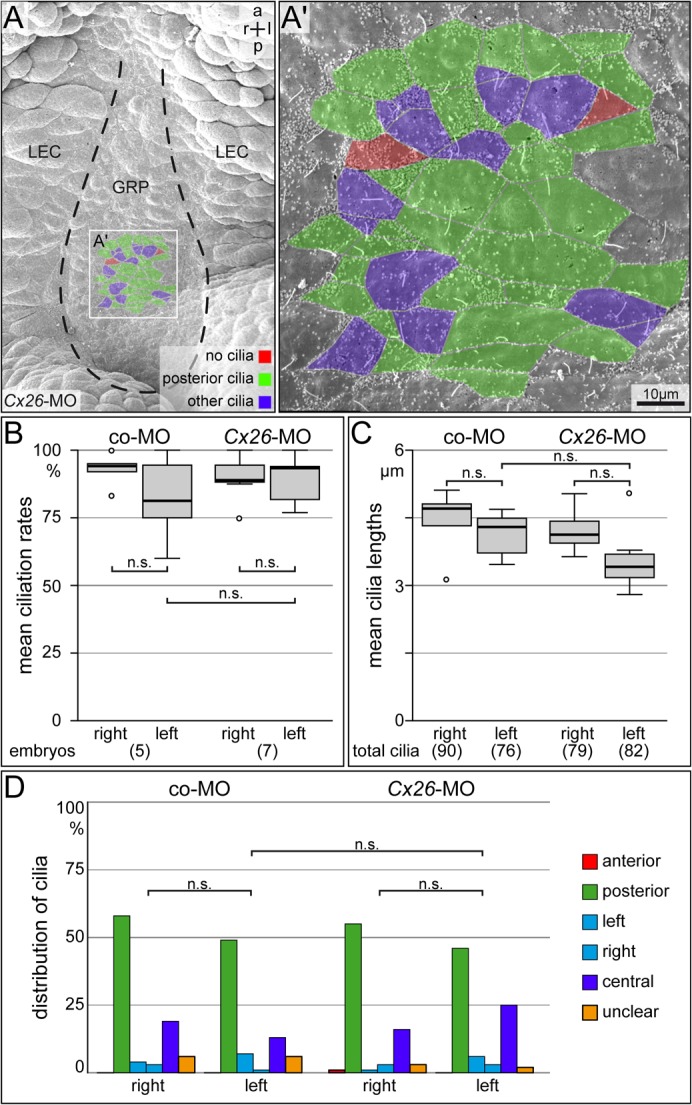
SEM-analysis of GRP ciliation and morphology in stage 17 dorsal explants of morphants injected with Cx26-MO or Co-MO into left blastomeres at the 4-cell stage. (A) Representative dorsal explant of Cx26 morphant specimen revealed normal cilia length, number of ciliated cells and cilia polarization. (B–D) Quantification of mean ciliation rates (B) and mean cilia lengths (C) of GRP cilia in a defined area (A′). (D) Cilia polarization (assessed in the same areas). Note that a not significant (n.s.) slight tendency towards fewer and shorter cilia was observed on the injected side in both Cx26 and co-MO morphants, but that differences between Cx26 morphants and co-MO injected specimens were not significant. Numbers in brackets indicate number of analyzed dorsal explants or cilia. Statistical significances were calculated using Student's t-test.
Discussion
Previous reports involved connexins and GJC in the establishment of molecular asymmetries during early chick and frog development (Levin and Mercola, 1999; Levin and Mercola, 1998). Dye transfer experiments in 8–16 cell Xenopus embryos had suggested that dorsal cells were coupled while ventral blastomeres were isolated. Inhibitor experiments had additionally shown that HepOH was able to interfere with LR development when the alcohol was applied before neurulation (stage 12) (Levin and Mercola, 1998). Based on these initial observations (Levin and Mercola, 1999; Levin and Mercola, 1998) and the later demonstration of asymmetries in ATP4 (Levin et al., 2002) and serotonin localization (Fukumoto et al., 2005) the ‘ion-flux’ model of symmetry breakage was proposed (Levin, 2005; Levin, 2007; Vandenberg and Levin, 2010). According to this model ATP4 sets up an asymmetric voltage gradient, which drives serotonin in an electrophoresis-like process through gap junctions to accumulate on the ventral side, where cells are not coupled and transfer comes to an end (Levin, 2005; Levin, 2007; Vandenberg and Levin, 2010). The present study challenges the proposed function of GJC in LR development. Incubation of embryos in HepOH-containing culture medium reproduced the previous findings (Levin and Mercola, 1998), although in our experiments we used two incubation periods and the later time frame (stage 6–20) was far more sensitive than the earlier one (stage 3/4–9). Importantly, we were able to extend the sensitive time period through flow stages by injecting HepOH directly into the gastrocoel. These injection experiments suggest that accessibility of HepOH to the relevant tissue is limited to time points prior to involution, which in the case of the GRP and the adjacent lateral endodermal cells has been completed at stage 12.5 and thus at the very moment when incubation experiments begin to fail (Shook et al., 2004). Theoretically, we might have targeted two independent processes by our incubation and injection experiments, i.e. pre- and post-flow. As ciliation and flow were unaltered in specimens incubated during pre-flow stages (data not shown), we reason that Cx26 knockdown, HepOH incubation and injection acted on the very same mechanism.
The asymmetric coupling of blastomeres at the 8–16 cell stage has previously been shown to be an artifact of light reflection and scattering and of the heavier pigmentation of ventral cells (Landesman et al., 2000). Using the same basic setup as Levin and colleagues (Levin and Mercola, 1998), these authors demonstrated in sectioned specimens that no asymmetries with respect to gap junctional coupling existed in the 16–128 cell embryo (Landesman et al., 2000). Our recent study on serotonin function in LR axis specification has revealed that serotonin signaling is required as a permissive factor for Wnt-induced specification of the superficial mesoderm (Beyer et al., 2012). This tissue, which before involution is positioned on top of the dorsal lip/organizer, is set aside during gastrulation (Shook et al., 2004). Loss of serotonin signaling consequently impaired GRP morphogenesis and leftward flow (Beyer et al., 2012). In addition, serotonin was found to be symmetrically distributed in the cleavage stage embryo (Beyer et al., 2012), arguing against the hypothesis of asymmetric gap junctional transfer of this small and charged molecule during early development. Finally, our unpublished investigation of ATP4 function in LR axis formation showed that symmetrically expressed ATP4a was required for Wnt/ß-catenin regulated Foxj1 induction in the superficial mesoderm and Wnt/PCP dependent cilia polarization at the GRP (Walentek et al., 2012). Taken together, these results question the ‘ion-flux’ model of symmetry breakage. Moreover, molecular asymmetries upstream of leftward flow are conceptually not required, as the superficial mesoderm is patterned by the organizer and the directionality of flow is determined by cilia polarization along the anterior-posterior axis (Schweickert et al., 2012).
Central to our investigation was the question which connexin gene(s) were expressed in the tissue of interest and how a specific gene knockdown would alter laterality, as opposed to unspecific inhibition of connexins through long-chain alcohols (Juszczak and Swiergiel, 2009). The co-expression of Cx26 and Cx32 in the endodermal lining of the gastrocoel during flow stages pinpointed these cells adjacent to the GRP as a possible route for the transfer of flow-generated cue(s) to the left LPM. Surprisingly, evidence from both gain- and loss-of-function experiments unequivocally pointed to Cx26 as the sole connexin gene responsible for the observed effects on LR asymmetry. Incidentally, knockout mice for Cx26 revealed embryonic lethality (Gabriel et al., 1998), although LR axis formation was not analyzed, while Cx32 knockout mice were viable and developed myelination defects only postnatally (Nelles et al., 1996; Scherer et al., 1998). While this manuscript was under review, a study in the mouse has demonstrated that Cx43 in gut endoderm is required for information relay from the PNC / node to the LPM (Viotti et al., 2012), demonstrating evolutionary conservation of mechanisms.
What may be the identity of the signal which acts Cx26-dependent downstream of flow and upstream of the Nodal signaling cascade? We like to suggest that Cx26 is involved in the propagation of an asymmetric calcium wave, which has been reported downstream of flow in mouse and zebrafish (McGrath et al., 2003; Sarmah et al., 2005; Slusarski and Pelegri, 2007), and which we have observed in post-flow stage rabbit embryos as well (M.B., unpublished). This wave initiates at the left margin of the respective ciliated epithelium (KV; PNC/node). Knockout mice for Pkd2, a gene expressed in the KV/PNC, which encodes the calcium channel Polycystin-2, lack both the asymmetric calcium signal and induction of Nodal in the left LPM (Pennekamp et al., 2002; McGrath et al., 2003). In zebrafish the calcium wave is required for the initiation of the Nodal cascade as well (Sarmah et al., 2005). Although an asymmetric calcium wave has not been reported in Xenopus as yet, it seems safe to predict that frog embryos display this feature as well, given the evolutionary conservation of events in species which apply leftward flow for symmetry breakage (Hamada, 2008; Blum et al., 2009).
A specific requirement of Cx26 for calcium-wave mediated LR development can be envisaged in at least three ways: (1) Cx26 may form homomeric channels in the endodermal gastrocoel epithelium; (2) Cx26 may form functionally relevant heteromeric channels with another yet unidentified connexin. Expression profiling should be able to identify possible candidates in future studies; (3) Cx26 may function as unpaired connexon channels. In the first two scenarios, calcium wave propagation is mediated through Cx26 homomeric or heteromeric intercellular channels. Correlations between connexin expression patterns and spreading of calcium waves have been well documented (Cotrina et al., 1998; Goodenough and Paul, 2003; Finkbeiner, 1992). In this scheme it is assumed that inositol 1,4,5-triposphate (IP3) diffuses through GJs to cause calcium release from the endoplasmatic reticulum (ER) to the cytoplasm (Goodenough and Paul, 2003).
Although these two options would certainly fulfill the requirements for calcium wave propagation and fit well to the published GJ and LR literature, we would like to discuss a third possibility, which seems attractive to us and worth to be investigated in the future. Unpaired hemichannels have been shown to be involved in paracrine signaling, for example in the release of glutamate or ATP into the extracellular space (Cotrina et al., 1998; Ye et al., 2003). Purinergic propagation of calcium waves has been described in astrocytes, osteocytes and chondrocytes (Guthrie et al., 1999; Cotrina et al., 2000; Jørgensen et al., 2002; Wann et al., 2012). In this view ATP binding to P2 purinergic receptors results in phospholipase C-mediated IP3 production, followed by calcium release from the ER. Calcium in turn leads to hemichannel-mediated ATP release into the extracellular space, resulting in calcium wave propagation to neighboring cells (Stout et al., 2004; Goodenough and Paul, 2003). A recent study has added yet another possible link to the transfer of asymmetric positional information from the midline to the left LPM. Purinergic calcium signaling in chondrocytes was shown to be dependent on the primary cilium and Pkd1, and this signaling was accompanied by up-regulated secretion of sulfated glycosaminoglycans (sGAGs) (Wann et al., 2012). A role for sGAGs in signal transfer from the mouse PNC or frog GRP to the respective LPM has been demonstrated by inhibition of sGAG synthesis or by removal of sGAGs from the embryo, which disrupted Nodal induction in the left LPM (Oki et al., 2007; Marjoram and Wright, 2011). Importantly, sGAGs were shown to be present in basement membrane-like structures between endoderm and LPM (Oki et al., 2009).
The following scenario could thus be envisaged. Leftward flow initiates an asymmetric calcium signal in the left marginal cells of the GRP (or KV/PNC in fish and mammals, respectively). Recent work in mouse and fish has suggested that a complex of Pkd2 and Pkd1l1 acts as the ciliary flow sensor in the lateral-most cells of KV and PNC/node (Field et al., 2011; Kamura et al., 2011), which would transfer the extracellular signal (flow) via calcium influx into the cell. As a direct response to flow, the Nodal inhibitor cerl2/charon/Coco becomes down-regulated in the sensing cells (Hojo et al., 2007; Kunimoto et al., 2012; Schweickert et al., 2010), resulting in a derepression of Nodal. Calcium spreads from the sensing cells to the adjacent lateral endodermal cells via Cx26-mediated purinergic wave propagation, accompanied by enhanced secretion of sGAGs to the basement membrane. Compelling evidence has been gathered in both mouse and frog that Nodal protein itself can be transferred along sGAGs from the midline to the LPM and within the LPM itself (Oki et al., 2007; Marjoram and Wright, 2011). Nodal in the left LPM initiates the asymmetric gene cascade which, subsequently, governs asymmetric organ morphogenesis and placement. Future studies in different model organisms have to show whether this rather bold proposal holds up to experimental verification.
Materials and Methods
HepOH treatment of embryos
Whole embryos were treated in culture medium, to which the alcohol was previously added. An emulsion of 1μl HepOH or 1μl UnOH in 0.1ml culture medium was freshly prepared before each experiment. The mixture was vortexed and sonicated until the liquid turned milky, followed by another 1∶100 dilution in buffer and additional sonication. Treatments were carried out in 12-well plates. Controls were cultivated in 0.01% ETOH. Following incubation embryos were carefully washed and transferred to 0.1×MBSH/1% agarose containing Petri dishes. Injection experiments were preformed at stage 15–20 using a Harvard Apparatus setup. Pure HepOH was used and the drop size was calibrated to 20 nl / injection. Pitx2c was evaluated at stage 28–32 and the organ situs was determined at stage 45.
Cloning of constructs
Full-length cDNAs encoding Cx26 and Cx32 were cloned by RT-PCR using primers derived from published sequences (#NM_001087009.1; #NM_001101749). For Cx26, primers Cx26for 5′- cgaattcatggattggggaacgc -3′ and Cx26rev 5′- acctcgagctaatgttctttctgtt -3′ were used, for amplification of Cx32 primers Cx32for 5′- ctgaattcatgaattgggcaggattata -3′ and Cx32rev 5′- ctctcgagttaggaggtagaacagtg -3′ were utilized. RFP and eGFP fusion constructs were cloned by PCR using plasmid DNAs and primers Cx26fusrev 5′- acctcgagatgttctttctgtt -3′ and Cx32fusrev 5′- ctctcgagggaggtagaacagtg -3′.
RNA in situ hybridization and SEM analysis
Embryos were fixed in MEMFA for 2hrs and processed following standard protocols. Digoxigenin-labeled (Roche) RNA probes were prepared from linearized plasmids using SP6 or T7 RNA polymerase (Promega). In situ hybridization was according to (Belo et al., 1997). Statistical calculations of gene expression patterns were performed using Pearson's chi-square test. SEM analysis was performed as described in (Sulik et al., 1994).
Microinjections
Embryos were injected at the 4–8 cell stage using a Harvard Apparatus setup. Drop size was calibrated to about 7–8 nl / injection. Morpholinos Cx26-MO: 5′- AGCGTTCCCCAATCCATTGTTACGG -3′, Cx26UTR-MO: 5′- ATATTGGTCTCTGTGCGCTGACTTC -3′ and Cx32-MO: 5′- TGGCGTATAATCCTGCCCAATTCAT -3′ were used at concentrations indicated. Synthetic mRNAs were prepared using the Ambion message machine kit. Injections were performed as described previously (Vick et al., 2009). Rhodamine-B dextran (0.5–1.0 μg/μl; Molecular Probes) was used as lineage tracer.
GRP and flow analysis
GRP ciliation, cilia length and polarization were assessed as described (Beyer et al., 2012). Flow was analyzed by time-lapse videography in dorsal explants to which fluorescent beads were added as described (Schweickert et al., 2007; Beyer et al., 2012).
Supplementary Material
Acknowledgments
Michael Levin and John Wallingford contributed plasmids, Philipp Vick determined parts of the lineage of injections at the 4–8 cell stage, and Vroni Staedele and Susanne Bogusch helped with some of the experiments. TB and TT were supported by PhD fellowship from the Landesgraduiertenfoerderung Baden-Wuerttemberg. Work in the Blum lab was supported by DFG grant BL-285/9.
Footnotes
Competing interests: The authors declare that there are no competing interests.
References
- Ayad W. A., Locke D., Koreen I. V., Harris A. L. (2006). Heteromeric, but not homomeric, connexin channels are selectively permeable to inositol phosphates. J. Biol. Chem. 281, 16727–16739 10.1074/jbc.M600136200 [DOI] [PubMed] [Google Scholar]
- Barrio L. C., Suchyna T., Bargiello T., Xu L. X., Roginski R. S., Bennett M. V., Nicholson B. J. (1991). Gap junctions formed by connexins 26 and 32 alone and in combination are differently affected by applied voltage. Proc. Natl. Acad. Sci. USA 88, 8410–8414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belo J. A., Bouwmeester T., Leyns L., Kertesz N., Gallo M., Follettie M., De Robertis E. M. (1997). Cerberus-like is a secreted factor with neutralizing activity expressed in the anterior primitive endoderm of the mouse gastrula. Mech. Dev. 68, 45–57 10.1016/S0925-4773(97)00125-1 [DOI] [PubMed] [Google Scholar]
- Bevans C. G., Kordel M., Rhee S. K., Harris A. L. (1998). Isoform composition of connexin channels determines selectivity among second messengers and uncharged molecules. J. Biol. Chem. 273, 2808–2816 10.1074/jbc.273.5.2808 [DOI] [PubMed] [Google Scholar]
- Beyer T., Danilchik M., Thumberger T., Vick P., Tisler M., Schneider I., Bogusch S., Andre P., Ulmer B., Walentek P. et al. (2012). Serotonin signaling is required for Wnt-dependent GRP specification and leftward flow in Xenopus. Curr. Biol. 22, 33–39 10.1016/j.cub.2011.11.027 [DOI] [PubMed] [Google Scholar]
- Blum M., Andre P., Muders K., Schweickert A., Fischer A., Bitzer E., Bogusch S., Beyer T., van Straaten H. W. M., Viebahn C. (2007). Ciliation and gene expression distinguish between node and posterior notochord in the mammalian embryo. Differentiation 75, 133–146 10.1111/j.1432-0436.2006.00124.x [DOI] [PubMed] [Google Scholar]
- Blum M., Weber T., Beyer T., Vick P. (2009). Evolution of leftward flow. Semin. Cell Dev. Biol. 20, 464–471 10.1016/j.semcdb.2008.11.005 [DOI] [PubMed] [Google Scholar]
- Britz-Cunningham S. H., Shah M. M., Zuppan C. W., Fletcher W. H. (1995). Mutations of the Connexin43 gap-junction gene in patients with heart malformations and defects of laterality. N. Engl. J. Med. 332, 1323–1329 10.1056/NEJM199505183322002 [DOI] [PubMed] [Google Scholar]
- Burn S. F., Hill R. E. (2009). Left-right asymmetry in gut development: what happens next? Bioessays 31, 1026–1037 10.1002/bies.200900056 [DOI] [PubMed] [Google Scholar]
- Capdevila J., Vogan K. J., Tabin C. J., Izpisúa Belmonte J. C. (2000). Mechanisms of left-right determination in vertebrates. Cell 101, 9–21 10.1016/S0092-8674(00)80619-4 [DOI] [PubMed] [Google Scholar]
- Casey B., Ballabio A. (1995). Connexin43 mutations in sporadic and familial defects of laterality. N. Engl. J. Med. 333, 941–942 [Letter to Editor] 10.1056/NEJM199510053331415 [DOI] [PubMed] [Google Scholar]
- Cotrina M. L., Lin J. H., Alves-Rodrigues A., Liu S., Li J., Azmi-Ghadimi H., Kang J., Naus C. C., Nedergaard M. (1998). Connexins regulate calcium signaling by controlling ATP release. Proc. Natl. Acad. Sci. USA 95, 15735–15740 10.1073/pnas.95.26.15735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotrina M. L., Lin J. H., López-García J. C., Naus C. C., Nedergaard M. (2000). ATP-mediated glia signaling. J. Neurosci. 20, 2835–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Boer T. P., Kok B., Neuteboom K. I. E., Spieker N., De Graaf J., Destrée O. H. J., Rook M. B., Van Veen T. A. B., Jongsma H. J., Vos M. A. et al. (2005). Cloning and functional characterization of a novel connexin expressed in somites of Xenopus laevis. Dev. Dyn. 233, 864–871 10.1002/dvdy.20420 [DOI] [PubMed] [Google Scholar]
- de Boer T. P., Kok B., Roël G., van Veen T. A. B., Destrée O. H. J., Rook M. B., Vos M. A., de Bakker J. M. T., van der Heyden M. A. G. (2006). Cloning, embryonic expression, and functional characterization of two novel connexins from Xenopus laevis. Biochem. Biophys. Res. Commun. 349, 855–862 10.1016/j.bbrc.2006.08.121 [DOI] [PubMed] [Google Scholar]
- Debrus S., Tuffery S., Matsuoka R., Galal O., Sarda P., Sauer U., Bozio A., Tanman B., Toutain A., Claustres M. et al. (1997). Lack of evidence for connexin 43 gene mutations in human autosomal recessive lateralization defects. J. Mol. Cell. Cardiol. 29, 1423–1431 10.1006/jmcc.1997.0380 [DOI] [PubMed] [Google Scholar]
- Feistel K., Blum M. (2008). Gap junctions relay FGF8-mediated right-sided repression of Nodal in rabbit. Dev. Dyn. 237, 3516–3527 10.1002/dvdy.21535 [DOI] [PubMed] [Google Scholar]
- Field S., Riley K.-L., Grimes D. T., Hilton H., Simon M., Powles-Glover N., Siggers P., Bogani D., Greenfield A., Norris D. P. (2011). Pkd1l1 establishes left-right asymmetry and physically interacts with Pkd2. Development 138, 1131–1142 10.1242/dev.058149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkbeiner S. (1992). Calcium waves in astrocytes-filling in the gaps. Neuron 8, 1101–1108 10.1016/0896-6273(92)90131-V [DOI] [PubMed] [Google Scholar]
- Fukumoto T., Kema I. P., Levin M. (2005). Serotonin signaling is a very early step in patterning of the left-right axis in chick and frog embryos. Curr. Biol. 15, 794–803 10.1016/j.cub.2005.03.044 [DOI] [PubMed] [Google Scholar]
- Gabriel H. D., Jung D., Bützler C., Temme A., Traub O., Winterhager E., Willecke K. (1998). Transplacental uptake of glucose is decreased in embryonic lethal connexin26-deficient mice. J. Cell Biol. 140, 1453–1461 10.1083/jcb.140.6.1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebbia M., Towbin J. A., Casey B. (1996). Failure to detect connexin43 mutations in 38 cases of sporadic and familial heterotaxy. Circulation 94, 1909–1912. [DOI] [PubMed] [Google Scholar]
- Goodenough D. A., Paul D. L. (2003). Beyond the gap: functions of unpaired connexon channels. Nat. Rev. Mol. Cell Biol. 4, 285–295 10.1038/nrm1072 [DOI] [PubMed] [Google Scholar]
- Gros J., Feistel K., Viebahn C., Blum M., Tabin C. J. (2009). Cell movements at Hensen’s node establish left/right asymmetric gene expression in the chick. Science 324, 941–944 10.1126/science.1172478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan X., Cravatt B. F., Ehring G. R., Hall J. E., Boger D. L., Lerner R. A., Gilula N. B. (1997). The sleep-inducing lipid oleamide deconvolutes gap junction communication and calcium wave transmission in glial cells. J. Cell Biol. 139, 1785–1792 10.1083/jcb.139.7.1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie P. B., Knappenberger J., Segal M., Bennett M. V., Charles A. C., Kater S. B. (1999). ATP released from astrocytes mediates glial calcium waves. J. Neurosci. 19, 520–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada H. (2008). Breakthroughs and future challenges in left-right patterning. Dev. Growth Differ. 50, Suppl 1S71–S78 10.1111/j.1440-169X.2008.01008.x [DOI] [PubMed] [Google Scholar]
- Hamada H., Meno C., Watanabe D., Saijoh Y. (2002). Establishment of vertebrate left-right asymmetry. Nat. Rev. Genet. 3, 103–113 10.1038/nrg732 [DOI] [PubMed] [Google Scholar]
- Hatler J. M., Essner J. J., Johnson R. G. (2009). A gap junction connexin is required in the vertebrate left-right organizer. Dev. Biol. 336, 183–191 10.1016/j.ydbio.2009.09.035 [DOI] [PubMed] [Google Scholar]
- Hirokawa N., Tanaka Y., Okada Y., Takeda S. (2006). Nodal flow and the generation of left-right asymmetry. Cell 125, 33–45 10.1016/j.cell.2006.03.002 [DOI] [PubMed] [Google Scholar]
- Hojo M., Takashima S., Kobayashi D., Sumeragi A., Shimada A., Tsukahara T., Yokoi H., Narita T., Jindo T., Kage T. et al. (2007). Right-elevated expression of charon is regulated by fluid flow in medaka Kupffer’s vesicle. Dev. Growth Differ. 49, 395–405 10.1111/j.1440-169X.2007.00937.x [DOI] [PubMed] [Google Scholar]
- Juszczak G. R., Swiergiel A. H. (2009). Properties of gap junction blockers and their behavioural, cognitive and electrophysiological effects: animal and human studies. Prog. Neuropsychopharmacol. Biol. Psychiatry 33, 181–198 10.1016/j.pnpbp.2008.12.014 [DOI] [PubMed] [Google Scholar]
- Jørgensen N. R., Henriksen Z., Sørensen O. H., Eriksen E. F., Civitelli R., Steinberg T. H. (2002). Intercellular calcium signaling occurs between human osteoblasts and osteoclasts and requires activation of osteoclast P2X7 receptors. J. Biol. Chem. 277, 7574–7580 10.1074/jbc.M104608200 [DOI] [PubMed] [Google Scholar]
- Kamura K., Kobayashi D., Uehara Y., Koshida S., Iijima N., Kudo A., Yokoyama T., Takeda H. (2011). Pkd1l1 complexes with Pkd2 on motile cilia and functions to establish the left-right axis. Development 138, 1121–1129 10.1242/dev.058271 [DOI] [PubMed] [Google Scholar]
- Kunimoto K., Yamazaki Y., Nishida T., Shinohara K., Ishikawa H., Hasegawa T., Okanoue T., Hamada H., Noda T., Tamura A. et al. (2012). Coordinated ciliary beating requires Odf2-mediated polarization of basal bodies via basal feet. Cell 148, 189–200 10.1016/j.cell.2011.10.052 [DOI] [PubMed] [Google Scholar]
- Landesman Y., Goodenough D. A., Paul D. L. (2000). Gap junctional communication in the early Xenopus embryo. J. Cell Biol. 150, 929–936 10.1083/jcb.150.4.929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landesman Y., Postma F. R., Goodenough D. A., Paul D. L. (2003). Multiple connexins contribute to intercellular communication in the Xenopus embryo. J. Cell Sci. 116, 29–38 10.1242/jcs.00182 [DOI] [PubMed] [Google Scholar]
- Lee J. D., Anderson K. V. (2008). Morphogenesis of the node and notochord: the cellular basis for the establishment and maintenance of left-right asymmetry in the mouse. Dev. Dyn. 237, 3464–3476 10.1002/dvdy.21598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M. (2005). Left-right asymmetry in embryonic development: a comprehensive review. Mech. Dev. 122, 3–25 10.1016/j.mod.2004.08.006 [DOI] [PubMed] [Google Scholar]
- Levin M. (2007). Gap junctional communication in morphogenesis. Prog. Biophys. Mol. Biol. 94, 186–206 10.1016/j.pbiomolbio.2007.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M., Mercola M. (1998). Gap junctions are involved in the early generation of left-right asymmetry. Dev. Biol. 203, 90–105 10.1006/dbio.1998.9024 [DOI] [PubMed] [Google Scholar]
- Levin M., Mercola M. (1999). Gap junction-mediated transfer of left-right patterning signals in the early chick blastoderm is upstream of Shh asymmetry in the node. Development 126, 4703–4714. [DOI] [PubMed] [Google Scholar]
- Levin M., Thorlin T., Robinson K. R., Nogi T., Mercola M. (2002). Asymmetries in H+/K+-ATPase and cell membrane potentials comprise a very early step in left-right patterning. Cell 111, 77–89 10.1016/S0092-8674(02)00939-X [DOI] [PubMed] [Google Scholar]
- Maisonneuve C., Guilleret I., Vick P., Weber T., Andre P., Beyer T., Blum M., Constam D. B. (2009). Bicaudal C, a novel regulator of Dvl signaling abutting RNA-processing bodies, controls cilia orientation and leftward flow. Development 136, 3019–3030 10.1242/dev.038174 [DOI] [PubMed] [Google Scholar]
- Marjoram L., Wright C. (2011). Rapid differential transport of Nodal and Lefty on sulfated proteoglycan-rich extracellular matrix regulates left-right asymmetry in Xenopus. Development 138, 475–485 10.1242/dev.056010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Männer J. (2009). The anatomy of cardiac looping: a step towards the understanding of the morphogenesis of several forms of congenital cardiac malformations. Clin. Anat. 22, 21–35 10.1002/ca.20652 [DOI] [PubMed] [Google Scholar]
- McGrath J., Somlo S., Makova S., Tian X., Brueckner M. (2003). Two populations of node monocilia initiate left-right asymmetry in the mouse. Cell 114, 61–73 10.1016/S0092-8674(03)00511-7 [DOI] [PubMed] [Google Scholar]
- Nelles E., Bützler C., Jung D., Temme A., Gabriel H. D., Dahl U., Traub O., Stümpel F., Jungermann K., Zielasek J. et al. (1996). Defective propagation of signals generated by sympathetic nerve stimulation in the liver of connexin32-deficient mice. Proc. Natl. Acad. Sci. USA 93, 9565–9570 10.1073/pnas.93.18.9565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oki S., Hashimoto R., Okui Y., Shen M. M., Mekada E., Otani H., Saijoh Y., Hamada H. (2007). Sulfated glycosaminoglycans are necessary for Nodal signal transmission from the node to the left lateral plate in the mouse embryo. Development 134, 3893–3904 10.1242/dev.009464 [DOI] [PubMed] [Google Scholar]
- Oki S., Kitajima K., Marques S., Belo J. A., Yokoyama T., Hamada H., Meno C. (2009). Reversal of left-right asymmetry induced by aberrant Nodal signaling in the node of mouse embryos. Development 136, 3917–3925 10.1242/dev.039305 [DOI] [PubMed] [Google Scholar]
- Paul D. L., Yu K., Bruzzone R., Gimlich R. L., Goodenough D. A. (1995). Expression of a dominant negative inhibitor of intercellular communication in the early Xenopus embryo causes delamination and extrusion of cells. Development 121, 371–381. [DOI] [PubMed] [Google Scholar]
- Pennekamp P., Karcher C., Fischer A., Schweickert A., Skryabin B., Horst J., Blum M., Dworniczak B. (2002). The ion channel polycystin-2 is required for left-right axis determination in mice. Curr. Biol. 12, 938–943 10.1016/S0960-9822(02)00869-2 [DOI] [PubMed] [Google Scholar]
- Sarmah B., Latimer A. J., Appel B., Wente S. R. (2005). Inositol polyphosphates regulate zebrafish left-right asymmetry. Dev. Cell 9, 133–145 10.1016/j.devcel.2005.05.002 [DOI] [PubMed] [Google Scholar]
- Scherer S. S., Xu Y. T., Nelles E., Fischbeck K., Willecke K., Bone L. J. (1998). Connexin32-null mice develop demyelinating peripheral neuropathy. Glia 24, 8–20 [DOI] [PubMed] [Google Scholar]
- Schier A. F. (2003). Nodal signaling in vertebrate development. Annu. Rev. Cell Dev. Biol. 19, 589–621 10.1146/annurev.cellbio.19.041603.094522 [DOI] [PubMed] [Google Scholar]
- Schweickert A., Weber T., Beyer T., Vick P., Bogusch S., Feistel K., Blum M. (2007). Cilia-driven leftward flow determines laterality in Xenopus. Curr. Biol. 17, 60–66 10.1016/j.cub.2006.10.067 [DOI] [PubMed] [Google Scholar]
- Schweickert A., Vick P., Getwan M., Weber T., Schneider I., Eberhardt M., Beyer T., Pachur A., Blum M. (2010). The nodal inhibitor Coco is a critical target of leftward flow in Xenopus. Curr. Biol. 20, 738–743 10.1016/j.cub.2010.02.061 [DOI] [PubMed] [Google Scholar]
- Schweickert A., Walentek P., Thumberger T., Danilchik M. (2012). Linking early determinants and cilia-driven leftward flow in left-right axis specification of Xenopus laevis: A theoretical approach. Differentiation 83, S67–S77 10.1016/j.diff.2011.11.005 [DOI] [PubMed] [Google Scholar]
- Shen M. M. (2007). Nodal signaling: developmental roles and regulation. Development 134, 1023–1034 10.1242/dev.000166 [DOI] [PubMed] [Google Scholar]
- Shook D. R., Majer C., Keller R. (2004). Pattern and morphogenesis of presumptive superficial mesoderm in two closely related species, Xenopus laevis and Xenopus tropicalis. Dev. Biol. 270, 163–185 10.1016/j.ydbio.2004.02.021 [DOI] [PubMed] [Google Scholar]
- Slusarski D. C., Pelegri F. (2007). Calcium signaling in vertebrate embryonic patterning and morphogenesis. Dev. Biol. 307, 1–13 10.1016/j.ydbio.2007.04.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosinsky G. (1995). Mixing of connexins in gap junction membrane channels. Proc. Natl. Acad. Sci. USA 92, 9210–9214 10.1073/pnas.92.20.9210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Splitt M. P., Burn J., Goodship J. (1995). Connexin43 mutations in sporadic and familial defects of laterality. N. Engl. J. Med. 333, 941–942 [Letter to Editor] 10.1056/NEJM199510053331415 [DOI] [PubMed] [Google Scholar]
- Stout C., Goodenough D. A., Paul D. L. (2004). Connexins: functions without junctions. Curr. Opin. Cell Biol. 16, 507–512 10.1016/j.ceb.2004.07.014 [DOI] [PubMed] [Google Scholar]
- Sulik K., Dehart D. B., Inagaki T., Carson J. L., Vrablic T., Gesteland K., Schoenwolf G. C. (1994). Morphogenesis of the murine node and notochordal plate. Dev. Dyn. 201, 260–278 10.1002/aja.1002010309 [DOI] [PubMed] [Google Scholar]
- Tabin C. (2005). Do we know anything about how left-right asymmetry is first established in the vertebrate embryo? J. Mol. Histol. 36, 317–323 10.1007/s10735-005-9000-y [DOI] [PubMed] [Google Scholar]
- van der Heyden M. A., Roeleveld L., Peterson J., Destrée O. H. (2001). Connexin43 expression during Xenopus development. Mech. Dev. 108, 217–220 10.1016/S0925-4773(01)00490-7 [DOI] [PubMed] [Google Scholar]
- Vandenberg L. N., Levin M. (2009). Perspectives and open problems in the early phases of left-right patterning. Semin. Cell Dev. Biol. 20, 456–463 10.1016/j.semcdb.2008.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg L. N., Levin M. (2010). Far from solved: a perspective on what we know about early mechanisms of left-right asymmetry. Dev. Dyn. 239, 3131–3146 10.1002/dvdy.22450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vick P., Schweickert A., Weber T., Eberhardt M., Mencl S., Shcherbakov D., Beyer T., Blum M. (2009). Flow on the right side of the gastrocoel roof plate is dispensable for symmetry breakage in the frog Xenopus laevis. Dev. Biol. 331, 281–291 10.1016/j.ydbio.2009.05.547 [DOI] [PubMed] [Google Scholar]
- Viotti M., Niu L., Shi S.-H., Hadjantonakis A.-K. (2012). Role of the gut endoderm in relaying left-right patterning in mice. PLoS Biol. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walentek P., Beyer T., Thumberger T., Schweickert A., Blum M. (2012). ATP4a is required for Wnt-dependent Foxj1 expression and leftward flow in Xenopus left-right development. Cell Rep. (in press) 10.1016/j.celrep.2012.03.005 [DOI] [PubMed] [Google Scholar]
- Wann A. K. T., Zuo N., Haycraft C. J., Jensen C. G., Poole C. A., McGlashan S. R., Knight M. M. (2012). Primary cilia mediate mechanotransduction through control of ATP-induced Ca2+ signaling in compressed chondrocytes. FASEB J. [Epub ahead of print] 10.1096/fj.11-193649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z.-C., Wyeth M. S., Baltan-Tekkok S., Ransom B. R. (2003). Functional hemichannels in astrocytes: a novel mechanism of glutamate release. J. Neurosci. 23, 3588–3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelin R., Schyr R. B.-H., Kot H., Zins S., Frumkin A., Pillemer G., Fainsod A. (2005). Ethanol exposure affects gene expression in the embryonic organizer and reduces retinoic acid levels. Dev. Biol. 279, 193–204 10.1016/j.ydbio.2004.12.014 [DOI] [PubMed] [Google Scholar]
- Yelin R., Kot H., Yelin D., Fainsod A. (2007). Early molecular effects of ethanol during vertebrate embryogenesis. Differentiation 75, 393–403 10.1111/j.1432-0436.2006.00147.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.