Abstract
This fMRI study investigated neural responses while making appraisals of self and other, across the social and academic domains, in children and adolescents with and without autism spectrum disorders (ASD). Compared to neurotypical youth, those with ASD exhibited hypoactivation of ventromedial prefrontal cortex during self-appraisals. Responses in middle cingulate cortex (MCC) and anterior insula (AI) also distinguished between groups. Stronger activity in MCC and AI during self-appraisals was associated with better social functioning in the ASD group. Although self-appraisals were significantly more positive in the neurotypical group, positivity was unrelated to brain activity in these regions. Together, these results suggest that multiple brain regions support making self-appraisals in neurotypical development, and function atypically in youth with ASD.
Keywords: Autism, Self, Ventral mPFC, Anterior insula, Middle cingulate cortex, Development
Introduction
Autism is a spectrum disorder characterized by a triad of symptoms including disrupted social and communicative abilities, as well as repetitive/stereotyped behaviors. Both philosophers and psychologists agree that our capacity to understand not only others, but also ourselves, is critical for successful social interactions (Harter 1999). Nevertheless, research has tended to emphasize the difficulties faced by individuals with autism spectrum disorder (ASD) in understanding and connecting with other people, to the relative neglect of differences in self-perception. This is surprising considering that, to some extent, the name of this disorder reflects the atypical self (autos) displayed by children with autism as first recognized by Leo Kanner and Hans Asperger (Lombardo et al. 2010; Lombardo and Baron-Cohen 2011). In this study, we build on a recent renewal of interest in this topic by using functional magnetic resonance imaging (fMRI) to explore the networks underlying the process of making self-appraisals in children and adolescents with ASD, as compared with their neurotypical (NT) peers.
Typical and Atypical Self-Development
The self is critical to social development and interpersonal relationships in many ways. This is undoubtedly due to its broad implication in a host of psychological processes, such as self-regulation, self-recognition, self-enhancement, self-esteem, and simulation (wherein the self is used as a basis for understanding others; Leary and Tangney 2003; Robins et al. 2008; Sedikides and Spencer 2007). In this study, we focus specifically on the process of making self-appraisals, which form the basis of self-concepts. Self-concepts perform many important functions throughout development and across the lifespan. Self-concepts organize evaluative and descriptive information about the self across multiple contexts, helping individuals make meaning out of personal interactions with their social and physical environments and maintain a sense of coherence and continuity (Harter 1999). Self-concepts are specific to particular domains, and help to organize and facilitate an individual’s behavior in that domain, but they are also related to an individual’s overall self-esteem (Harter 1999; Markus 1977). Negative self-concepts are associated with poor developmental outcomes, such as depression and loneliness in the social domain, or low achievement in the academic domain (Berndt and Burgy 1996; Bracken 1996; Marsh 1990).
Self-appraisals and other processes related to self-development in ASD, however, seem to differ in important ways from neurotypical development. While able to report on physical or behavioral qualities of the self, children with ASD refer less to social interactions or qualities and have significantly less rich social self-concepts in particular (Lee and Hobson 1998). That is, they incorporate less social comparative or interpersonal contextual information, even in self-concept domains that can be reported on equally well by children with and without ASD. Children with ASD also refer less frequently to the self using the pronoun “me” (Hobson et al. 2006; Lee et al. 1994; Lind and Bowler 2009a). In general, children with ASD exhibit less self-consciousness (Hobson et al. 2006; but see Williams and Happé 2010). Autobiographical memory is poorer in children with ASD (Bruck et al. 2007), and events experienced by the self are remembered less well by them than events happening to others (Millward et al. 2000), including greater difficulty remembering one’s own prior false beliefs (Williams and Happé 2009). A recent review (Lind 2010) noted that, relative to NT individuals, those with ASD generally exhibit more sparse autobiographical memories (see also Crane and Goddard 2008) as well as less memory for information processed with reference to the self. This self-reference effect in memory is reduced in adults with ASD (Lombardo et al. 2007; Toichi et al. 2002), and may be completely lacking in children with ASD (Henderson et al. 2009). However, physical representations of the self may be spared (Lind 2010; Uddin 2011), as children with ASD have not generally shown impairments in mirror self-recognition (Lind and Bowler 2009a) or memory for actions performed by the self (Lind and Bowler 2009b).
For individuals with ASD, the ability to make self-appraisals (and therefore develop self-concepts) may be linked to mentalizing abilities (or theory of mind). Children with ASD who possess relatively more advanced mentalizing abilities have more conventional understandings of embarrassment, a self-conscious emotion (Heerey et al. 2003; Hillier and Allinson 2002). It has also been suggested that mentalizing difficulties are in part responsible for the atypical nature of self-consciousness and self-reflection in individuals with ASD (Frith and Frith 1999; Lombardo et al. 2010; Lombardo and Baron-Cohen 2011). Along these lines, a study of self-referential processing in adults with ASD found that better memory for information processed with reference to the self, as well as more self-focused attention and self-consciousness, were all associated with enhanced mentalizing abilities (Lombardo et al. 2007). The authors concluded that being more self-focused was beneficial for individuals with ASD as it may support metacognition, introspection, and theory of mind.
Neural Correlates of Self-Appraisals
Before examining the neural underpinnings of making self-appraisals in children with and without ASD, here we briefly review the brain regions previously implicated in self-appraisal processes. There is a broad consensus that cortical midline structures (CMS) in the human brain are responsible for the cognitive, affective, and motivational processes that allow for self-appraisals (Mitchell 2009; Northoff and Bermpohl 2004; Northoff et al. 2006, 2011). The CMS include medial prefrontal cortex (mPFC, including ventral, anterior rostral, and dorsal aspects) and adjacent rostral anterior cingulate cortex (ACC) in the frontal lobe, as well as the precuneus, posterior cingulate cortex (PCC), and retrosplenial cortex (RSC) in the parietal lobe (together frequently referred to as medial posterior parietal cortex, mPPC). In NT adults, CMS are typically more responsive when making self-appraisals (reporting whether traits describe themselves) than when reporting whether various traits describe other familiar individuals, particularly in the frontal regions. Similarly, autobiographical memory retrieval preferentially engages CMS (Svoboda et al. 2006; Summerfield et al. 2010).
A handful of studies have investigated the developmental trajectory of CMS engagement in making self-appraisals. The first demonstrated that NT children (at 10 years of age) engaged mPFC significantly more than NT adults (Pfeifer et al. 2007). However, both groups showed greater activity in the precuneus or PCC for appraisals of a fictional familiar other (Harry Potter) as compared to self-appraisals. This pattern of enhanced mPPC activity when making other-appraisals is increasingly common (Kennedy and Courchesne 2008a; Lombardo et al. 2010). A study of NT boys (ages 7–13 years) found that recall of psychological trait words increased with age when the words were referenced to the self, but not when the words were referenced to the subjects’ mother, and greater self-reference effects were linked to increased responses in mPFC (rostral and ventral ACC), among other regions (Ray et al. 2009). Finally, NT adolescents (ages 11–14 years) engaged mPFC (including rostral and dorsal ACC) more than did NT adults when making self-appraisals (Pfeifer et al. 2009). Interestingly, the adolescents also engaged the temporo-parietal junction [TPJ; a region associated with mentalizing (Saxe 2010)] during self-appraisals, but adults only did so during a process known as making reflected self-appraisals (thinking about what others think of us), suggesting mentalizing is an important contribution to self-perception during adolescence. Together, these results suggest that mPFC may be critical when making self-appraisals, and also identify a more extended network relevant to self-development including TPJ (as well as anterior insula and ventral striatum; for a review, see Pfeifer and Peake 2012).
To our knowledge, there have been only two studies thus far examining the functioning of CMS during the process of self-appraisals in adults with ASD. When reporting whether traits described one’s self versus one’s mother (Kennedy and Courchesne 2008a), adults with ASD did not show greater activity in CMS during self-appraisals relative to other-appraisals. However, neither did NT adults, making the finding in adults with ASD difficult to interpret. A lack of differentiation between self and other in mPFC has been observed in prior studies, most frequently when the participants were very close or similar to the targets for comparison (Chen et al. 2010; Krienen et al. 2010; Ochsner et al. 2005; Schmitz et al. 2004). Another study used a more distant target for comparison (the British Queen) and found that NT adults showed greater activity in ventral mPFC when making self-appraisals than other-appraisals, as well as significantly more activity in this region than adults with ASD (Lombardo et al. 2010). This study also called attention to the potential role of middle cingulate cortex (MCC). Compared to adults with ASD, NT adults engaged MCC more when reporting whether psychological attributes (vs. physical attributes) described the self, relative to the same contrast about others. The authors interpreted this as indicating a potential role for MCC in mentalizing about the self, as suggested by a supplementary meta-analysis they conducted which found that two regions most robustly distinguished self from other: ventral mPFC and MCC. Ventral mPFC is also consistently less active across a variety of social tasks in ASD, as opposed to non-social tasks, as revealed by a recent meta-analysis (Di Martino et al. 2009).
Finally, these two studies add to a growing body of evidence suggesting that CMS function atypically in adults with ASD during rest, in other words, independent of task-driven activity (Assaf et al. 2010; Cherkassky et al. 2006; Kennedy and Courchesne 2008a, b; Monk et al. 2009). The CMS are part of a network that is typically more active in adults at rest than during tasks that are externally oriented, suggesting that activity in this network represents the default mode of brain functioning (Greicius et al. 2003; Raichle and Snyder 2007). A recent study also demonstrated weaker connectivity between PCC and most other nodes of the default network in adolescents with ASD, relative to NT adolescents (Weng et al. 2010).
Current Study
Taken together, the prior literature suggests that in autism, CMS are likely to exhibit atypical patterns of responses while engaging in the process of making self-appraisals. However, to our knowledge, this has yet to be investigated in children and adolescents with ASD. Here, we compared neural activity while making appraisals of one’s self and a familiar but distant other (Harry Potter), in two different domains: social competence and academic abilities. We hypothesized that children and adolescents with ASD would show less activity when making self-appraisals, particularly in ventral mPFC as this region has most consistently demonstrated atypical patterns of functioning in ASD. Our a priori regions of interest (ROIs), based on the literature reviewed above, therefore included (1) ventral mPFC, as well as more anterior rostral and dorsal aspects of mPFC; (2) adjacent rostral and dorsal ACC; (3) MCC; (4) medial posterior parietal cortex (precuneus, PCC, and RSC); (5) TPJ; (6) anterior insula; and (7) ventral striatum.
Methods
Participants
Our sample included 18 youth (17 males; 2 left-handed) with ASD, and 18 matched NT youth (17 males; 2 left-handed, 1 ambidextrous). Groups did not significantly differ by gender, age, IQ (full-scale or verbal), or mean motion during the fMRI scan (see Table 1). All participants had a full-scale IQ ≥ 80, as determined by either the Wechsler Intelligence Scale for Children (WISC; Wechsler 1991) or the Wechsler Scale of Abbreviated Intelligence (WASI; Wechsler 1999). Further exclusionary criteria included head trauma, significant gross brain abnormalities, or a history of neurological or psychological disorders, substance abuse, or other serious medical conditions.
Table 1.
Participant characteristics by group
| NT | ASD | |
|---|---|---|
| M (SD) | M (SD) | |
| Age in years | 13.3 (2.8) | 14.9 (2.0) |
| Full-scale IQ | 108.1 (11.7) | 107.0 (7.6) |
| Verbal IQ | 113.1 (15.5) | 107.9 (14.8) |
| Mean absolute motion (mm) | 0.25 (0.19) | 0.33 (0.39) |
The participants with ASD were recruited primarily through UCLA’s Center for Autism Research and Treatment, fliers distributed to the local community and throughout the greater Los Angeles area, and from a pool of prior participants in research studies conducted at UCLA. The NT participants were recruited via the latter two strategies. Parents provided written consent and participants provided written assent according to procedures approved by the UCLA Institutional Review Board. All participants with ASD had a prior clinical diagnosis that was confirmed at the UCLA Autism Evaluation Center, using either the Autism Diagnostic Observation Schedule (ADOS; Lord et al. 2000) or the Autism Diagnostic Interview (ADI; Lord et al. 1994), as well as best clinical judgment (based upon DSM-IV diagnostic criteria). Specifically, all participants in the ASD group met criteria for autism based on the ADI and best clinical judgment; based on the ADOS, nine participants from the ASD group met criteria for autism and six met criteria for ASD.
Procedures
fMRI Paradigm
Participants were trained prior to the fMRI session on how to complete a person evaluation task where they were asked, in different blocks, whether short phrases described two specific targets: themselves, or a familiar fictional character (Harry Potter). These short phrases have been used in previous developmental social neuroscience research, and are described fully elsewhere (a complete list is available from Pfeifer et al. 2007). The phrases, adapted from developmentally-appropriate measures of self-concept, assessed two domains: social competence (“Social”—example: “I make friends easily”) and verbal academic abilities (“Academic”—example: “I’m a bad speller”). In one run (“Self”), participants were asked to report via button press (yes/no) whether the phrases described themselves. In another run (“Other”), participants were asked to report whether the phrases described Harry Potter. As in previous studies, phrases were presented only as auditory stimuli and lasted approximately 1 s each, followed by 3 s during which participants could respond before the next phrase was presented. Domains were presented in 40 s blocks within runs, which alternated with 12 s of rest. Each block began with 6 s of instruction reminding participants of the task during that block (“Do you think these phrases describe you/Harry Potter?”), and contained 10 trials (5 positive and 5 negative) that were ordered in a pseudorandom fashion. Two blocks per domain were presented in each run, in alternation. The order of the two domains (Social or Academic) within runs, as well as the order of the two targets (Self or Other) across runs, was counterbalanced between participants in each group. Responses as well as latencies were recorded for all participants (technological errors resulted in missing data for four participants total, two participants from each group).
Questionnaires
Parents of both NT and ASD participants were asked to complete the Social Responsiveness Scale (SRS) about the kind and severity of symptoms commonly displayed by youth with ASD (Constantino et al. 2003). All participants also completed a short questionnaire about Harry Potter. Each participant reported reading at least one book, or watching at least three movies, from the series. Furthermore, all participants had an equal level of familiarity with Harry Potter, based on their self-reports of equivalent knowledge about him on a 5-point scale (Ms = 3.53 and 3.47 for ASD and NT groups, respectively, t(30) = .183, NS). The groups also did not differ on their liking of Harry Potter on a 5-point scale (Ms = 3.60 and 3.24 for ASD and NT, respectively, t(30) = 1.13, NS).
fMRI Data Acquisition
Images were acquired using a Siemens Trio 3 Tesla MRI scanner. Participants were informed about the need to remain extremely still during the scan, and foam padding and medical tape were used to aid in motion reduction. For each participant, a 2D spin-echo scout localizer (TR = 8.6 ms, TE = 4.0 ms, matrix size 256 × 256, FoV = 25 cm) was acquired to allow prescription of the slices to be obtained in the remaining scans. The paradigm consisted of two functional scans, each lasting 4 min 4 s, during which time 122 images were collected (TR = 2,000 ms, TE = 28 ms, matrix size 64 × 64, FoV = 19.2 cm, 34 slices, 3 mm in-plane resolution, 4 mm thick). Additionally, a high-resolution structural scan was acquired co-planar with the functional scans (TR = 5,000 ms, TE = 34 ms, matrix size 128 × 128, FoV = 19.2 cm, 34 slices, 1.5 mm in-plan resolution, 4 mm thick).
fMRI Data Analysis
All structural and functional brain images were first skull-stripped using FSL’s Brain Extraction Tool (http://www.fmrib.ox.ac.uk/analysis/research/bet/bet.pdf; Smith 2002), and manually reoriented to the AC-PC before being preprocessed and analyzed using Statistical Parametric Mapping (SPM8; http://www.fil.ion.ucl.ac.uk/spm). Functional data for each participant were: (a) realigned to correct for head motion using a least squares approach and a 6 parameter (rigid body) spatial transformation; (b) coregistered to their respective high-resolution structural image using a rigid-body transformation in 3-dimensions; (c) spatially normalized into a Talairach-compatible atlas using 12-parameter affine transformation; and (d) smoothed using an 6-mm full width, half-maximum isotropic Gaussian Kernel.
Preprocessed images for each participant were then analyzed to estimate condition effects according to the general linear model, using a canonical hemodynamic response function convolved with the timing parameters of the box function described above (40 s experimental blocks), a high-pass filter to eliminate low frequency noise, and an autoregressive model (AR(1)) to estimate intrinsic autocorrelation of the data. Resulting contrast images of each simple effect against rest were entered into a second-level random effects 2 × 2 × 2 ANCOVA to allow for inferences to be made at the population level (Friston et al. 1999), with group (NT or ASD) as the between subjects factor, target (self vs. other) and domain (social vs. academic) as within subjects factors, and age as the covariate, to account for developmental changes over the age range of our participants. Resulting interactions were further interrogated using one-sample t-tests (for within subject interactions) or two-sample t-tests (for between subject interactions), and were masked by the relevant contrast. ROI analyses were conducted using MarsBaR (http://marsbar.sourceforge.net/), by extracting from clusters resulting from the 2 × 2 × 2 ANCOVA that fell within our a priori regions of interest specified above.
We used 3dClustSim, a Monte Carlo simulation bootstrapping program implemented in the AFNI library, to achieve correction for multiple comparisons. 3dClustSim accounts for the voxel-wise and cluster-volume thresholds to establish a family-wise error (FWE) rate of 5 %. Only regions with FWE-corrected p <0.05 at a combined magnitude and spatial extent are reported. These values were calculated by 3dClustSim using the mask from the 2 × 2 × 2 ANCOVA, and equaled 23 voxels (functional resolution, 3 × 3 × 3 mm) at p <.005 for magnitude.
Results
Behavioral Data
Responses and latencies were entered into ANOVAs with two within-subject factors, target (Self or Other) and domain (Social or Academic), and one between-subject factor, group (NT or ASD). Age was entered as a covariate in all analyses. Responses were coded as positive appraisals if the participant responded yes to a positively-valenced phrase, or no to a negatively-valenced phrase. Positivity of appraisals was then expressed as a percentage of total appraisals to which the participant responded. There were no significant differences between groups in the number of phrases that were missing data within and across targets and domains, indicating that the task was appropriate for both groups to complete. Indeed, very little data were missing (on average, 1 item or less per target, per domain, per group).
Positivity
Results demonstrated that there was a significant interaction between group and target (F(1, 29) = 11.6, p = .002). Post hoc comparisons indicated that NT youth made significantly more positive self-appraisals than did youth with ASD whereas youth with ASD made significantly more positive other-appraisals than did NT youth (see Table 2). Furthermore, NT youth showed a strong self-enhancing bias, as they made significantly more positive self-appraisals than other-appraisals. In contrast, youth with ASD made more positive other-appraisals than self-appraisals, although this was only marginally significant. There was also a significant interaction between target and age (F(1, 29) = 4.5, p = 0.042), such that positivity towards the other (but not the self) decreased with age. No other main effects or interactions were significant.
Table 2.
Mean positivity of evaluations by group, target, and domain
| NT | ASD | |
|---|---|---|
| M (SE) | M (SE) | |
| Self | 0.80 (0.03) | 0.64 (0.03) |
| Self-social | 0.88 (0.06) | 0.59 (0.06) |
| Self-academic | 0.73 (0.05) | 0.70 (0.05) |
| Other | 0.56 (0.05) | 0.71 (0.05) |
| Other-social | 0.55 (0.06) | 0.74 (0.07) |
| Other-academic | 0.58 (0.07) | 0.69 (0.07) |
Positivity equals the number of positive items endorsed and negative items rejected, divided by the total number of items responded to (resulting in a possible range of 0–1). Statistics reported from age covaried analysis
Latencies
Results demonstrated that there were no significant main effects of target, domain, group, or age. There were also no significant interactions between any of these factors.
fMRI Data
As described in the method section above, contrasts between each condition (Self-Social, Self-Academic, Other-Social, and Other-Academic) and the resting baseline were entered into a 2 × 2 × 2 ANCOVA with one between-subject factor, group (NT or ASD); two within-subject factors, target (Self or Other) and domain (Social or Academic); and one covariate, age. Significant findings (main effects or interactions) resulting from this model were subsequently interrogated using one or two-sample t-tests, in which age was again a covariate.
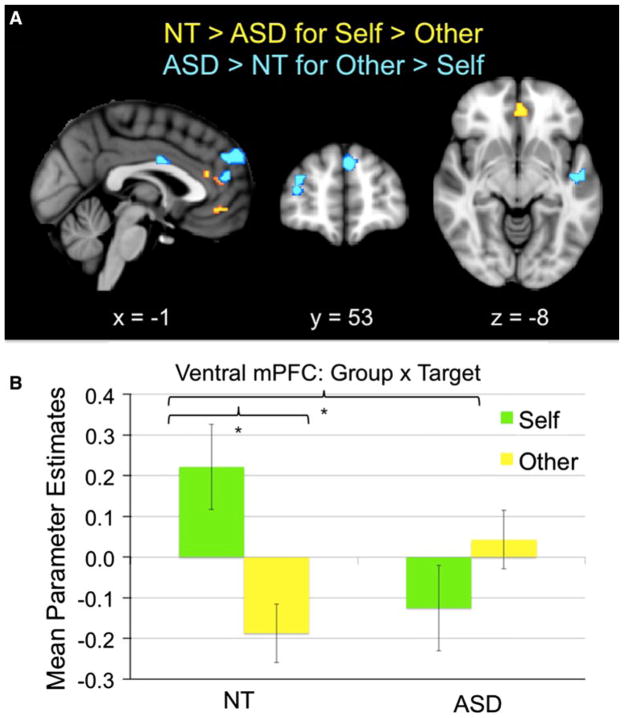
We were most interested in whether making appraisals of self and other differed, at a neural level, between NT youth and those with ASD; this would be indicated by a significant two-way interaction between group and target. Results demonstrated that there was indeed a significant interaction between group and target in several of our a priori regions of interest, including mPFC (dorsal, anterior rostral, and ventral), MCC, and anterior insula (see Table 3 for all results). Specifically, two-sample t-tests demonstrated that NT children and adolescents engaged mPFC (ventral, dorsal, and anterior rostral), MCC, and left anterior insula significantly more when making appraisals of self than other, in comparison to children and adolescents with ASD (NT >ASD for Self >Other, masked by NT Self >Other; see Fig. 1a, b; Table 3). Meanwhile, children and adolescents with ASD recruited significantly greater dorsal mPFC, left anterior insula, right inferior frontal gyrus (IFG), and right dorsolateral prefrontal cortex (dlPFC), during appraisals of other relative to self, in comparison to NT children and adolescents (ASD >NT for Other >Self, masked by ASD Other >Self; see Table 3; Fig. 1a). The two remaining complementary contrasts (ASD >NT for Self >Other, masked by ASD Self >Other; and NT >ASD for Other >Self, masked by NT Other >Self) did not result in any significant clusters of activation.
Table 3.
Significant clusters of activity from the two-way interaction between group and target
| Region | BA | x | y | z | k | F | |
|---|---|---|---|---|---|---|---|
| Group × target interaction: TD >ASD for Self >Other (age covariate) | |||||||
| Precentral gyrus | 8 | R | 33 | −7 | 46 | 131 | 18.77 |
| Middle frontal gyrus | 6 | L | −30 | 8 | 31 | 129 | 17.21 |
| Middle frontal gyrus | 6 | L | −33 | 2 | 55 | 48 | 13.42 |
| MCC | 24 | −3 | −1 | 28 | 69 | 15.69 | |
| Rostral PFC | 10 | R | 18 | 65 | 19 | 36 | 15.53 |
| Dorsal mPFC | 9 | −3 | 53 | 34 | 51a | 13.96 | |
| Anterior rostral mPFC | 10/32 | 0 | 47 | 16 | 30 | 12.45 | |
| Ventral mPFC | 32/10 | −3 | 44 | −8 | 12a | 11.68 | |
| Dorsolateral PFC | 10 | R | 21 | 44 | 19 | 64 | 13.84 |
| IFG | 45 | R | 45 | 14 | 19 | 61 | 12.70 |
| Dorsal striaum | R | 27 | 20 | 13 | 31 | 12.27 | |
| Anterior insula | L | −30 | 11 | 10 | 53 | 11.55 | |
| ASD >TD for Self >Other (age covariate) | |||||||
| No significant clusters | |||||||
| Region | BA | x | y | z | k | t | |
|---|---|---|---|---|---|---|---|
| TD >ASD for Self >Other (age covariate, masked by TD Self >Other) | |||||||
| Middle frontal gyrus | 6 | L | −30 | 8 | 37 | 58 | 4.54 |
| Precentral gyrus | 8 | R | 33 | −7 | 46 | 36 | 4.46 |
| Anterior insula | L | −24 | 29 | 16 | 63 | 4.20 | |
| Ventral mPFC | 32/10 | 0 | 41 | −8 | 50a | 4.00 | |
| Middle cingulate cortex | 24 | L | −12 | 14 | 22 | 24 | 3.56 |
| ASD >TD for Other >Self (age covariate, masked by ASD Other >Self) | |||||||
| Middle frontal gyrus | 6 | L | −30 | 8 | 37 | 103 | 4.53 |
| Middle frontal gyrus | 6 | L | −36 | 2 | 55 | 48 | 3.65 |
| Precentral gyrus | 8 | R | 33 | −7 | 46 | 45 | 4.41 |
| Anterior insula | L | −24 | 29 | 16 | 52 | 4.17 | |
| IFG | 45 | R | 42 | 14 | 16 | 41 | 3.70 |
| MCC | −12 | 14 | 22 | 25 | 3.55 | ||
| Dorsal striatum | R | 27 | 23 | 13 | 39 | 3.52 | |
| Dorsolateral PFC | 10 | R | 21 | 41 | 19 | 42 | 3.46 |
| Dorsolateral PFC | 9 | R | 27 | 35 | 37 | 23 | 3.35 |
| Dorsal mPFC | 9 | −3 | 53 | 31 | 21a | 3.31 | |
BA refers to putative Brodmann’s Area; x, y, and z refer to the left–right, anterior–posterior, and inferior–superior dimensions, respectively; t and F refers to the t and F statistic, respectively, at those coordinates (local maxima or submaxima); MCC, PFC, mPFC, and IFG refer to middle cingulate cortex, prefrontal cortex, medial prefrontal cortex, and inferior frontal gyrus, respectively
A cluster that survived the extent threshold (>23 voxels) in either the 2 × 2 × 2 ANCOVA or the masked t test, but not both
Fig. 1.
a Significant clusters resulting from the two-way interaction between group and target, specifically the directional contrast NT >ASD (for Self >Other). Images are thresholded at p <.005, 23 voxels (which achieves correction for multiple comparisons as calculated by 3dClusterSim). For display purposes, hot colors represent the contrast when masked by the Self >Other contrast from NT participants, thus depicting clusters in which NT >ASD (for Self >Other); cool colors represent the contrast when masked by the Other >Self contrast from ASD participants, thus depicting clusters in which ASD >NT (for Other >Self). x, y, and z refer to the left–right, anterior–posterior, and inferior–superior dimensions, respectively. b Mean parameter estimates of activity from the ventral mPFC (resulting from two-way interaction) cluster depicted in (a), for each target, by group. Asterisk denotes means that significantly differ (p <.05, two-tailed)
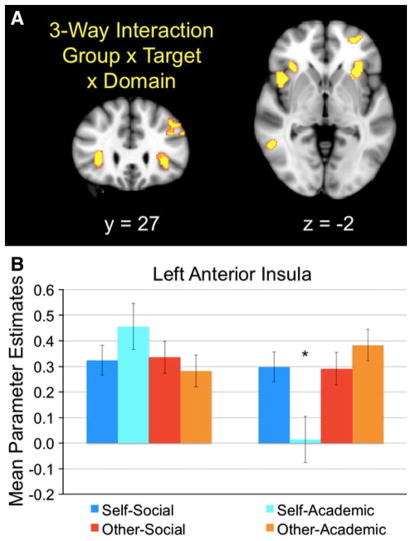
We also explored whether there was a significant three-way interaction between group, target, and domain. The 2 × 2 × 2 ANCOVA identified a significant three-way interaction in bilateral anterior insula and bilateral dlPFC (see Table 4; Fig. 2a). In general, these regions were significantly hyporesponsive in Self-Academic for youth with ASD (see Fig. 2b). There was no significant interaction between group and domain, and no significant main effect of group.
Table 4.
Significant clusters of activity from the three-way interaction between group, target, and domain
| Region | BA | x | y | z | k | F | |
|---|---|---|---|---|---|---|---|
| Group × target × domain (age covariate) | |||||||
| IFG/anterior insula | 47/13 | R | 42 | 14 | −5 | 66 | 22.79 |
| Anterior insula | R | 33 | 26 | 4 | 41 | 15.51 | |
| Anterior insula/IFG | 13/47 | L | −33 | 26 | 1 | 68 | 16.35 |
| Dorsolateral PFC | 9/10/46 | L | −33 | 50 | 31 | 97 | 14.62 |
| Dorsolateral PFC | 10/46/9 | R | 45 | 41 | 31 | 40 | 14.25 |
| IFG | 46 | R | 48 | 41 | 10 | 38 | 13.75 |
| Precentral gyrus | 6/22/44 | L | −57 | 8 | 4 | 23 | 13.55 |
| Ventrolateral PFC | 10 | L | −30 | 53 | −2 | 23 | 12.13 |
| Middle temporal gyrus | 20 | R | 54 | −52 | −2 | 26 | 15.76 |
BA refers to putative Brodmann’s Area; x, y, and z refer to the left–right, anterior–posterior, and inferior–superior dimensions, respectively; F refers to the F statistic, respectively, at those coordinates (local maxima or submaxima); IFG and PFC refer to inferior frontal gyrus and prefrontal cortex, respectively
Fig. 2.
a Significant clusters resulting from the three-way interaction between group, target, and domain in bilateral insula. Images are thresholded at p <.005, 23 voxels (which achieves correction for multiple comparisons as calculated by 3dClusterSim). y and z refer to the anterior–posterior and inferior–superior dimensions, respectively. b Mean parameter estimates of activity from the left anterior insula cluster depicted in (a), for each target across domains, by group. Asterisk denotes means that significantly differ (p <.05, two-tailed)
Brain-Behavior Correlations
Finally, we sought to determine whether neural responses in our a priori ROIs were associated with individual differences in social impairment for participants with ASD. We included the subset of ROIs from our full a priori list that were observed to result (as clusters) from the target × group interaction analyses summarized above: mPFC (ventral and dorsal aspects), MCC, and left anterior insula. As described in the methods section above, we used MarsBaR to extract parameter estimates of activity in these regions for the two target conditions (Self and Other). Age was partialled out of these correlational analyses. Within participants with ASD, higher levels of social impairment (indicated by higher scores on the social subscale of the ADOS) were correlated with lower levels of neural activity in MCC and left anterior insula (rs(16) = −0.46 and −0.51, one-tailed ps = .027, and .015, respectively) during Self >Other (see Fig. 3). In addition, higher levels of social responsiveness (indicated by lower scores on the SRS) were correlated with higher levels of activity in ventral mPFC ROI during Self >Other appraisals, but only in NT youth, not those with ASD (rs(16) = −0.45 and −0.01, ps = .031 and NS, for NT and ASD groups, respectively). Positivity was unrelated to brain activity in any of these ROIs. Of note, the differential (Self >Other) response to the process of making appraisals in the ventral mPFC ROI increased with age, but only significantly so for youth with ASD (rs(16) = 0.53 and 0.27, ps = .012 and .139, for ASD and NT groups, respectively).
Fig. 3.
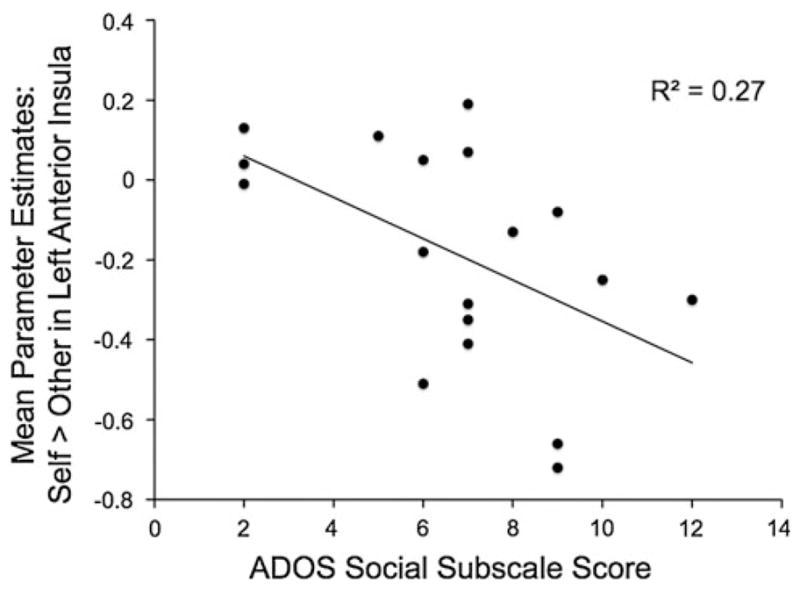
The significant correlation between more activity in the left anterior insula during appraisals of self (relative to appraisals of other) and better social functioning (as indexed by lower scores on the social subscale of the ADOS; higher scores indicate more impairment)
Discussion
This study documented the neural responses exhibited by children and adolescents with ASD when making appraisals of themselves as well as a familiar but distant other, and compared these with the neural responses exhibited by NT children and adolescents. Consistent with our primary hypothesis, we found that youth with ASD exhibited atypical functioning of ventral mPFC during the process of making self-appraisals. In the youth with ASD, symptom severity on the social subscale of the ADOS was also inversely correlated with responses in MCC and anterior insula during self versus other, two other brain regions whose response profiles differed as a function of group.
Ventral mPFC
In NT children and adolescents, ventral mPFC activity was significantly greater when making appraisals of self than other, but this pattern was absent in the ASD group—responses in ventral mPFC did not significantly differ between self and other appraisals in the ASD group. Furthermore, ventral mPFC activity was significantly greater in NT than ASD youth during self-appraisals, relative to other-appraisals. This pattern extends previous research which found that ventral mPFC distinguished between making appraisals of self and other in NT adults, but not in those with ASD (Lombardo et al. 2010; note the similarity between peak voxels). Another prior study likewise found that ventral mPFC did not distinguish between self and other in adults with ASD (Kennedy and Courchesne 2008a), although it should be noted that the “other” used in this design was someone with whom participants were likely to be very close: their mother. Closeness or intimacy between self and other appears to reduce the differentiation observed in this region (Chen et al. 2010; Krienen et al. 2010; Ochsner et al. 2005; Schmitz et al. 2004). The results of this study make it clear that this atypical pattern, namely a lack of differentiation in ventral mPFC between self and other during the appraisal process, is already present in children and adolescents with ASD, although the correlation between increasing age and self >other differentiation in ventral mPFC for the ASD group suggests that the magnitude of this effect may diminish over time.
What is the implication of this early lack of self-other differentiation in ventral mPFC in ASD? This will be an important target for future research, but one possibility is that, because of a less “peer-oriented” style of behavior (Sigman and Ruskin 1999; for review, see McConnell 2002), there is less intrinsic motivation in children or adolescents with ASD to engage in the kinds of social comparative processes that provide the basis for differentiation between self and other. Another reason to hypothesize motivational factors may be at work is that ventral mPFC has been implicated in more motivational and affective facets of self-referential processing, relative to dorsal mPFC (Moran et al. 2006; Ochsner et al. 2005; Pfeifer and Peake 2012). Compared to NT adults, NT children engage more ventral and dorsal mPFC when making self-appraisals than other-appraisals (Pfeifer et al. 2007), but the lack of self-other differentiation was only observed here in the ventral mPFC.
MCC
As previously discussed, regions implicated in self-appraisal processes traditionally include mPFC (dorsal, anterior rostral, and ventral aspects), adjacent rostral ACC, and mPPC (precuneus, PCC, and RSC). This study joins a growing body of research that suggests it may be time to expand this list: another relevant region to consider, also located along the cortical midline, is MCC. This region was found to respond in opposite ways when making self-appraisals than other-appraisals across the two groups. Specifically, the pattern of responses indicated that while NT youth activated MCC more during appraisals of self than other, youth with ASD activated MCC more during appraisals of other than self. To the extent youth with ASD demonstrated more activity in MCC during appraisals of self than other, they showed less severe impairments in the social domain (in other words, the more they showed the NT pattern, the better their social skills). This is consistent with previous research in adults with ASD, where greater engagement of MCC during appraisals of self than other was associated with less parent-reported autistic symptomatology (Lombardo et al. 2010).
Recent work using paradigms that tap into fairness and trust built over multiple rounds of interactions have strongly suggested that MCC activity is observed when individuals make a decision or response for one’s self in these economic exchange tasks, while ACC and PCC activity is associated with responses or decisions made by others (Chiu et al. 2008; Tomlin et al. 2006). This self-specific response is hypothesized to support consideration of how one’s own actions and intentions will be perceived by one’s interaction partner in the game (“reputation management”; Frith and Frith 2008). In high-functioning adult males with ASD, this self-specific response in MCC was on average absent, and relatively greater self-specific responses in MCC during the trust game were associated with lower levels of autistic symptomatology on the Autism Diagnostic Interview (Chiu et al. 2008). This same region of MCC is also engaged when imagining actions or emotions as if they were happening to one’s self (first-person perspective; Jackson et al. 2006; Lamm et al. 2007; Singer et al. 2004). Our finding that MCC responses are greater when making appraisals of self than other in NT youth but not those with ASD, and that greater self-specific responses in MCC were associated with less social dysfunction as measured by the ADOS, suggests that this discriminating function of MCC is already present in late childhood and early adolescence. Future research should continue to focus on the role of MCC in facilitating self-appraisals and how one may appear to others, as well as how atypical functioning of this region relates to the challenges experienced by individuals with ASD, especially because the cingulate is often found to exhibit reduced connectivity in ASD (Schulte-Ruther et al. 2011).
Anterior Insula
Several analyses indicated the anterior insula was another key region that responded differently when making appraisals of self and other, in social and academic domains, across our two groups. There was a significant three-way interaction between group, target, and domain in bilateral anterior insula. Here, the general pattern was that children and adolescents with ASD engaged this region less during academic self-appraisals than all other conditions, while NT youth engaged this region equally across all conditions. More broadly, left anterior insula was also identified, as part of a larger cluster, by the interaction between group and target. Children and adolescents with ASD engaged this region significantly more when making appraisals of other than self, compared to NT youth who engaged this region significantly more when making appraisals of self than other. Just like in MCC, scores on the social subscale of the ADOS indicating less social impairment were associated with more engagement of this region during self-appraisals than other-appraisals.
The anterior insula is notable for possessing large numbers of von Economo neurons, which may be responsible for connecting this region with the cingulate cortex, and some relate the possession of these special neurons to the capacity for self-awareness (Craig 2009). More generally, Craig (2009) reports that the anterior insula is frequently implicated in various aspects of agency, interoception (emotional awareness and physical sensations), decision-making, and visual self-recognition. It is puzzling that the children and adolescents with ASD did not engage this region during self-appraisals in the academic domain, suggesting that they were reporting on personal attributes without the accompanying sense of how possessing those attributes “felt” internally, and implying they may have acquired this trait information through an atypical route. In addition, the anterior insula (particularly in the left hemisphere) has been identified via studies and meta-analyses as specifically responsive to self-related stimuli (Modinos et al. 2009; Northoff et al. 2011; Qin et al. 2012). However, the anterior insula is also one of the most commonly activated regions in fMRI studies (Yarkoni et al. 2011). In the future, exploring additional domains of self-concept might help to shed light on these group differences in the contributions of anterior insula to appraising self and others.
mPPC
Research has steadily documented atypical functioning of mPPC in autism. For example, a recent meta-analysis showed that mPPC is hypoactive in adults with ASD during social tasks, along with the right anterior insula and peri-genual ACC/anterior rostral mPFC (Di Martino et al. 2009). Furthermore, mPPC shows atypical functional connectivity in ASD (Assaf et al. 2010; Cherkassky et al. 2006; Monk et al. 2009; Uddin et al. 2010). Subregions of mPPC exhibit varied anatomical connectivity, in ways that are consistent with patterns of functional connectivity: prominent links are observed with dorsal and ventral mPFC, MCC, and TPJ (Margulies et al. 2009). Interestingly, there were no differences observed at the group level in engagement of mPPC during this task between youth with and without ASD, suggesting this pattern of hypore-sponsivity may emerge later than the group differences already visible in mPFC, MCC, and anterior insula function when making appraisals of self and other. Future research should attempt to better characterize the developmental trajectory of group differences in mPPC functioning (task-dependent as well as independent, resting-state connectivity).
Positivity
Analyses of the behavioral responses indicated that NT youth were significantly more positive about themselves relative to a specific target other (Harry Potter), and also significantly more positive about themselves when compared directly to children and adolescents with ASD. The NT children and adolescents showed a self-enhancement bias (Sedikides and Gregg 2008), defined as perceiving themselves more positively than others in a social comparative fashion (rather than seeing themselves more positively than others see them due to limited self-insight; for discussion, see Kwan et al. 2008). The social comparison variety of self-enhancement is widely associated with positive adjustment and self-esteem (Brown 1986; Taylor and Brown 1988).
Meanwhile, the youth with ASD trended towards more positive appraisals of other than self. Such a pattern can be seen in depressed individuals (Noles et al. 1985). It should be noted that Harry Potter was chosen as the other target, in part, because he is rather average socially and academically. He has some good friends, but also gets teased and picked on by others with some frequency. He does well in some classes, but only aces a few, and is rather poor at several others. One could hypothesize that perhaps the children and adolescents with ASD perceived this average level of competence to be superior to their own (which may have been accurate, particularly in the social domain), and thus they evaluated him more positively than themselves. Regardless, it is important to note that differences in positivity were not significantly correlated with variability in responses in our a priori ROIs during the task. Future research should continue to focus on the valence of self-evaluations in individuals with ASD, and specifically explore under what circumstances individuals with ASD may make positive social comparisons.
Conclusion
In conclusion, this fMRI study documented hypoactivation of ventral mPFC during the process of making self-appraisals in children and adolescents with ASD. Self-other differentiation in ventral mPFC increased with age in youth with ASD, and was associated with more social responsiveness in NT youth. This suggests that there is the potential for improvement in youth with ASD, and perhaps indicates they suffer from a delay in developing the social or cognitive processes necessary to facilitate self-other differentiation in this region of ventral mPFC, or a delay in underlying structural development of ventral mPFC (or structural/functional connectivity between it and supporting regions). Other regions whose activity distinguished between groups included MCC and anterior insula. Children and adolescents with ASD who exhibited more normative patterns of activity in MCC and anterior insula during this task exhibited less severe social dysfunction. Together, these findings help to better delineate the time-course of atypical neurodevelopment in brain regions that are critical to self and social perception. In addition, the importance of endeavoring to understand this trajectory is highlighted by the significant differences in the valence of self-evaluations made by youth with ASD. Negative social self-evaluations are known to predispose children and adolescents to a wide range of undesirable outcomes, including loneliness and depression (Berndt and Burgy 1996; Bracken 1996), which are often perpetuated via cycles of peer rejection (Coie and Cillessen 1993); meanwhile, negative academic self-evaluations confer risk for academic disengagement and low achievement (Marsh 1990; Harter 1999). Preventing the development of negative self-evaluations in both social and nonsocial domains, and understanding the implications of possessing them (not only at the behavioral level, but also the neural level), should be a powerfully motivating stimulus for future research and interventions in populations with ASD and other developmental disorders. In particular, it will be important to determine whether the neural systems underlying other aspects of self-processing, such as autobiographical memory retrieval or self-conscious emotions, also exhibit atypical patterns in children and adolescents with autism spectrum disorders. This will allow us to determine how the self and its development, much more broadly construed, are impacted in autism.
Acknowledgments
This work was supported by the National Institute of Child Health and Human Development [P50 HD055784] and grants from the National Institutes of Health Loan Repayment Program [WFMY9703 and NAOT6886 to J.H.P.]. For generous support the authors also wish to thank the Brain Mapping Medical Research Organization, Brain Mapping Support Foundation, Pierson-Lovelace Foundation, Ahmanson Foundation, Tamkin Foundation, Jennifer Jones-Simon Foundation, Capital Group Companies Charitable Foundation, Robson Family, William M. and Linda R. Dietel Philanthropic Fund at the Northern Piedmont Community Foundation, Northstar Fund, and the FPR-UCLA Center for Culture, Brain, and Development. This project was in part also supported by grants (RR12169, RR13642 and RR00865) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH); its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCR or NIH.
Contributor Information
Jennifer H. Pfeifer, Email: jpfeifer@uoregon.edu, Department of Psychology, 1227 University of Oregon, Eugene, OR 97403-1227, USA
Junaid S. Merchant, Department of Psychology, 1227 University of Oregon, Eugene, OR 97403-1227, USA
Natalie L. Colich, Department of Psychology, Stanford University, Stanford, CA, USA. Ahmanson-Lovelace Brain Mapping Center, David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, CA, USA
Leanna M. Hernandez, Ahmanson-Lovelace Brain Mapping Center, David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, CA, USA
Jeff D. Rudie, Neuroscience Interdepartmental Program, David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, CA, USA
Mirella Dapretto, Ahmanson-Lovelace Brain Mapping Center, David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, CA, USA. Center for Autism Research and Treatment, Semel Institute for Neuroscience and Human Behavior, David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, CA, USA. Department of Psychiatry and Biobehavioral Sciences, David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, CA, USA. FPR-UCLA Center for Culture, Brain, and Development, University of California, Los Angeles, Los Angeles, CA, USA.
References
- Assaf M, Jagannathan K, Calhoun VD, Miller L, Stevens MC, Sahl R, et al. Abnormal functional connectivity of default mode sub-networks in autism spectrum disorder patients. Neuroimage. 2010;53(1):247–256. doi: 10.1016/j.neuroimage.2010.05. 067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndt TJ, Burgy L. Social self-concept. In: Bracken BA, editor. Handbook of self-concept: Developmental, social, and clinical considerations. New York: Wiley; 1996. pp. 171–209. [Google Scholar]
- Bracken B, editor. Handbook of self-concept. New York: Wiley; 1996. [Google Scholar]
- Brown JD. Evaluations of self and others: Self-enhancement biases in social judgments. Social Cognition. 1986;4:353–376. doi: 10.1521/soco.1986.4.4.353. [DOI] [Google Scholar]
- Bruck M, London K, Landa R, Goodman J. Autobiographical memory and suggestibility in children with autism spectrum disorder. Development and Psychopathology. 2007;19(1):73–95. doi: 10.1017/S0954579407070058. [DOI] [PubMed] [Google Scholar]
- Chen AC, Welsh RC, Liberzon I, Taylor SF. ‘Do I like this person?’ A network analysis of midline cortex during a social preference task. Neuroimage. 2010;51(2):930–939. doi: 10.1016/j.neuroimage.2010.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherkassky VL, Kana RK, Keller TA, Just MA. Functional connectivity in a baseline resting-state network in autism. NeuroReport. 2006;17(16):1687–1690. doi: 10.1097/01.wnr. 0000239956.45448.4c. [DOI] [PubMed] [Google Scholar]
- Chiu PH, Kayali MA, Kishida KT, Tomlin D, Klinger LG, Klinger MR, et al. Self responses along cingulate cortex reveal quantitative neural phenotype for high-functioning autism. Neuron. 2008;57(3):463–473. doi: 10.1016/j.neuron.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coie JD, Cillessen AHN. Peer rejection: Origins and effects on children’s development. Current Directions in Psychological Science. 1993;2:89–92. [Google Scholar]
- Constantino JN, Davis SA, Todd RD, Schindler MK, Gross MM, Brophy SL, et al. Validation of a brief quantitative measure of autistic traits: Comparison of the social responsiveness scale with the autism diagnostic interview-revised. Journal of Autism and Developmental Disorders. 2003;33(4):427–433. doi: 10.1023/A:1025014929212. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel—now? The anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Crane L, Goddard L. Episodic and semantic autobiographical memory in adults with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2008;38(3):498–506. doi: 10.1007/s10803-007-0420-2. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Ross K, Uddin LQ, Sklar AB, Castellanos FX, Milham MP. Functional brain correlates of social and nonsocial processes in autism spectrum disorders: An activation likelihood estimation meta-analysis. Biological Psychiatry. 2009;65(1):63–74. doi: 10.1016/j.biopsych.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Price CJ, Buchel C, Worsley KJ. Multisubject fMRI studies and conjunction analyses. Neuroimage. 1999;10:385–396. doi: 10.1006/nimg.1999.0484. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U. Interacting minds—a biological basis. Science. 1999;286(5445):1692. doi: 10.1126/science.286.5445.1692. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U. Implicit and explicit processes in social cognition. Neuron. 2008;60(3):503–510. doi: 10.1016/j.neuron.2008.10.032. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proceedings of the National academy of Sciences of the United States of America. 2003;100(1):253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harter S. The construction of the self: A developmental perspective. New York, NY: The Guilford Press; 1999. [Google Scholar]
- Heerey EA, Keltner D, Capps LM. Making sense of self-conscious emotion: Linking theory of mind and emotion in children with autism. Emotion (Washington, DC) 2003;3(4):394–400. doi: 10.1037/1528-3542.3.4.394. [DOI] [PubMed] [Google Scholar]
- Henderson HA, Zahka NE, Kojkowski NM, Inge AP, Schwartz CB, Hileman CM, et al. Self-referenced memory, social cognition, and symptom presentation in autism. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2009;50(7):853–861. doi: 10.1111/j.1469-7610.2008.02059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillier A, Allinson L. Beyond expectations: Autism, understanding embarrassment, and the relationship with theory of mind. Autism: The International Journal of Research and Practice. 2002;6(3):299–314. doi: 10.1177/1362361302006003007. [DOI] [PubMed] [Google Scholar]
- Hobson PR, Chidambi G, Lee A, Meyer J. Foundations for self-awareness: An exploration through autism. Monographs of the Society for Research in Child Development. 2006;71(2):vii–166. doi: 10.1111/j.1540-5834.2006.00387.x. [DOI] [PubMed] [Google Scholar]
- Jackson PL, Brunet E, Meltzoff AN, Decety J. Empathy examined through the neural mechanisms involved in imagining how I feel versus how you feel pain. Neuropsychologia. 2006;44(5):752–761. doi: 10.1016/j.neuropsychologia.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Kennedy DP, Courchesne E. Functional abnormalities of the default network during self- and other-reflection in autism. Social Cognitive and Affective Neuroscience. 2008a;3(2):177–190. doi: 10.1093/scan/nsn011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DP, Courchesne E. The intrinsic functional organization of the brain is altered in autism. Neuroimage. 2008b;39(4):1877–1885. doi: 10.1016/j.neuroimage.2007.10.052. [DOI] [PubMed] [Google Scholar]
- Krienen FM, Tu P, Buckner RL. Clan mentality: Evidence that the medial prefrontal cortex responds to close others. The Journal of Neuroscience. 2010;30(41):13906–13915. doi: 10.1523/JNEUROSCI.2180-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan VS, John OP, Robins RW, Kuang LL. Conceptualizing and assessing self-enhancement bias: A componential approach. Journal of Personality and Social Psychology. 2008;94(6):1062–1077. doi: 10.1037/0022-3514.94.6.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Batson CD, Decety J. The neural substrate of human empathy: Effects of perspective-taking and cognitive appraisal. Journal of Cognitive Neuroscience. 2007;19(1):42–58. doi: 10.1162/jocn.2007.19.1.42. [DOI] [PubMed] [Google Scholar]
- Leary MR, Tangney JP. Handbook of self and identity. Guilford Press; 2003. The self as an organizing construct in the behavioral and social sciences; pp. 3–14. [Google Scholar]
- Lee A, Hobson RP. On developing self-concepts: A controlled study of children and adolescents with autism. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1998;39(8):1131–1144. [PubMed] [Google Scholar]
- Lee A, Hobson RP, Chiat S. I, you, me, and autism: An experimental study. Journal of Autism and Developmental Disorders. 1994;24(2):155–176. doi: 10.1007/BF02172094. [DOI] [PubMed] [Google Scholar]
- Lind SE. Memory and the self in autism: A review and theoretical framework. Autism: The International Journal of Research and Practice. 2010;14(5):430–456. doi: 10.1177/1362361309358700. [DOI] [PubMed] [Google Scholar]
- Lind SE, Bowler DM. Delayed self-recognition in children with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2009a;39(4):643–650. doi: 10.1007/s10803-008-0670-7. [DOI] [PubMed] [Google Scholar]
- Lind SE, Bowler DM. Recognition memory, self-other source memory, and theory-of-mind in children with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2009b;39(9):1231–1239. doi: 10.1007/s10803-009-0735-2. [DOI] [PubMed] [Google Scholar]
- Lombardo MV, Barnes JL, Wheelwright SJ, Baron-Cohen S. Self-Referential cognition and empathy in autism. PLoS One. 2007;2(9):e883. doi: 10.1371/journal.pone.0000883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo MV, Baron-Cohen S. The role of the self in mindblindness in autism. Consciousness and Cognition. 2011;20(1):130–140. doi: 10.1016/j.concog.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Lombardo MV, Charkrabarti B, Bullmore ET, Sadek SA, Pasco G, Wheelwright SJ, et al. Atypical neural self-representation in autism. Brain. 2010;133(2):611–624. doi: 10.1093/brain/awp306. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–223. doi: 10.1023/A:1005592401947. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Margulies DS, Vincent JL, Kelly C, Lohmann G, Uddin LQ, Biswal BB, et al. Precuneus shares intrinsic functional architecture in humans and monkeys. Proceedings of the National Academy of Sciences. 2009;106(47):20069–20074. doi: 10.1073/pnas.0905314106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus H. Self-schemata and processing information about the self. Journal of Personality and Social Psychology. 1977;35(2):63–78. [Google Scholar]
- Marsh HW. Causal ordering of academic self-concept and academic achievement: A multiwave, longitudinal panel analysis. Journal of Educational Psychology. 1990;82:646–656. [Google Scholar]
- McConnell SR. Interventions to facilitate social interaction for young children with autism: Review of available research and recommendations for educational intervention and future research. Journal of Autism and Developmental Disorders. 2002;32(5):351–372. doi: 10.1023/A:1020537805154. [DOI] [PubMed] [Google Scholar]
- Millward C, Powell S, Messer D, Jordan R. Recall for self and other in autism: Children’s memory for events experienced by themselves and their peers. Journal of Autism and Developmental Disorders. 2000;30(1):15–28. doi: 10.1023/A: 1005455926727. [DOI] [PubMed] [Google Scholar]
- Mitchell JP. Inferences about other minds. Philosophical Transactions of the Royal Society B. 2009;364:1309–1316. doi: 10.1098/rstb.2008.0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modinos G, Ormel J, Aleman A. Activation of Anterior Insula during Self-Reflection. PLoS One. 2009;4(2):e4618. doi: 10.1371/journal.pone.0004618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk CS, Peltier SJ, Wiggins JL, Weng SJ, Carrasco M, Risi S, et al. Abnormalities of intrinsic functional connectivity in autism spectrum disorders. Neuroimage. 2009;47(2):764–772. doi: 10.1016/j.neuroimage.2009.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran JM, Macrae CN, Heatherton TF, Wyland CL, Kelley WM. Neuroanatomical evidence for distinct cognitive and affective components of self. Journal of Cognitive Neuroscience. 2006;18(9):1586–1594. doi: 10.1162/jocn.2006.18.9.1586. [DOI] [PubMed] [Google Scholar]
- Noles SW, Cash TF, Winstead BA. Body image, physical attractiveness, and depression. Journal of Consulting and Clinical Psychology. 1985;53:88–94. doi: 10.1037//0022-006x.53.1.88. [DOI] [PubMed] [Google Scholar]
- Northoff G, Bermpohl F. Cortical midline structures and the self. Trends in Cognitive Sciences. 2004;8(3):102–107. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain–a meta-analysis of imaging studies on the self. Neuroim-age. 2006;31(1):440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Northoff G, Qin P, Feinberg TE. Brain imaging of the self—conceptual, anatomical, and methodological issues. Consciousness and Cognition. 2011;20:52–64. doi: 10.1016/j.concog. 2010.09.011. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Beer JS, Robertson ER, Cooper JC, Gabrieli JD, Kihsltrom JF, et al. The neural correlates of direct and reflected self-knowledge. Neuroimage. 2005;28(4):797–814. doi: 10.1016/j.neuroimage.2005.06.069. [DOI] [PubMed] [Google Scholar]
- Pfeifer JH, Lieberman MD, Dapretto M. “I know you are but what am I?!”: Comparing the neural bases of self- and social knowledge retrieval in children and adults. Journal of Cognitive Neuroscience. 2007;19:1323–1337. doi: 10.1162/jocn.2007.19.8.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, Masten CL, Borofsky LA, Dapretto M, Lieberman MD, Fuligni AJ. Neural correlates of direct and reflected self-appraisals in adolescents and adults: When social perspective-taking informs self-perception. Child Development. 2009;80:1016–1034. doi: 10.1111/j.1467-8624.2009.01314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, Peake SJ. Self-development: Integrating cognitive, social, and neuroimaging perspectives. Developmental Cognitive Neuroscience. 2012 doi: 10.1016/j.dcn.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin P, Liu Y, Shi J, Wang Y, Duncan N, Gong Q, et al. Dissociation between anterior and posterior cortical regions during self-specificity and familiarity: A combined fMRI-meta-analytic study. Human Brain Mapping. 2012;33:154–164. doi: 10.1002/hbm.21201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ. A default mode of brain function: A brief history of an evolving idea. Neuroimage. 2007;37(4):1083–1090. doi: 10.1016/j.neuroimage. 2007.02.041. discussion 1097–1099. [DOI] [PubMed] [Google Scholar]
- Ray RD, Shelton AL, Hollon NG, Michel BD, Frankel CB, Gross JJ, et al. Cognitive and neural development of individuated self-representation in children. Child Development. 2009;80(4):1232–1242. doi: 10.1111/j.1467-8624.2009.01327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins RW, Tracy JL, Trzesniewski KH. Handbook of personality: Theory and research. 3. Guilford Press; 2008. Naturalizing the self; pp. 375–398. [Google Scholar]
- Saxe R. The right temporo-parietal junction: A specific brain region for thinking about thoughts. In: Leslie A, German T, editors. Handbook of theory of mind. 2010. [Google Scholar]
- Schmitz TW, Kawahara-Baccus TN, Johnson SC. Metacognitive evaluation, self-relevance, and the right prefrontal cortex. Neuroimage. 2004;22:941–947. doi: 10.1016/j.neuroimage.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Schulte-Ruther M, Greimel E, Markowitsch HJ, Kamp-Becker I, Remschmidt H, Fink GR, et al. Dysfunctions in brain networks supporting empathy: An fMRI study in adults with autism spectrum disorders. Social Neuroscience. 2011;6:1–21. doi: 10.1080/17470911003708032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedikides C, Spencer SJ, editors. The self: Frontiers in social psychology. New York, NY: Psychology Press; 2007. [Google Scholar]
- Sedikides C, Gregg AP. Self-enhancement: Food for thought. Perspectives on Psychological Science. 2008;3(2):102. doi: 10.1111/j.1745-6916.2008.00068.x. [DOI] [PubMed] [Google Scholar]
- Sigman M, Ruskin E. Continuity and change in the social competence of children with autism, Down syndrome, and developmental delays. Monographs of the Society for Research in Child Development. 1999;64:1–130. doi: 10.1111/1540-5834.00002. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerfield JJ, Hassabis D, Maguire E. Differential engagement of brain regions within a ‘core’ network during scene construction. Neuropsychologia. 2010;48:1501–1509. doi: 10.1016/j.neuropsychologia.2010.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda E, McKinnon MC, Levine B. The functional neuroanatomy of autobiographical memory: A meta-analysis. Neuropsychologia. 2006;44:2189–2208. doi: 10.1016/j.neuropsychologia.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Brown JD. Illusion and well-being: A social psychological perspective on mental health. Psychological Bulletin. 1988;103:193–210. [PubMed] [Google Scholar]
- Toichi M, Kamio Y, Okada T, Sakihama M, Youngstrom EA, Findling RL, et al. A lack of self-consciousness in autism. The American Journal of Psychiatry. 2002;159(8):1422–1424. doi: 10.1176/appi.ajp.159.8.1422. [DOI] [PubMed] [Google Scholar]
- Tomlin D, Kayali MA, King-Casas B, Anen C, Camerer CF, Quartz SR, et al. Agent-Specific responses in the cingulate cortex during economic exchanges. Science (New York, NY) 2006;312(5776):1047–1050. doi: 10.1126/science.1125596. [DOI] [PubMed] [Google Scholar]
- Uddin LQ. The self in autism: An emerging view from neuroimaging. Neurocase. 2011;17(3):201–208. doi: 10.1080/13554794.2010.509320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Menon V. Typical and atypical development of functional human brain networks: Insights from resting-state FMRI. Frontiers in Systems Neuroscience. 2010;4:21. doi: 10.3389/fnsys.2010.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. WISC-III manual. New York: The Psychological Corporation; 1991. [Google Scholar]
- Wechsler D. The Wechsler Abbreviated Scale of intelligence-UK. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- Weng SJ, Wiggins JL, Peltier SJ, Carrasco M, Risi S, Lord C, et al. Alterations of resting state functional connectivity in the default network in adolescents with autism spectrum disorders. Brain Research. 2010;1313:202–214. doi: 10.1016/j.brainres.2009.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DM, Happé F. What did I say? Versus what did I think? Attributing false beliefs to self amongst children with and without autism. Journal of Autism and Developmental Disorders. 2009;39(6):865–873. doi: 10.1007/s10803-009-0695-6. [DOI] [PubMed] [Google Scholar]
- Williams D, Happé F. Recognising ‘social’ and ‘non-social’ emotions in self and others: A study of autism. Autism: The International Journal of Research and Practice. 2010;14(4):285–304. doi: 10.1177/1362361309344849. [DOI] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nature Methods. 2011;8:665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]