Abstract
NADPH oxidases synthesize reactive oxygen species that may participate in fibrosis progression. NOX4 and NOX2 are NADPH oxidases expressed in the kidneys, with the former being the major renal isoform, but their contribution to renal disease is not well understood. Here, we used the unilateral urinary obstruction model of chronic renal injury to decipher the role of these enzymes using wild-type, NOX4-, NOX2-, and NOX4/NOX2-deficient mice. Compared with wild-type mice, NOX4-deficient mice exhibited more interstitial fibrosis and tubular apoptosis after obstruction, with lower interstitial capillary density and reduced expression of hypoxia-inducible factor-1α and vascular endothelial growth factor in obstructed kidneys. Furthermore, NOX4-deficient kidneys exhibited increased oxidative stress. With NOX4 deficiency, renal expression of other NOX isoforms was not altered but NRF2 protein expression was reduced under both basal and obstructed conditions. Concomitant deficiency of NOX2 did not modify the phenotype exhibited by NOX4-deficient mice after obstruction. NOX4 silencing in a mouse collecting duct (mCCDcl1) cell line increased TGF-β1–induced apoptosis and decreased NRF2 protein along with expression of its target genes. In addition, NOX4 silencing decreased hypoxia-inducible factor-1α and expression of its target genes in response to hypoxia. In summary, these results demonstrate that the absence of NOX4 promotes kidney fibrosis, independent of NOX2, through enhanced tubular cell apoptosis, decreased microvascularization, and enhanced oxidative stress. Thus, NOX4 is crucial for the survival of kidney tubular cells under injurious conditions.
CKD is a worldwide health problem defined as an alteration of kidney function usually estimated by GFR decline or histologic damage to the kidney.1 CKD is associated with high mortality and morbidity as well as tremendous costs associated with renal replacement when patients reach ESRD.
Interstitial fibrosis and glomerulosclerosis are hallmarks of CKD. Tubulointerstitial fibrosis is better correlated with loss of kidney function than glomerulosclerosis independently of primary injury.2 Tubulointerstitial fibrosis arises from numerous, confounding events. These include tubular atrophy, via necrosis or apoptosis, and local hypoxia, due to the loss of peritubular capillaries, which are early events.3–5 Tubular cells are both targets of tubulointerstitial fibrosis and important players in its progression via secretion of chemotactic factors and cytokines.
Oxidative stress has been associated with aging and CKD progression.6,7 Increased levels of kidney reactive oxygen species (ROS), such as angiotensin II–induced and diabetic nephropathies, are found in CKD.8–13 However, low levels of ROS also play important roles in oxygen sensing, neoangiogenesis, and cell survival pathways, which may play protective roles against kidney fibrosis.14,15 NADPH oxidases (NOXs) are enzymes that produce ROS as primary products.16 The NOX family is composed of seven members (NOX1–5 and DUOX 1 and 2). Among these, NOX1, NOX2, and NOX4 are expressed in both mice and human kidney, whereas NOX5 is only expressed in human kidney. NOX4 is highly expressed in the tubular cell compartment, where its physiologic role is unknown.17 This isoform is mainly regulated by its controlled abundance.18 The role of NOX4 in kidney injury is not yet elucidated. Increased renal NOX4 expression has been shown in diabetic nephropathy and its silencing has been demonstrated to be protective.8,19 However, NOX4 may participate to oxygen sensing and exhibit anti-inflammatory properties via regulation of the NRF2 pathway in the heart.15,20
To determine the role of NOX4 and NOX2 in kidney fibrosis progression, we performed unilateral ureteral obstruction (UUO) on wild-type (WT), and NOX4 knockout (KO) mice as well as on NOX2 and NOX4/NOX2 KO mice. Indeed, UUO is a well described model of renal tubular stress leading to kidney fibrosis.21,22 We demonstrate that deletion of NOX4 is associated with increased kidney fibrosis in obstructed kidneys. This effect was associated with enhanced tubular cell apoptosis as well as defective hypoxia-inducible factor-1α (HIF-1α) oxygen sensing and NRF2 antioxidant pathways. NOX4 is therefore crucial for kidney tubular cell survival under stressed conditions.
Results
NOX4 Deficiency Increases Tubulointerstitial Fibrosis after UUO
Gross kidney morphology and histologic organization were not altered by NOX4 deficiency. Kidney size and ratio of kidney weight to body weight were similar in NOX4 KO compared with WT mice (1.4%±0.3% versus 1.4%±0.4%; P=0.53). Serum creatinine levels were similar in NOX4 KO versus WT animals (8.3±0.7 versus 8.5±1.4 μmol/L; P>0.05). Differentiation of cortex and external and internal medulla were similar in WT and NOX4 KO mice (Supplemental Figure 1A). Glomerular and tubular structures of NOX4 KO mice were undistinguishable from those of WT mice (Supplemental Figure 1B). Labeling of NaPi2a and AQP2 demonstrated that the overall kidney tubule structure and differentiation were similar in each group of animals (Supplemental Figure 1C).
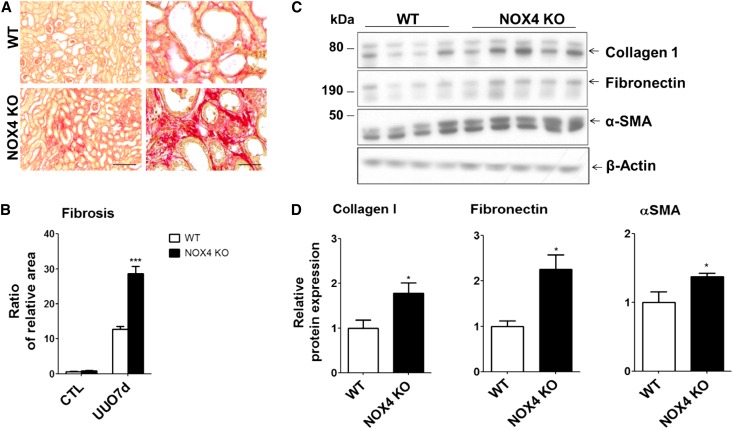
To evaluate the role of NOX4 in interstitial fibrosis observed after kidney tubule injury, UUO was performed. After 7 days of UUO, both WT and NOX4 KO mice exhibited tubulointerstitial fibrosis detected by argentic and Masson trichrome staining (Supplemental Figure 2, A and B). Increased tubulointerstitial fibrosis in the kidney cortex of NOX4 KO mice compared with that of WT mice was revealed by quantification of unpolarized (Figure 1, A and B) and polarized (Supplemental Figure 2, C and D) Sirius red staining that allows reliable semiquantitative measurement of kidney fibrosis.23 Increased Sirius red staining was associated with increased expression of classic markers of fibrosis such as collagen-1, fibronectin, and α-smooth muscle actin (αSMA), measured by Western blotting in obstructed kidney cortex of NOX4 KO mice compared with WT mice (Figure 1, C and D). Loading control was performed using Coomassie staining (Supplemental Figure 3). Contralateral, nonobstructed kidneys did not display significant fibrosis.
Figure 1.
Tubulointerstitial fibrosis is increased in obstructed kidneys of NOX4 KO mice. (A) Representative images of unpolarized Sirius red staining of obstructed kidneys (UUO7d) from WT and NOX4 KO animals after 7 days of UUO. Scale bar, 200 µm on left and 50 µm on right. (B) Renal cortex quantification of unpolarized Sirius red staining performed in contralateral (CTL) and obstructed (UUO7d) kidneys from WT and NOX4 KO mice (WT, n=9; NOX4 KO, n=9). Results are expressed as the mean ratio of stained area to the total tissue area over the mean value obtained in WT ± SEM. (C) Representative Western blot analysis of collagen-1, fibronectin, and α-SMA performed in obstructed kidneys (UUO7d) from WT and NOX4 KO mice after 7 days of UUO. (D) Densitometric quantification of collagen-1, fibronectin, and α-SMA protein expression (WT, n=7; NOX4 KO, n=9). Results are expressed as the mean ratio of individual values over the mean value obtained in WT ± SEM. *P<0.05; ***P<0.005.
Therefore, NOX4 deficiency does not alter kidney development and basal architecture but promotes tubulointerstitial fibrosis in response to tubular stress.
NOX4 Deficiency Increases Tubular Cell Apoptosis without Altering Proliferation, Resulting in Tubular Atrophy in Obstructed Kidneys
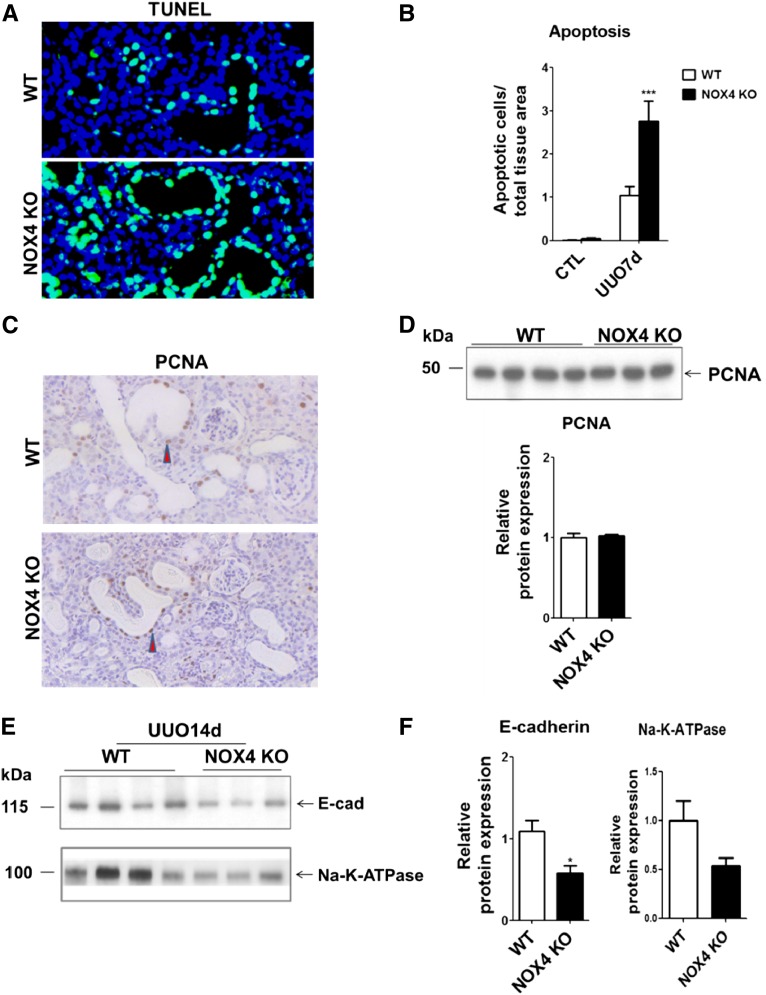
Tubular cell apoptosis is an early event of UUO that induces loss of normal structures and formation of atubular glomeruli.3 Extensive tubular cell apoptosis was observed by terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling (TUNEL) assay after 7 days in obstructed kidneys (Figure 2A). Epithelial cell apoptosis was observed in both the proximal tubule and collecting duct (Supplemental Figure 4). Cortical tubular epithelial cell apoptosis in NOX4 KO mice was significantly higher than in WT mice (Figure 2B). Tubular cell apoptosis was not detected in contralateral, nonobstructed kidneys. Tubular cell proliferation, assessed by proliferating cell nuclear antigen immunostaining and Western blotting, was increased in the cortex of obstructed kidneys as compared with contralateral, nonobstructed kidneys (data not shown) but was not influenced by NOX4 deficiency (Figure 2, C and D). Enhanced apoptosis that is not compensated by increased cell proliferation should result in tubular atrophy. E-cadherin and Na-K ATPase expression, assessed by Western blotting after 14 days of UUO, were lower in obstructed kidneys of NOX4 KO mice compared with WT mice (Figure 2, E and F), suggesting that tubular epithelial mass was decreased. Therefore, NOX4 deficiency promotes tubular epithelial cell apoptosis and tubular atrophy in response to UUO.
Figure 2.
Tubular cell apoptosis and atrophy are increased in obstructed kidneys of NOX4 KO mice. (A) Merged TUNEL and DAPI staining performed in kidneys from WT and NOX4 KO mice after 7 days of UUO. (B) Renal cortex quantification of TUNEL-positive apoptotic cells in renal cortex of WT and NOX4 KO mice after 7 days of UUO (WT, n=9; NOX4 KO, n=9). Results are expressed as the mean ratio of apoptotic cells to total tissue area over the mean density of apoptotic cells measured in WT ± SEM. (C) Representative images of PCNA immunohistochemical staining performed in obstructed kidneys (UUO7d) from WT and NOX4 KO mice after 7 days of UUO (arrowheads indicate PCNA-positive nuclei). Scale bar, 200 µm. (D) PCNA Western blot analysis (upper panel) and densitometric quantification (lower panel) performed in obstructed kidneys (UUO7d) from WT and NOX4 KO mice after 7 days. Results are expressed as the mean ratio of individual values over the mean value obtained in WT ± SEM. (E and F) E-cadherin and Na-K ATPase Western blot analysis and densitometric quantification performed in obstructed kidneys (UUO14d) from WT and NOX4 KO mice after 14 days of UUO (WT, n=4; NOX4 KO, n=3). The results are expressed as the mean ratio of individual values over the mean value obtained in WT ± SEM. *P<0.05; ***P<0.005. DAPI, 4',6-diamidino-2-phenylindole; CTL, contralateral; PCNA, proliferating cell nuclear antigen.
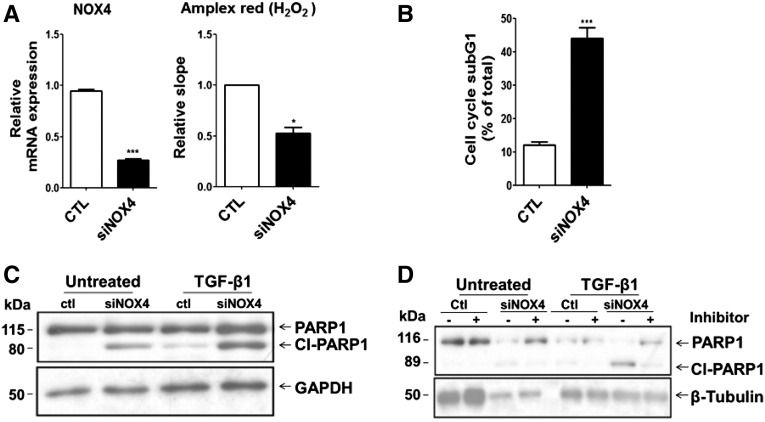
To determine whether NOX4 deficiency directly influences tubular cell apoptosis, we used cultured mCCDcl1 cells, a mouse collecting duct cell line expressing NOX4, transfected either with scramble siRNA or siRNA targeting NOX4. The efficiency of NOX4 silencing in mCCDcl1 cells was demonstrated by a >70% decrease of NOX4 mRNA expression and by decreased H2O2 production, as measured by Amplex red (Figure 3A). Silencing of NOX4 increased the proportion of subG1 phase apoptotic cells detected by flow cytometry analysis (Figure 3B). In NOX4-silenced cells, apoptosis was increased by TGF-β1 treatment (Figure 3C), as detected by Western blot analysis of poly (ADP-ribose) polymerase-1 cleavage, a marker of late apoptosis. To determine whether enhanced apoptosis is mediated through a classic or alternative pathway, we used a pharmacologic inhibitor of caspase-9. Poly (ADP-ribose) polymerase-1 cleavage in NOX4-depleted cells was partially reversed by caspase-9 inhibition (Figure 3D), suggesting that the intrinsic mitochondrial apoptotic pathway is enhanced by NOX4 deficiency in response to TGF-β1. These results confirm a major role of NOX4 in tubular cell survival under stress conditions.
Figure 3.
NOX4 depletion enhances apoptosis in mCCDcl1 cells subjected to TGF-β1 and hypoxia. (A) NOX4 mRNA depletion efficiency (left) and H2O2 production (right), respectively, measured by real-time PCR and by Amplex red, performed in mCCDcl1 cells transfected with scramble RNA (ctl) or siRNA specifically targeting NOX4 (siNOX4). (B) FACS analysis of subG1 population performed in siNOX4 or scramble transfected mCCDcl1 cells after 96 hours of propidium iodide DNA staining after transfection. Results are expressed as the percentage of subG1 phase cells to the total. (C) PARP-1 cleavage was detected by Western blot analysis performed in mCCDcl1 cells transfected with scramble RNA (ctl) or siRNA targeting NOX4 (siNOX4) in the presence or not of 2 ng/ml of TGFβ-1 for 30 hours. Glyceraldehyde 3-phosphate dehydrogenase was used as loading control. (D) PARP-1 cleavage was detected by Western blot analysis performed in mCCDcl1 cells transfected with scramble RNA (ctl) or siRNA targeting NOX4 (siNOX4) and treated or not with 2 ng/ml of TGF-β1 for 24 hours in the presence (+) or absence (-) of a specific caspase-9 inhibitor. α-tubulin was used as loading control. *P<0.05; ***P<0.005. CTL, contralateral; PARP-1, poly (ADP-ribose) polymerase-1.
NOX4 Deficiency Decreases Capillary Density in Obstructed Kidneys and Impairs HIF-1α and Vascular Endothelial Growth Factor Induction
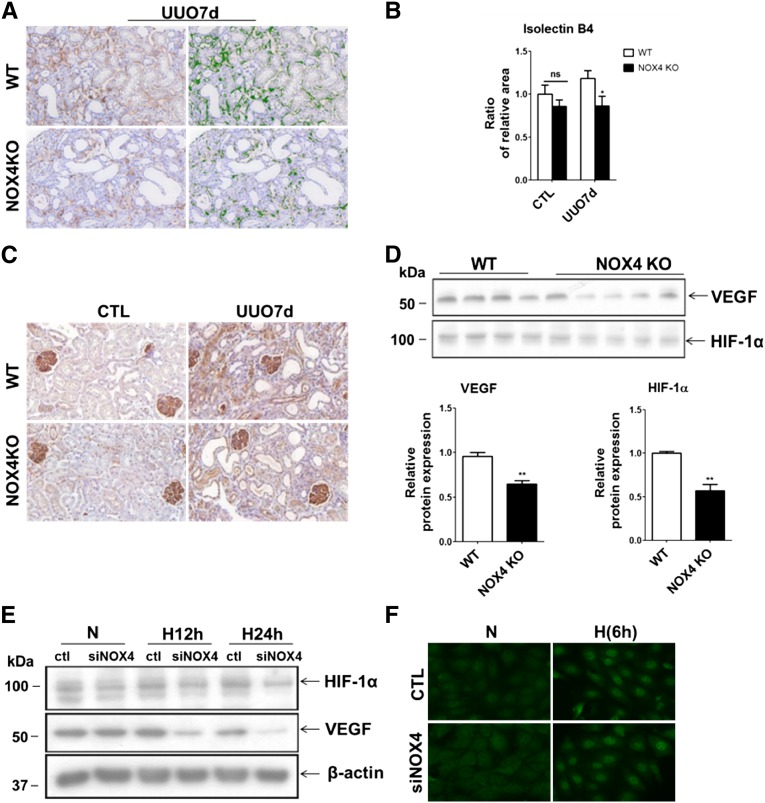
Peritubular capillary density correlates with tubulointerstitial fibrosis progression in CKD.24 Because NOX4 is involved in vascular endothelial growth factor (VEGF) signaling and angiogenesis,14 we hypothesized that angiogenesis could be altered in NOX4-deficient mice. In obstructed kidneys, capillary density, measured by isolectin B staining, was significantly reduced in NOX4 KO mice compared with WT mice (Figure 4, A and B). In contralateral, nonobstructed kidneys, peritubular capillary density was similar between WT mice and NOX4 KO mice (Figure 4, A and B). Expression of VEGF, a classic modulator of angiogenesis, was attenuated in obstructed kidneys of NOX4 KO mice compared with WT mice, as revealed by immunohistochemistry (Figure 4C) and Western blot (Figure 4D). VEGF expression was similar in contralateral, nonobstructed kidneys (Supplemental Figure 5A). Because HIF-1α is a major regulator of VEGF production, we hypothesized that NOX4 may directly regulate HIF-1α expression in tubular cells. As assessed by Western blot, HIF-1α expression was lower in obstructed kidneys of NOX4 KO mice compared with WT mice (Figure 4D). HIF-1α expression was similar in contralateral, nonobstructed kidneys of NOX4 KO and WT mice (Supplemental Figure 5B).
Figure 4.
Capillary density is decreased in obstructed kidneys of NOX4 KO mice. (A) Representative images of isolectin B4 staining of endothelial cells performed in obstructed kidneys from WT and NOX4 KO mice after 7 days of UUO (right). The stained area is outlined in green by the Metamorph software and is further quantified (left). (B) Renal cortex quantification of capillary density performed in contralateral (CTL) and obstructed (UUO7d) kidneys from WT and NOX4 KO mice after 7 days of UUO. Results are expressed as the mean ratio of stained area to the total tissue area over the mean value obtained in WT ± SEM (WT, n=5; NOX4 KO, n=6). (C) Representative images of VEGF immunostaining performed in contralateral (CTL, left) and obstructed (UUO7d, right) kidneys from WT and NOX4 KO mice after 7 days of UUO. (D) VEGF and HIF-1α Western blot analysis (upper panel) and densitometric quantification (lower panel) performed in obstructed kidneys (UUO7d) from WT and NOX4 KO mice after 7 days of UUO. Results are expressed as the mean ratio of individual values over the mean value obtained in WT ± SEM. (E) Representative HIF-1α and VEGF Western blot analysis performed in mCCDcl1 cells transfected with scramble RNA (ctl) or siRNA specifically targeting NOX4 (siNOX4) and subjected or not (N) to 12 hours (H12h) or 24 hours (H24h) of hypoxia. β-actin was used as a loading control. (F) Representative images of HIF-1α immunofluorescence analysis performed in mCCDcl1 cells transfected with scramble RNA (control) or siRNA specifically targeting NOX4 (siNOX4) and subjected or not (N) to 6 hours (H6h) of hypoxia. ns, P>0.05; *P<0.05; **P<0.01. ns, not significant.
Silencing of NOX4 in mCCDcl1 cells exposed to hypoxia decreased HIF-1α protein expression compared with control cells, whereas basal HIF-1α expression was unchanged (Figure 4E). The induction of VEGF, PDK1, Glut1, and PAI1 mRNA expression by hypoxia was significantly lower in NOX4-depleted cells compared with control cells (data not shown). VEGF protein expression was also decreased in NOX4-depleted cells subjected to hypoxia compared with control cells (Figure 4E). Basal VEGF protein expression appeared unchanged by NOX4 silencing. Finally, although HIF stabilization was impaired, some level of hypoxia-induced HIF-1α nuclear localization was still observed in NOX4-depleted cells (Figure 4F) submitted to hypoxia. Therefore, NOX4 modulates HIF-1α stabilization and target gene expression in response to tubular hypoxia.
NOX4 Deficiency Increases Oxidative Stress and Decreases the Antioxidant Defense Response
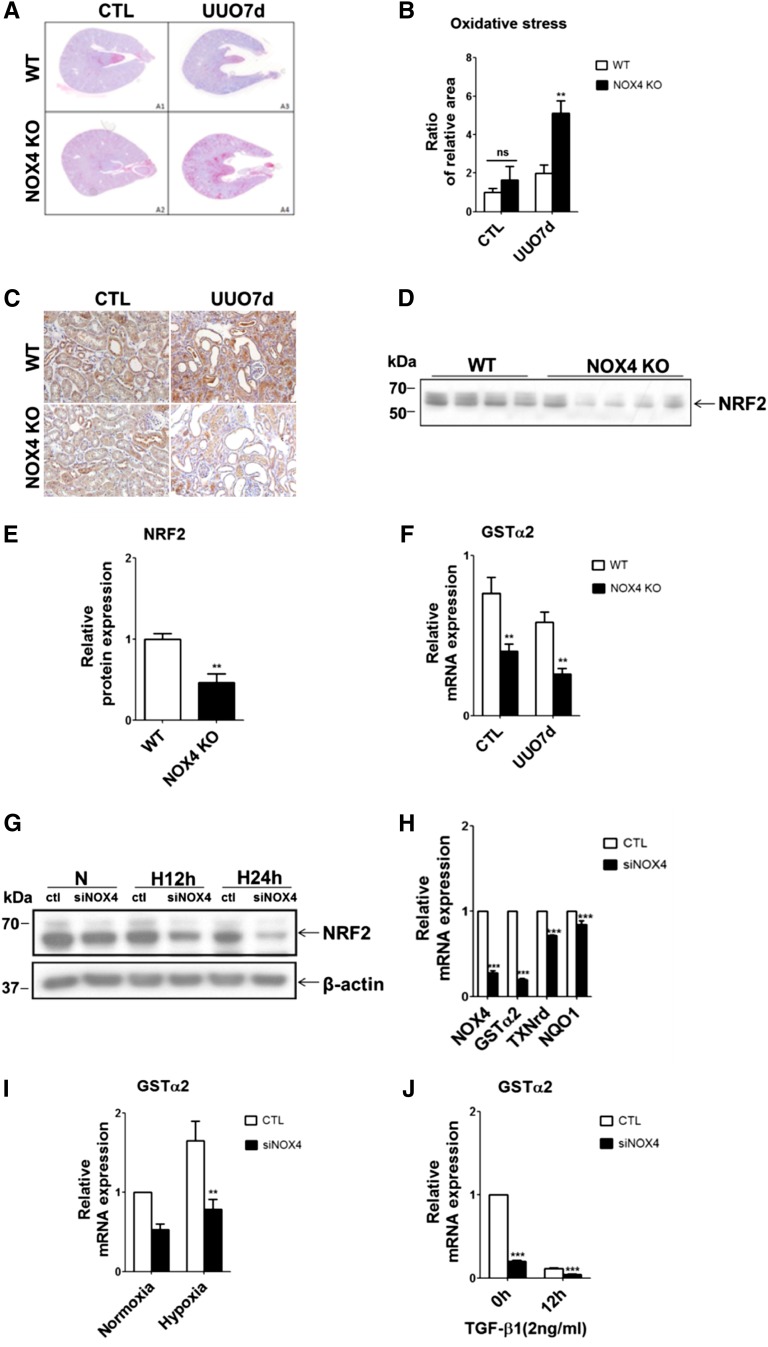
Because NOX4 generates ROS, oxidative stress would be expected to decrease in kidneys of NOX4 KO mice. Oxidative stress, as measured by 8-OH-2’-dG staining, a marker of oxidized DNA, was increased in the cortex of obstructed kidneys from all animals (Figures 5A). However, oxidative stress was higher in kidney cortex of NOX4 KO mice compared with WT mice (Figure 5B). A similar degree of oxidative stress was observed in contralateral, nonobstructed kidneys of WT mice and NOX4 KO mice (Figure 5A). Similarly, nitrotyrosine staining increased in the cortex of obstructed kidneys of NOX4 KO mice compared with WT mice (Supplemental Figure 6). Oxidative stress results either from exaggerated ROS production or decreased antioxidant defenses. NOX4 has been demonstrated to regulate NRF2, a major transcription factor involved in the antioxidant response,25 in cardiomyocytes.20 As assessed by immunohistochemistry and Western blot, reduced NRF2 expression was observed in obstructed kidneys of NOX4 KO mice compared with those of WT mice (Figures 5, C–E). Interestingly, NRF2 protein expression was also lower in contralateral, nonobstructed kidneys of NOX4 KO mice compared with WT mice (Figure 5C and Supplemental Figure 5C). Glutathione S-transferase α (GSTα), a key NRF2 target gene, is highly expressed in the proximal tubule. Real-time PCR experiments showed that GSTα mRNA expression was decreased in both control and obstructed kidneys of NOX4 KO mice compared with WT mice (Figure 5F).
Figure 5.
Oxidative stress is increased in obstructed kidneys of NOX4 KO mice. (A) Representative images of 8-OH-2’-dG immunostaining of oxidized DNA performed in contralateral (A1 and A2) and obstructed (A3 and A4) kidneys from WT and NOX4 KO mice after 7 days of UUO. (B) Renal cortex semi-quantification of oxidative stress performed in contralateral (CTL) and obstructed (UUO7d) kidneys from WT and NOX4 KO mice after 7 days of UUO (WT, n=5; NOX4 KO, n=6). Results are expressed as the ratio of stained area to the total tissue area over the mean value obtained in WT ± SEM. (C) Representative images of NRF2 immunostaining performed in contralateral and obstructed kidneys from WT and NOX4 KO mice after 7 days of UUO. (D and E) NRF2 Western blot analysis (D) and densitometric quantification (E) performed in obstructed kidneys (UUO7d) from WT and NOX4 KO mice after 7 days of UUO. Results are expressed as the mean ratio of individual values over the mean value obtained in WT ± SEM. (F) Relative GSTα2 mRNA expression performed in contralateral (CTL) and obstructed (UUO7d) kidneys from WT and NOX4 KO mice after 7 days of UUO (WT, n=5; NOX4 KO, n=5). Results are expressed as relative expression reported to p0 expression as a housekeeping gene in WT ± SEM. (G) Representative NRF2 Western blot analysis performed in mCCDcl1 cells transfected with scramble RNA (ctl) or siRNA specifically targeting NOX4 (siNOX4) and subjected or not (N) to 12 hours (H12h) or 24 hours (H24h) of hypoxia. β-actin was used as a loading control. (H) Relative GSTα2, NQO1, and TXNrd mRNA expression performed in mCCDcl1 transfected with scramble RNA (CTL) or siRNA specifically targeting NOX4 (siNOX4). Results are expressed as relative expression reported to P0 expression as a housekeeping gene in WT ± SEM. (I and J) Relative GSTα2 mRNA expression performed in mCCDcl1 cells transfected with scramble RNA (CTL) or siRNA specifically targeting NOX4 (siNOX4) and subjected or not (N) to 24 hours (H24h) of hypoxia (I) or 12 hours of TGF-β1 treatment (J). Results are expressed as relative expression reported to p0 expression as a housekeeping gene in WT ± SEM. ns, P>0.05; *P<0.05; **P<0.01; ***P<0.005. NQO1, quinone oxidoreductase; TXNrd, thioredoxin reductase; ns, not significant.
NOX4 silencing in mCCDcl1 cells decreased expression of classic NRF2 target genes, such as GSTα2, thioredoxin reductase, and quinone oxidoreductase (Figure 5, G and H), under baseline conditions. Decreased NRF2 protein expression was observed when cells were exposed to hypoxia (Figure 5G). GSTα mRNA expression, a marker of NRF2 activity, was consistently lower after NOX4 silencing under both baseline and hypoxia conditions as well as after TGF-β1 treatment (Figure 5, I and J). Therefore, NOX4 deficiency decreases the antioxidant defense response mediated by the NRF2 pathway.
NOX2 Does Not Compensate for NOX4 Deficiency in Obstructed Kidneys
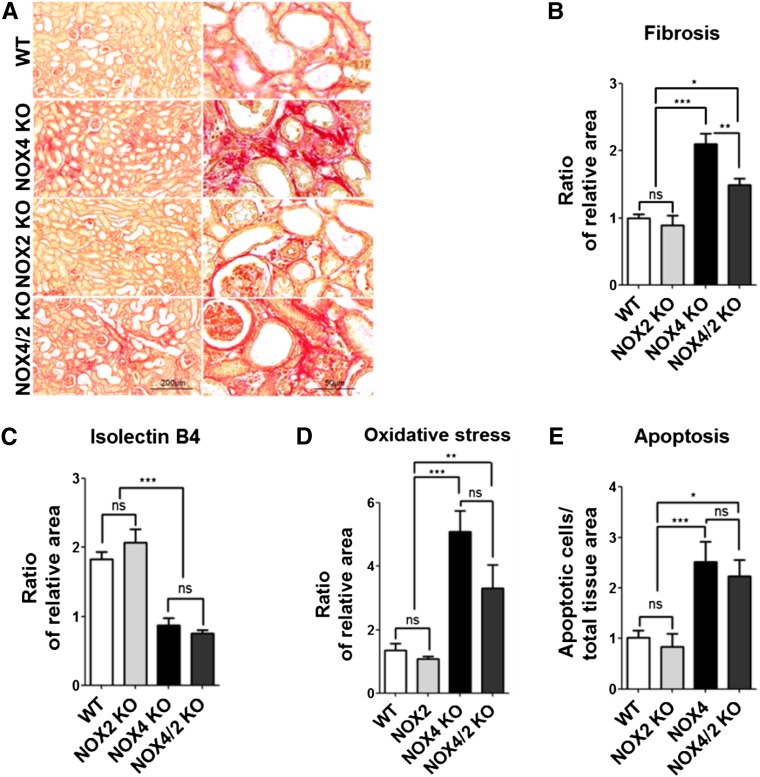
We next determined whether compensation of NOX4 deficiency by NOX2 activation may explain, at least in part, the phenotype observed in obstructed kidneys of NOX4 KO mice. NOX2 mRNA expression, as measured by real-time PCR, was unchanged in obstructed kidneys from NOX4 KO mice compared with WT mice (Supplemental Figure 7). We then compared the effect of UUO in NOX2, NOX4, and NOX4/NOX2 KO mice. As revealed by Sirius red staining, increased tubulointerstitial fibrosis was observed in the cortex of obstructed kidneys of double NOX4/NOX2 KO mice compared with NOX2 KO and WT mice, but was not different to that observed in NOX4 KO mice (Figure 6, A and B). Interstitial capillary density (Figure 6C) and VEGF expression (data not shown) were also decreased in the cortex of obstructed kidneys to the same extent as in NOX4 KO and NOX4/NOX2 KO mice compared with NOX2 KO and WT mice. Oxidative stress, assessed by 8-OH-2’-dG (Figure 6D), and apoptosis, detected by TUNEL assay (Figure 6E), were higher in the cortex of obstructed kidneys of NOX4 and NOX4/NOX2 KO mice compared with NOX2 KO and WT mice. Although the extent of tubulointerstitial fibrosis and oxidative stress displayed a trend toward attenuation in double NOX2/NOX4 KO mice compared with NOX4 KO mice, the observed differences were not statistically significant. Morphologic alterations observed in NOX2 KO mice were undistinguishable from those observed in WT mice (Figure 6, A–E). In addition, real-time PCR experiments showed that low levels of NOX1 and DUOX2 expression were unchanged in NOX4 KO mice compared with WT mice (Supplemental Figure 7).
Figure 6.
NOX2 is not responsible for increased fibrosis in obstructed kidneys of NOX4 KO mice. (A) Representative images of unpolarized Sirius red staining of obstructed kidney (UUO7d) from WT, NOX2, NOX4, and NOX4/NOX2 KO mice after 7 days of UUO. Scale bar, 200 µm on left and 50 µm on right. (B) Renal cortex quantification of unpolarized Sirius red staining performed in obstructed kidneys (UUO7d) from WT, NOX2, NOX4, and NOX4/NOX2 mice after 7 days of UUO (WT, n=9; NOX2 KO, n=6; NOX4 KO, n=9; NOX4/NOX2 KO, n=6). Results are expressed as the mean ratio of stained area to the total tissue area over the mean value obtained in WT ± SEM. (C) Renal cortex quantification of capillary density performed in obstructed kidneys (UUO7d) from WT, NOX2, NOX4, and NOX4/NOX2 KO mice after 7 days of UUO (WT, n=7; NOX2 KO, n=6; NOX4 KO, n=8; NOX4/NOX2 KO, n=6). Results are expressed as the mean ratio of stained area to the total tissue area over the mean value obtained in WT ± SEM. (D) Renal cortex semi-quantification of 8-OH-2’-dG immunostaining performed in obstructed kidneys (UUO7d) from WT, NOX2, NOX4, and NOX4/NOX2 KO mice after 7 days of UUO (WT, n=9; NOX2 KO, n=6; NOX4 KO, n=9; NOX4/NOX2 KO, n=6). Results are expressed as the ratio of stained area to the total tissue area over the mean value obtained in WT ± SEM. (E) Renal cortex quantification of TUNEL-positive apoptotic cells performed in renal cortex of obstructed kidneys (UUO7d) from WT, NOX2, NOX4, and NOX4/NOX2 KO mice after 7 days of UUO (WT, n=9; NOX2 KO, n=6; NOX4 KO, n=9; NOX4/NOX2, n=6). Results are expressed as the mean ratio of apoptotic cells to total tissue area over the mean density of apoptotic cells measured in WT ± SEM. ns, P>0.05; *P<0.05; **P<0.01; ***P<0.005. ns, not significant.
Discussion
NOX4 has been suspected to participate in kidney fibrosis generation, suggesting that its inhibition might be protective at least in some types of CKD.8,12,13 Our results show that in the UUO model, NOX4 rather plays a protective role because kidney fibrosis was clearly enhanced in NOX4 KO animals. The exaggerated fibrosis was due to enhanced tubular cell apoptosis, decreased vascularization, and enhanced oxidative stress in NOX4 KO animals. Therefore, NOX4 plays a major role in cell survival, oxygen sensing, and major detoxifying pathways in kidney tubular cells.
Tubular apoptosis is one of the earliest and most important events in the pathogenesis of UUO26 and leads to atrophy of the tubular compartment, atubular glomeruli, and fibrosis.3 We observed that NOX4 deficiency enhances tubular cell apoptosis and aggravates tubular atrophy. We further demonstrate that enhanced apoptosis is directly dependent on NOX4 deficiency. Indeed, NOX4 depletion in cultured renal epithelial cells directly promoted apoptosis mediated by TGF-β1, a cytokine that is classically induced in the setting of UUO.27 The decreased ability of tubular cells to survive in UUO in the absence of NOX4 may also be related to a disruption of HIF-1α and NRF2 pathways, as described below.
Rarefaction of kidney capillaries is a good indicator of kidney interstitial fibrosis in both experimental and human kidney disease.4,5 Decreased peritubular capillary density in obstructed kidneys of NOX4 KO animals may significantly contribute to increased fibrosis through enhanced local hypoxia. This alteration of microvascularization is in line with the role of NOX4 in angiogenesis described in the heart15 as well as in some tumors.14 We further demonstrate that NOX4 depletion in kidney tubular cells impairs HIF-1α protein expression in response to hypoxia, resulting in reduced VEGF expression, likely accounting for decreased microvascularization. NOX4 is therefore an important regulator of HIF-1α expression, probably via H2O2-dependent HIF-1α stabilization.28 NOX4 depletion may thereby enhance fibrosis in UUO by impeding VEGF production and adequate kidney microvascularization.
Finally, we demonstrate that oxidative stress is not decreased but rather enhanced in NOX4 KO animals subjected to UUO. NOX4 appears to play an antioxidant role despite its function in H2O2 production. We show that the extent of kidney injury is similar between NOX2 KO and WT animals after ureteral obstruction. However, kidney fibrosis is increased in NOX4/NOX2 KO mice compared with WT mice. Therefore, even though NOX2 may generate some level of oxidative stress in NOX4 KO mice, NOX2 is only marginally responsible for increased injury observed in obstructed kidneys of NOX4 KO mice. NOX4 may play an indirect, antioxidant function by regulating the NRF2 pathway in kidney tubular cells. H2O2 is an important regulator of NRF2 stability via oxidation of KEAP1.29 It is therefore plausible that NOX4-related H2O2 production controls KEAP1 oxidation and therefore NRF2 stability in kidney tubular cells. Therefore, in the absence of NOX4, the global antioxidant defense against enhanced ROS production might be decreased in kidney tubular cells. The exact mechanisms of these effects will deserve further studies.
Our results show that NOX4 deficiency is detrimental under conditions of tubular stress are at variance with results obtained in cultured myofibroblasts and experimental models of diabetic nephropathy.8,10,12,13 Several observations may explain this discrepancy. First, tubular cells express high levels of NOX4 in contrast to podocytes and myofibroblasts that express much lower levels of the enzyme under physiologic conditions. NOX4 might play a different function in kidney tubular cells, where its physiologic role is more likely preponderant. In addition, pharmacologic inhibitors may exert additional, nontargeted effects. Finally, decreased VEGF production observed in the absence of NOX4 might be protective in early diabetic nephropathy, but not in other forms of CKD.30,31
In conclusion, we show that under conditions of ureteral obstruction, deletion of NOX4 increases interstitial fibrosis through enhanced oxidative stress, decreased microvascularization, and enhanced tubular cell apoptosis. Thus, NOX4 contributes to kidney tubular cell survival by regulating HIF1α and NRF2 pathways.
Concise Methods
Animals
WT, NOX4, and gp91phox/NOX2 KO mice were on a C57BL/6J background. NOX2 KO mice were obtained from The Jackson Laboratory. NOX4 KO mice were obtained in collaboration with K.H.K. They were generated as previously described.32 We obtained NOX4/NOX2 double-KO mice by crossing NOX2 KO mice and NOX4+/− mice. Genotyping was performed by PCR using the following primers: NOX4F, 5′-TCATGACAGTTGGGGACAAA-3′; NOX4R, 5′-CGGCTACATGCACACCTGAGAA-3′; and NOX4neoR, 5′-AACGTCGTGACTGGGAAAAC-3′, which generated a 352 bp fragment for KO and a 219 bp for WT.
UUO
Experiments were performed on WT, NOX4 KO, NOX2 KO, and NOX4/NOX2 double-KO littermate mice aged 6–12 weeks. All animal experiments were approved by the Institutional Ethical Committee of Animal Care in Geneva and Cantonal authorities. Male mice weighing 22–31 g were anesthetized by intraperitoneal injection of ketamine and xylazine (100 mg/kg and 5 mg/kg, respectively) (GRAEUB, Swissmedic, Bayer Healthcare). Left ureters were visualized through a flank incision and double-ligated with 6–0 silk. Mice were sacrificed 7 or 14 days after surgery and tissue samples from obstructed and contralateral kidneys were collected for histology, immunohistochemistry, Western blotting, and real-time PCR analysis.
Cell Culture and Transfection
mCCDcl1 cells were cultured as described previously.33 Cells were seeded in six-well plates 24 hours before transfection and were then transfected with 120 pmol of small interfering RNAs (siRNAs) and 5 μl of lipofectamine 2000 (Invitrogen) for 72 or 96 hours. The siRNA duplexes (Invitrogen) were provided as purified and annealed duplexes. The following sequences were used: siNOX4, 5′-UUUAGGGACAGCCAAAUGAGCAGGC-3′; and scramble, 5′-GCCACUCGUUUGUCGCCCUUGUAAA-3′.
Hypoxia Induction
Cells were seeded in six-well plates and put in a plastic pouch with a paper gas-generating sachet (AnaeroGen Compact) during the time described in the Results. This system allows a reduction of the oxygen content in the pouch below 1% within 30 minutes.
Mouse Kidney Histology and Immunohistochemistry
Changes in renal morphology were examined on 4% paraformaldehyde (Alfa Aesar) fixed and paraffin-embedded tissue samples. Kidney sections 5 µm in thickness were stained with hematoxylin and eosin, Masson trichrome, and Sirius red to assess the kidney structure and fibrosis. Immunohistochemistry was performed using the citrate buffer (0.1 mol/L, pH 6) microwave-based antigen target retrieval technique, and the EnVision Flex kit (Dako) or the Alkaline Phosphatase Substrate kit (Reactolab, Vector Laboratories) were used to reveal the antigen-antibody complex.
TUNEL Assay
TUNEL staining was performed to detect apoptotic nuclei using the DeadEnd Fluorometric TUNEL System (Promega) according to the manufacturer’s instructions. The slides were analyzed using the fluorescent microscope coupled to a camera (Axiocam). TUNEL-positive cells (nuclei) were counted using image analysis software (Metamorph) and expressed as ration of TUNEL-positive cells to the total tissue area. For illustrative purposes, contrast of the representative image only was enhanced.
Quantification of Histochemistry
Quantitative analysis of histostaining experiments of 8-OHdG, Isolectin B4, Sirius red, and TUNEL staining were carried out on slides using Metamorph software. Basically, all slides were scanned with Mirax Midi (Zeiss) coupled to the Axiocam MRm (Zeiss), and five images were taken in different regions of the renal cortex of each slide using a Mirax viewer. These images were afterward analyzed with Metamorph image analysis software.
The positive areas were matched and ratios of positive (stained) area/total tissue area were analyzed in different animals. Data were expressed as the ratio of positive area to the total area examined and reported to the mean of WT ± SEM. Groups statistics were analyzed by the t test and one- and two-way ANOVA/Bonferroni multiple comparison tests, respectively, for two or three groups.
RNA Extraction and Real-Time PCR
Total RNA was isolated using the NucleoSpin RNA II (Macherey-Nagel) according to the manufacturer’s instructions. One microgram of RNA was used to synthesize cDNA using the qScript cDNA SuperMix (Quanta Biosciences) according to the manufacturer’s instructions. Real-time PCR was performed on 5–10 ng of cDNA using 3 ng of each primer and 6 µl of SYBR Green Master Mix (Applied Biosystems) to obtain a final reaction volume of 12 µl. Triplicate amplification reactions were performed with the StepOne Plus Real-Time PCR System (AB Applied Biosystems).
Western Blot Analyses
mCCDcl1 cells or kidney tissue samples were homogenized in 100 µl or 1 ml, respectively, of lysis buffer (20 mM Tris-HCl, 2 mM EDTA, 30 mM NaF, 30 mM NaPP, 2mM Na3VO4, 0.1% SDS, 1% Triton-X-100, protease inhibitors). Protein concentrations were determined using both the bicinchoninic acid protein assay (Thermo Scientific, Pierce) and Coomassie Blue gel staining (Thermo Scientific GelCode blue stain reagent). Proteins were subjected to SDS-PAGE and blotted onto polyvinylidene difluoride membranes (Immobilon-P, Millipore) using standard methods.34 Horseradish peroxidase-conjugated secondary antibodies (1:20,000; BD Biosciences Pharmingen) were used for detection of immunoreactive proteins by chemiluminescence (horseradish peroxidase substrate; Immobilon Millipore). Protein levels were quantified using ImageJ Java-based image processing software after background substraction. Loading control of kidney extracts was performed by Coomassie blue staining coupled to the bicinchoninic acid assay (Supplemental Figure 2).
Immunofluorescence Microscopy
Confluent cells grown on glass coverslips were rinsed twice with PBS, fixed with 4% (wt/vol) paraformaldehyde (Alfa Aesar) for 25 minutes at room temperature, and then permeabilized with 0.1% Triton X-100 for 3 minutes. After blocking for 30 minutes with 1% (wt/vol) BSA in PBS, cells were incubated overnight with monoclonal anti-HIF-1α antibody diluted 1:200 in PBS containing 0.1% BSA and 0.01 Triton X-100. After three washes in PBS, cells were incubated with a secondary anti-mouse IgG Alexa 488–conjugated antibody diluted 1:800 in PBS containing 0.1% BSA and 0.01 Triton X-100 (Molecular Probes). Coverslips were mounted on glass slides with Vectashield (Vector Laboratories), and fluorescence images were captured at 488 nm with an Axiovert 200M Zeiss microscope at ×40 magnification using Openlab software (Improvision).
Cell-Cycle Analyses
Cell-cycle analysis was carried out by propidium iodide staining and flow cytometry. Twenty-four hours after siRNA transfection, cells were maintained in serum-free medium for 3 days. Cells were collected and then added to the attached cells harvested by trypsinization. Cells were resuspended in PBS, fixed with ice-cold 50% ethanol, and stained in PBS solution, containing 50 µg/ml of PI, 0.1% Triton X-100, and 100 µg/ml of RNase A. Cells were analyzed for DNA content using ACCURI C6 flow cytometer. Cell distribution among cell-cycle phases and the percentage of apoptotic cells were evaluated using CFlow C6 software.
Primers and antibodies used are reported in the Supplemental Methods.
Disclosures
K.H.K. is cofounder of a company producing NOX inhibitors.
Supplementary Material
Acknowledgments
During revision of this manuscript, a paper by Babelova et al. titled “Role of Nox4 in Murine Models of Kidney Disease” was accepted for publication.
This work was supported by grants from the Swiss National Science Foundation (31_127181), the Novartis Foundation, and the Ernst and Lucie Schmidheiny Foundation to S.d.S.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012040373/-/DCSupplemental.
References
- 1.Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, Gansevoort RT, Kasiske BL, Eckardt KU: The definition, classification and prognosis of chronic kidney disease: A KDIGO Controversies Conference report. Kidney Int 80: 17–28, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Schainuck LI, Striker GE, Cutler RE, Benditt EP: Structural-functional correlations in renal disease. II. The correlations. Hum Pathol 1: 631–641, 1970 [DOI] [PubMed] [Google Scholar]
- 3.Forbes MS, Thornhill BA, Chevalier RL: Proximal tubular injury and rapid formation of atubular glomeruli in mice with unilateral ureteral obstruction: A new look at an old model. Am J Physiol Renal Physiol 301: F110–F117, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steegh FM, Gelens MA, Nieman FH, van Hooff JP, Cleutjens JP, van Suylen RJ, Daemen MJ, van Heurn EL, Christiaans MH, Peutz-Kootstra CJ: Early loss of peritubular capillaries after kidney transplantation. J Am Soc Nephrol 22: 1024–1029, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohashi R, Shimizu A, Masuda Y, Kitamura H, Ishizaki M, Sugisaki Y, Yamanaka N: Peritubular capillary regression during the progression of experimental obstructive nephropathy. J Am Soc Nephrol 13: 1795–1805, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Singh DK, Winocour P, Farrington K: Oxidative stress in early diabetic nephropathy: Fueling the fire. Nat Rev Endocrinol 7: 176–184, 2011 [DOI] [PubMed] [Google Scholar]
- 7.He W, Wang Y, Zhang MZ, You L, Davis LS, Fan H, Yang HC, Fogo AB, Zent R, Harris RC, Breyer MD, Hao CM: Sirt1 activation protects the mouse renal medulla from oxidative injury. J Clin Invest 120: 1056–1068, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gorin Y, Block K, Hernandez J, Bhandari B, Wagner B, Barnes JL, Abboud HE: Nox4 NAD(P)H oxidase mediates hypertrophy and fibronectin expression in the diabetic kidney. J Biol Chem 280: 39616–39626, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Ricono JM, Wagner B, Gorin Y, Arar M, Kazlauskas A, Choudhury GG, Abboud HE: PDGF receptor-beta modulates metanephric mesenchyme chemotaxis induced by PDGF AA. Am J Physiol Renal Physiol 296: F406–F417, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mandal CC, Ganapathy S, Gorin Y, Mahadev K, Block K, Abboud HE, Harris SE, Ghosh-Choudhury G, Ghosh-Choudhury N: Reactive oxygen species derived from Nox4 mediate BMP2 gene transcription and osteoblast differentiation. Biochem J 433: 393–402, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Block K, Gorin Y, New DD, Eid A, Chelmicki T, Reed A, Choudhury GG, Parekh DJ, Abboud HE: The NADPH oxidase subunit p22phox inhibits the function of the tumor suppressor protein tuberin. Am J Pathol 176: 2447–2455, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bondi CD, Manickam N, Lee DY, Block K, Gorin Y, Abboud HE, Barnes JL: NAD(P)H oxidase mediates TGF-beta1-induced activation of kidney myofibroblasts. J Am Soc Nephrol 21: 93–102, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eid AA, Gorin Y, Fagg BM, Maalouf R, Barnes JL, Block K, Abboud HE: Mechanisms of podocyte injury in diabetes: Role of cytochrome P450 and NADPH oxidases. Diabetes 58: 1201–1211, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xia C, Meng Q, Liu LZ, Rojanasakul Y, Wang XR, Jiang BH: Reactive oxygen species regulate angiogenesis and tumor growth through vascular endothelial growth factor. Cancer Res 67: 10823–10830, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Zhang M, Brewer AC, Schröder K, Santos CX, Grieve DJ, Wang M, Anilkumar N, Yu B, Dong X, Walker SJ, Brandes RP, Shah AM: NADPH oxidase-4 mediates protection against chronic load-induced stress in mouse hearts by enhancing angiogenesis. Proc Natl Acad Sci U S A 107: 18121–18126, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bedard K, Krause KH: The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87: 245–313, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Geiszt M, Kopp JB, Várnai P, Leto TL: Identification of renox, an NAD(P)H oxidase in kidney. Proc Natl Acad Sci U S A 97: 8010–8014, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Serrander L, Cartier L, Bedard K, Banfi B, Lardy B, Plastre O, Sienkiewicz A, Fórró L, Schlegel W, Krause KH: NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J 406: 105–114, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Block K, Gorin Y, Abboud HE: Subcellular localization of Nox4 and regulation in diabetes. Proc Natl Acad Sci U S A 106: 14385–14390, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brewer AC, Murray TV, Arno M, Zhang M, Anilkumar NP, Mann GE, Shah AM: Nox4 regulates Nrf2 and glutathione redox in cardiomyocytes in vivo. Free Radic Biol Med 51: 205–215, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chevalier RL, Forbes MS, Thornhill BA: Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney Int 75: 1145–1152, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Higgins DF, Kimura K, Bernhardt WM, Shrimanker N, Akai Y, Hohenstein B, Saito Y, Johnson RS, Kretzler M, Cohen CD, Eckardt KU, Iwano M, Haase VH: Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest 117: 3810–3820, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farris AB, Adams CD, Brousaides N, Della Pelle PA, Collins AB, Moradi E, Smith RN, Grimm PC, Colvin RB: Morphometric and visual evaluation of fibrosis in renal biopsies. J Am Soc Nephrol 22: 176–186, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Serón D, Alexopoulos E, Raftery MJ, Hartley B, Cameron JS: Number of interstitial capillary cross-sections assessed by monoclonal antibodies: Relation to interstitial damage. Nephrol Dial Transplant 5: 889–893, 1990 [DOI] [PubMed] [Google Scholar]
- 25.Venugopal R, Jaiswal AK: Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc Natl Acad Sci U S A 93: 14960–14965, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chevalier RL: Growth factors and apoptosis in neonatal ureteral obstruction. J Am Soc Nephrol 7: 1098–1105, 1996 [DOI] [PubMed] [Google Scholar]
- 27.Kaneto H, Morrissey J, Klahr S: Increased expression of TGF-beta 1 mRNA in the obstructed kidney of rats with unilateral ureteral ligation. Kidney Int 44: 313–321, 1993 [DOI] [PubMed] [Google Scholar]
- 28.Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM, Schumacker PT: Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1α during hypoxia: A mechanism of O2 sensing. J Biol Chem 275: 25130–25138, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Fourquet S, Guerois R, Biard D, Toledano MB: Activation of NRF2 by nitrosative agents and H2O2 involves KEAP1 disulfide formation. J Biol Chem 285: 8463–8471, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flyvbjerg A, Dagnaes-Hansen F, De Vriese AS, Schrijvers BF, Tilton RG, Rasch R: Amelioration of long-term renal changes in obese type 2 diabetic mice by a neutralizing vascular endothelial growth factor antibody. Diabetes 51: 3090–3094, 2002 [DOI] [PubMed] [Google Scholar]
- 31.de Vriese AS, Tilton RG, Elger M, Stephan CC, Kriz W, Lameire NH: Antibodies against vascular endothelial growth factor improve early renal dysfunction in experimental diabetes. J Am Soc Nephrol 12: 993–1000, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Carnesecchi S, Deffert C, Donati Y, Basset O, Hinz B, Preynat-Seauve O, Guichard C, Arbiser JL, Banfi B, Pache JC, Barazzone-Argiroffo C, Krause KH: A key role for NOX4 in epithelial cell death during development of lung fibrosis. Antioxid Redox Signal 15: 607–619, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gaeggeler HP, Gonzalez-Rodriguez E, Jaeger NF, Loffing-Cueni D, Norregaard R, Loffing J, Horisberger JD, Rossier BC: Mineralocorticoid versus glucocorticoid receptor occupancy mediating aldosterone-stimulated sodium transport in a novel renal cell line. J Am Soc Nephrol 16: 878–891, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Carranza ML, Féraille E, Favre H: Protein kinase C-dependent phosphorylation of Na(+)-K(+)-ATPase alpha-subunit in rat kidney cortical tubules. Am J Physiol 271: C136–C143, 1996 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.