Abstract
The development of advanced materials that facilitate hyaline cartilage formation and regeneration in aging populations is imperative. Critical to the success of this endeavor is the optimization of ECM production from clinically relevant cells. However, much of the current literature focuses on the investigation of primary bovine chondrocytes from young calves, which differ significantly than osteoarthritic cells from human sources. This study examines the levels of extracellular matrix (ECM) production using various levels of type I collagen and hyaluronic acid in poly(ethylene glycol) dimethacrylate (PEGDM) hydrogels in total knee arthroplasties, compared with the results from bovine chondrocytes. The addition of type 1 collagen in both the presence and absence of low levels of hyaluronic acid increased ECM production and/or retention in scaffolds containing either bovine or human chondrocytes. These findings are supported consistently with colorimetric quantification, whole mount extracellular matrix staining for both cell types, and histological staining for glycoaminoglycans and collagen of human chondrocyte containing samples. While exhibiting similar trends, the relative ECM productions levels for the primary human chondrocytes are significantly less than the bovine chondrocytes which reinforces the need for additional optimization.
INTRODUCTION
By 2030, it is projected that 67 million Americans will suffer from osteoarthritis (OA), a disease characterized by the degeneration of cartilage and underlying bone in a joint,1 with 25 million progressing to severe forms which limit their physical activity.2 Current surgical interventions such as microfracture, autologous chondrocyte transplantation, use of filling material, total knee arthroplasty, etc., typically produce fibrous cartilage, which degrades with constant loading over time instead of more durable hyaline cartilage found in healthy joints. More conservative surgical interventions are less effacious with increasing patient age,3–5 leaving middle age patients possessing focal defects without a treatment option capable of preserving the joint and orthopedic surgeons reluctant to do more invasive arthroplasty. The Osteoarthritis Research Society International in concert with the United States Food and Drug Administration has decided the further development of OA treatments should focus on the structural modification of articular cartilage to prevent joint degeneration in addition to the alleviation of symptoms.6
The extracellular matrix (ECM) of adult articular cartilage is composed of type II collagen (Col 2) fibrils intertwined with a glycosoaminoglycan (GAG) proteoglycan network which collectively form a polyelectrolyte hydrogel that combines tensile strength with deformability to distribute applied loads across the tissue. If either Col 2 or the negatively charged, highly hydrated proteoglycan matrix is damaged, tissue degradation will ensue since mature cartilage has a limited ability to self-heal.7,8 However, immature cartilage has been known to spontaneously repair damage.9–11 The difference in healing potential is most likely due to shifts during development which take the cartilage ECM from a simple isotropic tissue with a high cell density and homogeneous distribution of collagen fibrils to an organized, anisotropic tissue with a low density of chondrocytes growing in vertical columns and specific zonal arrangements of collagen fibrils.12
Emulating portions of the developmental process using tissue engineering offers the potential to generate biomimetic hyaline cartilage with mechanical properties similar to native materials. However, most tissue engineering strategies focus on either synthetic or natural systems in isolation.13,14 Each of these strategies has possesses advantages and drawbacks. Synthetic materials offer a consistent base material which can be expressly tailored for the application but possess little or no inherent biological signaling motifs. Native materials possess biological signaling motifs but suffer from batch to batch variation, limited mechanical properties, and potential immune rejection. We hypothesize that the inclusion of natural ECM components in synthetic poly(ethylene glycol) dimethacrylate (PEGDM) matrices leverages the respective advantages of both systems while limiting their inherent drawbacks. PEGDM matrices are bioinert, providing structural support to cells without direct biological signaling. The addition of the ECM molecules, type I collagen (Col I) and hyaluronic acid (HA), provides biological signaling elements without having to play a structural role in the matrix and are the most common constituents of scaffolds used in current clinical matrix assisted autologous chondrocyte transplantations.15–17 Although Col I is not typically associated with chondrogenesis, it has been previously shown that bovine and human chondrocytes can remodel Col I into more suitable ECM components.13 In our studies, primary bovine and human chondrocytes from middle age patients undergoing total knee replacement were cultured in 10% PEGDM hydrogels containing varying concentrations of type I collagen (Col I) and hyaluronic acid (HA). The effects of the matrix components on ECM production and tissue formation were examined over 6 weeks of in vitro culture. The results show clear trends of ECM production and retention between the human and bovine cell sources. In general, the primary human chondrocytes from patients of advanced aged are significantly less responsive that the chondrocytes from freshly killed calves. While ECM production and retention can clearly be increased using hybrid, bioactive polymer hydrogels, significant improvements are needed to further optimize proteoglycan production and proliferative activity, both of which will facilitate expanded clinical use.
EXPERIMENTAL METHODS
Cell Isolation
All studies involving human tissue were IRB-approved at each of the institutions involved. Chondrocytes were isolated from the tibial plateaus and femoral condoyles of patients undergoing total knee arthroplasty (average age: 45 years; range: 39–62 years; total knees (female): 7(4)) and 2–3 week old bovine calves. Isolated tissue was placed in 4 mg/mL collagenase in Hank’s buffered salt solution for at least 4 h and washed twice with phosphate buffered saline (Invitrogen, San Diego, CA). Bovine chondrocytes were expanded for 1 week in Opti-MEM I reduced-serum medium (Opti-MEM) (Invitrogen) containing 50 μg/mL ascorbate (Sigma-Aldrich, St. Louis, MO) and 100 μg/mL primocin (Invitrogen). Human chondrocytes were encapsulated in hydrogels immediately after isolation.
Hydrogel Preparation
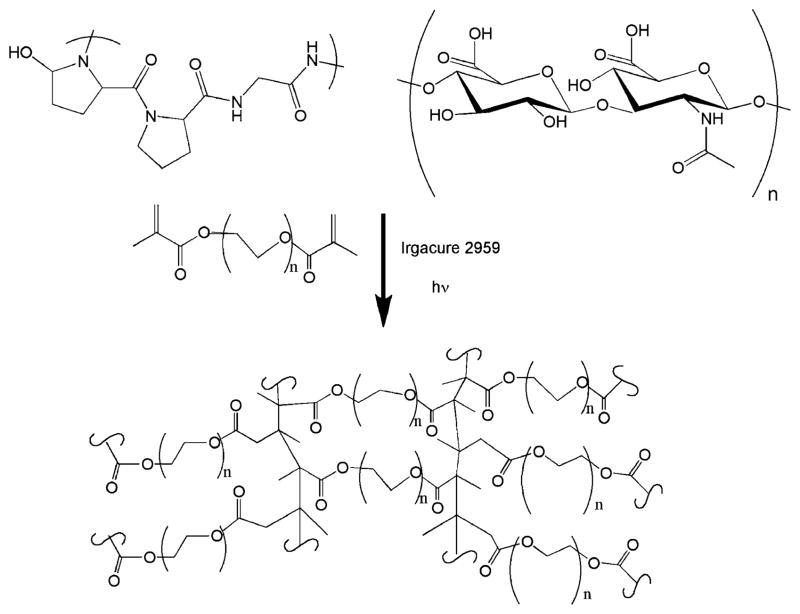
PEGDM (~8000 g/mol) was purified via forced air dialysis and lyophilized (Monomer-Polymer & Dajac Laboratories, Trevose, PA). PEGDM-Opti-MEM solutions (10 mass %) were prepared containing 0.1% Irgacure 2959 (Ciba Specialty Chemicals, Basel, Switzerland). For ECM additive testing, 1% Col I (BD Biosciences, Bedford, MA) with and without 0.5% or 1% HA (MW 1.63 × 106 g/mol) (Sigma-Aldrich) was added to the 10% PEGDM Opti-MEM solution. Hydrogels were photopolymerized using ~2.3 mJ/cm2 UVA light for 5 min (Figure 1) and then placed in Opti-MEM I reduced-serum medium for culture. For cell culture experiments, 1 million bovine or human chondrocytes were encapsulated in a 100 μL of 10% PEGDM Opti-MEM solution with or without ECM additives to create samples (~1 cm diameter, 0.3 cm thick) which were cultured up to 6 weeks in Opti-MEM I reduced-serum medium containing 50 μg/mL ascorbate and 100 μg/mL primocin at 37 °C in a 5% CO2 incubator. The media was changed three times a week.
Figure 1.
Schematic of hydrogel components and fabrication showing the interconnected network of PEGDM formed upon cross-linking. Physical and chemical properties are varied via mass fraction of PEGDM, FW of PEGDM, and relative mass fractions of collagen I and hyaluronic acid that are included.
Hydrogel Characterization
For swelling studies, samples were weighed and measured immediately after photopolymerization then placed in Opti-MEM at 37 °C in a 5% CO2 incubator for 24 h. After 24 h, the samples were blotted dry before being weighed and measured again. The samples were then placed in a freeze-dryer and lyophilized prior to being weighed again. The swelling ratio, q, was determined by taking the ratio of the swollen mass of the hydrogel to the mass of the hydrogel after freeze-drying. The mesh size was determined as described by Canal and Peppas14 using the equation ξ = V2,s−1/3lCn1/2n1/2 with the alteration proposed by Anseth15 and Hubbel.16 V2,s is the equilibrium polymer volume fraction in the gel, l is the bond length (1.50 Å),16 Cn is the characteristic ratio of PEG,17 and n is the number of bonds between cross-links.
To determine the storage and loss modulus, samples (25 mm diameter, round) were cast between two glass slides with 500 μL of the appropriate ECM PEGDM solution and photopolymerized under conditions identical to those described previously. After swelling overnight in Opti-MEM, the storage modulus of the samples was measured with a ARES-G2 rheometer (TA Instruments, Newcastle, DE) using 25 mm serrated parallel plates with strain amplitude of 1% and 30 N constant normal force to prevent slippage over an angular frequency sweep from 100 to 1 rad/s with 10 points per decade. Moduli data are reported at an angular frequency of 1 rad/s as the gels did not show a frequency-dependent response.
Histological Staining
Following predefined culture periods, samples were fixed overnight in 4% paraformaldehyde (Sigma). Samples for histological sectioning were transferred to 70% ETOH for at least 1 h, 80% ETOH for 1.5 h, 95% ETOH for 12 h, ETOH for 1.5 h twice, and xylene for 1 h. Samples were then placed in 60 °C paraffin for 12 h and embedded in a paraffin block for sectioning.18 Blocks were removed from a −20 °C freezer and cut into 7 μm sections. After 2 days of drying in a 37 °C oven, samples were stained with 0.5% Alcian blue with 0.1% nuclear fast Red or picro-sirius red solution with Mayer’s hematoxylin (Fisher Biological, Pittsburgh, PA) and viewed on a CKX41 microscope (Olympus, Center Valley, PA). After rehydration, immunochemistry samples were incubated in 0.5% pepsin for 10 min at 30 °C for antigen retrieval. Nonspecific antibody binding was blocked by incubating in 10% goat serum. Samples were then exposed to collagen type 2 (1:200) antibody, followed by appropriate secondary antibodies (Col 2) conjugated to Alexa Flour 488 (1:500, Invitrogen). DAPI (Sigma) was used to stain the cell nuclei. The Col 2 antibody (II-II6B3) developed by Thomas F. Linsenmayer was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by the Department of Biology at The University of Iowa.
Biochemistry
Sulfated glycosaminoglycans (sGAGs) were quantified with dimethylmethylene blue (DMB), and collagen content was quantified using dimethylaminobenzaldehyde (DAB) to observe chloramines T-oxidized hydroxyproline as previously described.14,23 Briefly, samples were homogenized with a Tissue-Tearor (BioSpec Products, Inc., Bartlesville, OK) and then digested with proteinase K overnight at 60 °C. Samples for sGAGs detection were added to DMB solution at ratio of 1:10, mixed, and read at 535 nm. Samples for hydroxyproline detection were dehydrated, autoclaved at 120 °C with 2 N NaOH for 20 min, oxidized with chloramines T solution for 25 min at room temperature on an orbital shaker at 100 rpm, and then incubated with DAB for 20 min at 65 °C. All biochemistry was first normalized against the same sample type cultured in media without encapsulated cells for the same duration followed by normalization to total amount of protein in the sample and then to 10% PEGDM samples.
Statistics
All experiments were conducted at least three times (n ≥ 3). All quantitative data are presented as the average ± standard deviation. One-way analysis of variance (ANOVA) with post hoc analysis were performed where applicable. Significance was set at a p-value of less than 0.05.
RESULTS
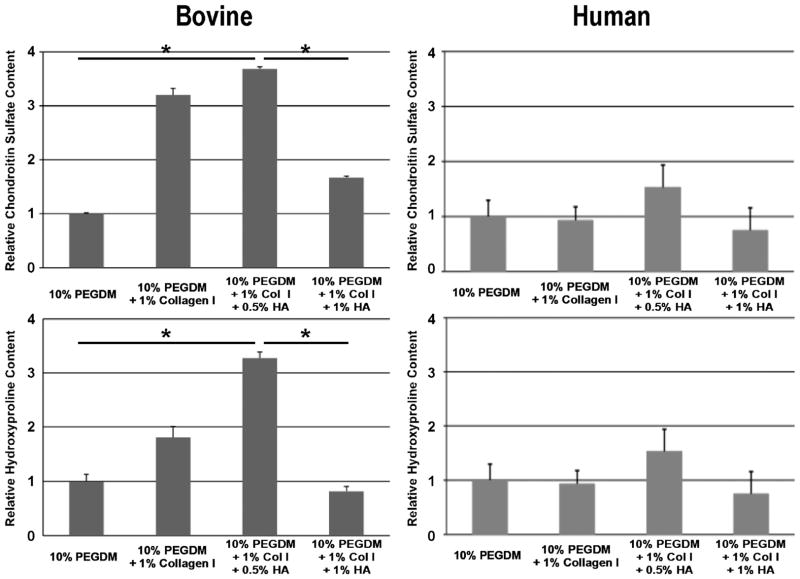
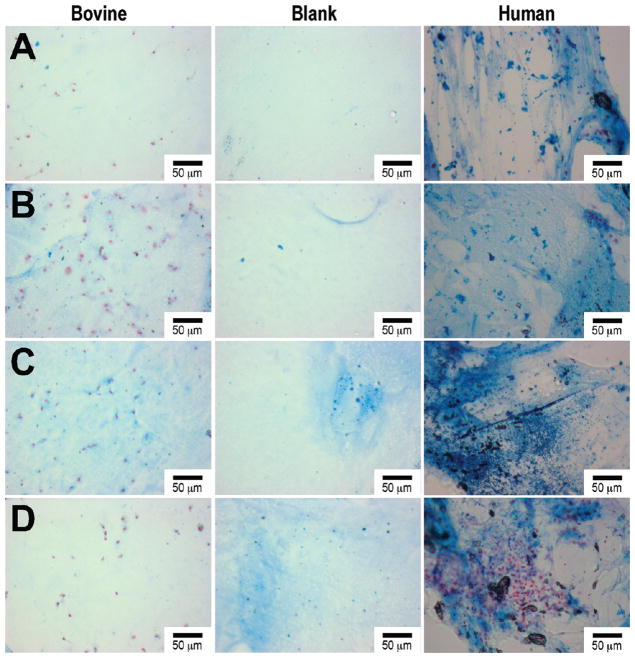
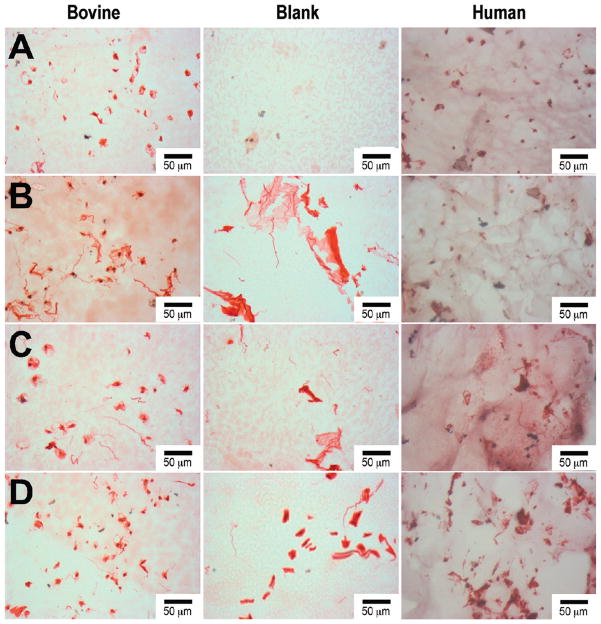
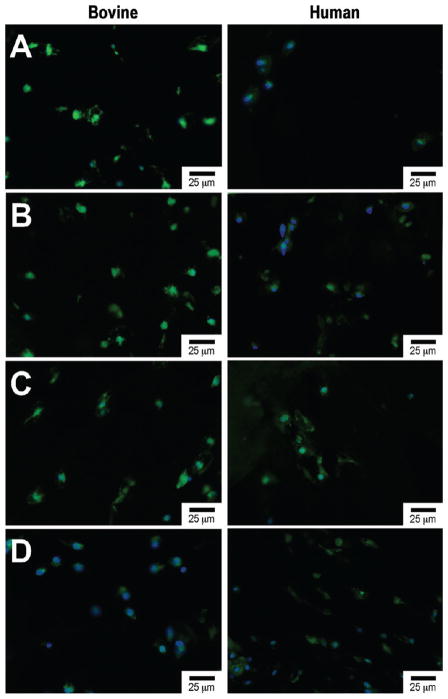
Table 1 summarizes the material characterization data. The 0.5% HA/Col I containing samples have a significantly higher swelling ratio and mesh size compared to 1% HA/Col I, Col I, and PEGDM only samples. This is accompanied by a significant reduction in the storage modulus in the HA containing samples compared to the Col I and PEGDM only samples. Col I samples exhibited a slightly increased storage modulus compared to PEGDM only samples. Biochemical quantification of sGAGs and hydroxyproline (Figure 2) indicates PEGDM matrices seeded with bovine chondrocytes in 0.5% HA and/or 1% Col I hydrogels contain roughly 2.6 times and 1.9 times more chondroitin sulfate, respectively, than matrices containing only PEGDM and 2.4 times and 1.8 times more hydroxyproline respectively than matrices containing only PEGDM after 6 weeks of cultures. Staining of histological sections for sGAGs (Figure 3), collagen (Figure 4), and type II collagen (Figure 5) supports the biochemical quantifications and indicates the inclusion of Col I (3B, 4B, and 5B) and 0.5% HA with Col I (3C, 4C, and 5C) in the matrices promotes bovine chondrocyte ECM accumulation compared to other tested samples containing bovine chondrocytes.
Table 1.
Summary of Material Characterizationa
| sample | swelling ratio | mesh size (Å) | storage modulus (Pa) | loss modulus (Pa) |
|---|---|---|---|---|
| 10% PEGDM | 13.5 ± 1.0 | 120.2 ± 7.0 | 1578 ± 60 | 793 ± 78 |
| 10% PEGDM + 1% Col I | 13.2 ± 1.1b | 114.4 ± 6.2 | 2214 ± 189 | 545 ± 103b |
| 10% PEGDM + 1% Col I + 0.5% HA | 15.9 ± 0.4c | 132.7 ± 1.8b | 1275 ± 51c | 852 ± 109 |
| 10% PEGDM + 1% Col I + 1.0% HA | 13.3 ± 1.9 | 112.3 ± 6.5 | 1238 ± 34c | 865 ± 6 |
All data points are reported as average ± standard deviation.
Indicates a p-value <0.05 relative to 10% PEGDM samples.
Indicates p-value <0.01 relative to 10% PEGDM samples.
Figure 2.
Quantification of sulfated glycoaminoglycans and hydroxyproline production in hydrogels encapsulating bovine and human chondrocytes after 6 weeks of culture, respectively. Asterisk indicates a p-value <0.05. While the respective samples trend the similarly, the statistical differences are minimal in the human cells. However, the relative ECM productions by the OA human cells are distinctly less than the bovine chondrocytes.
Figure 3.
Histological Alcian blue staining of bovine and human chondrocytes after 6 weeks of encapsulated culture in poly(ethylene glycol) dimethacrylate (PEGDM) hydrogels: (A) PEGDM, (B) PEGDM with 1% collagen type 1, (C) PEGDM with 1.0% collagen type 1 and 0.5% hyaluronic acid, (D) PEGDM with 1.0% collagen type 1 and 1.0% hyaluronic acid. The blank sections depict the background staining in the individual samples. Scale bar = 50 μm.
Figure 4.
Histological sirius red staining of bovine and human chondrocytes after 6 weeks of encapsulated culture in poly(ethylene glycol) dimethacrylate (PEGDM) hydrogels: (A) PEGDM, (B) PEGDM with 1% collagen type 1, (C) PEGDM with 1.0% collagen type 1 and 0.5% hyaluronic acid, (D) PEGDM with 1.0% collagen type 1 and 1.0% hyaluronic acid. The blank sections depict the background staining in the individual samples. Scale bar = 50 μm.
Figure 5.
Fluorescent image with collagen 2 (green) and nuclear (blue) staining of bovine and human chondrocytes after 6 weeks of encapsulated culture in poly(ethylene glycol) dimethacrylate (PEGDM) hydrogels: (A) PEGDM, (B) PEGDM with 1% collagen type 1, (C) PEGDM with 1.0% collagen type 1 and 0.5% hyaluronic acid, (D) PEGDM with 1.0% collagen type 1 and 1.0% hyaluronic acid.
After 6 weeks of culture, human chondrocytes samples showed a similar trend of sGAGs (Figure 2) and hydroxyproline concentration as the bovine chondrocytes samples. However, these trends were not significant. Staining of histological sections for sGAGs (Figure 3), collagen (Figure 4), and type II collagen (Figure 5) shows the inclusion of Col I (3B, 4B, and 5B) and 0.5% HA with Col I (3C, 4C, and 5C) in the matrices promotes human chondrocyte ECM accumulation compared to other tested samples containing human chondrocytes.
DISCUSSION
Biomimetic, hybrid tissue engineering scaffolds represent a potential advance in the viable treatment options for middle aged patients with isolated defects who often find themselves between conventional biological treatments and arthroplasty. The development of advanced hybrid tissue engineering scaffolds requires identifying and optimizing the material characteristics, which maximize ECM production in synthetic polymer systems. Although shown by us and others to be supportive of cartilage ECM production, PEGDM hydrogels do not include any speciffic biological signaling motifs which facilitate the formation of mature cartilage.19 This study is the first we are aware of that examines ECM interactions using multiple ECM molecules within a synthetic hydrogel encapsulating middle aged human chondrocytes compared to immature bovine chondrocytes, the common cell source for cartilage tissue engineering studies, in growth factor and serum free media.
Previous studies have found the inclusion of a single ECM protein, such as chondroitin sulfate, type II collagen, Col I and HA, in PEG-based hydrogels did not significantly affect the swelling ratio or mechanical properties of the gels.20–22 However, our results showed significant changes from PEGDM samples within certain test groups (Table 1). The reduction in the storage modulus in the HA containing samples compared to the Col I and PEGDM only samples are attributed to an increase in solution viscosity caused by HA chain entanglement, which most likely inhibits the formation of cross-links during photoinitiation. As the concentration of HA increases, hydrogen bonds between HA molecules increasing the stability of the hydrogel.23 The increased storage modulus observed in Col I samples PEGDM only samples is attributed to the formation of a collagen fibril network, which reinforces the PEGDM hydrogel structure.
The differences between our results and previous studies are most likely due to differences in base materials, fabrication, and testing parameters and modalities. It is important to note that the variations in characterization parameters caused by the inclusion of the ECM components may be contributing to the biological results through changes in mass transport and mechanical strength.24,25 For example, the 0.5% HA with 1% Col 1 samples have a higher swelling ratio and mesh size than the other samples which could allow for increased mass transport and improved cellular health and tissue formation compared to the other samples. The subtle differences in mechanical properties appear to have an insignificant effect on the biological data as they do not correlate with the differences in biological outcomes.
Bovine chondrocytes encapsulated in samples containing 1% Col I or 1% Col I and 0.5% HA showed increased ECM accumulation compared to the other tested samples (Figure 2). This accumulation could arise from increased ECM production, increased retention due to a defined hydrogel network structure, or a decrease in the recycling of ECM due to its isolation in the synthetic gel. Previous studies of samples containing Col I have shown similar results with encapsulated goat mesenchymal stem cell and bovine chondrocytes.21,22 However, previous studies with HA containing samples show mixed results.21,22,26–30 A study with human chondrocytes showed a dose dependent suppression of Aggrecan and Sox9 mRNA expression,27 indicating that the effect of HA on chondrocyte ECM synthesis is most likely dose dependent. Based on our results (Figures 2–5), low levels of HA stimulate chondrogenic ECM production while higher levels inhibit production. However, variations in the amount and type of synthetic base polymer alter gene expression and mechanical properties of the formed tissue when chondrocytes are encapsulated in hydrogels.31 Substantial differences in culture media exist between the individual studies in the literature (inclusion of serum, growth factors, etc.). These factors make direct comparison and definitive conclusions difficult.
Although the immature bovine chondrocytes are significantly more metabolically active than mature human chondrocytes, we observed similar trends in ECM synthesis with both cell types (Figures 2–5). The reduced response of human chondrocytes compared to bovine chondrocytes is most likely related to disease or age-related changes to the chondrocytes which reduce their ability to maintain homeostasis and make them less responsive to anabolic signals.32–34 Maximizing ECM production in OA human chondrocytes is critical as autologous cell sourcing from a cartilage biopsy will remain the most clinically relevant cell population in the near term due to safety and regulatory concerns. While autologous stem cell transplantation may eventually yield a clinically relevant option, cell sourcing will continue to be a limitation to advancing surgical and nonsurgical tissue engineering options.
CONCLUSIONS
The inclusion of low concentrations of Col I and HA increases ECM production in both bovine and OA human chondrocytes encapsulated in PEGDM hydrogels. Our data clearly show that working with clinically relevant cell sources is important and extrapolations from results using other cell sources including mesenchymal stems cells must be viewed with caution. Future work will examine more detailed measurement of the effect of ECM fragments on gene expression and examine further the mechanistic activations within the signaling pathways triggering these responses.
Acknowledgments
The authors thank the Mahan Packing Company in Bristolville, OH, for supplying all bovine samples, LeeAnn Spearing of Summa Health Systems for facilitating surgical selections and specimen transfers, and Ryan Sieve for assisting with data processing. The authors acknowledge Professor Robert A. Weiss for use of an Ares G2 rheometer for mechanical measurements. Our research was supported by the University of Akron Research Foundation (M.L.B.), the Akron Functional Materials Center (M.L.B.), and Summa Health Systems Hospital Foundation (W.E.H.).
Footnotes
The authors declare no competing financial interest.
References
- 1.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, Gabriel S, Hirsch R, Hochberg MC, Hunder GG, Jordan JM, Katz JN, Kremers HM, Wolfe F, Workgroup NAD. Arthritis Rheum. 2008;58(1):26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hootman JM, Helmick CG. Arthritis Rheum. 2006;54(1):226–229. doi: 10.1002/art.21562. [DOI] [PubMed] [Google Scholar]
- 3.Steadman JR, Briggs KK, Rodrigo JJ, Kocher MS, Gill TJ, Rodkey WG. Arthroscopy. 2003;19(5):477–484. doi: 10.1053/jars.2003.50112. [DOI] [PubMed] [Google Scholar]
- 4.Asik M, Ciftci F, Sen C, Erdil M, Atalar A. Arthroscopy. 2008;24(11):1214–1220. doi: 10.1016/j.arthro.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 5.Kreuz PC, Erggelet C, Steinwachs MR, Krause SJ, Lahm A, Niemeyer P, Ghanem N, Uhl M, Südkamp N. Arthroscopy. 2006;22(11):1180–1186. doi: 10.1016/j.arthro.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 6.Lane NE, Brandt K, Hawker G, Peeva E, Schreyer E, Tsuji W, Hochberg MC. Osteoarth Cartilage. 2011;19(5):478–482. doi: 10.1016/j.joca.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 7.Hollander AP, Heathfield TF, Webber C, Iwata Y, Bourne R, Rorabeck C, Poole AR. J Clin Invest. 1994;93(4):1722–1732. doi: 10.1172/JCI117156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hollander AP, Dickinson SC, Kafienah W. Stem Cells. 2010;28(11):1992–1996. doi: 10.1002/stem.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vasara AI, Hyttinen MM, Pulliainen O, Lammi MJ, Jurvelin JS, Peterson L, Lindahl A, Helminen HJ, Kiviranta I. Osteoarth Cartilage. 2006;14(10):1066–1074. doi: 10.1016/j.joca.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Kim HKW, Moran ME, Salter RB. J Bone Jt Surg. 1991;73(9):1301–1315. [PubMed] [Google Scholar]
- 11.Wei X, Messner K. J Biomed Mater Res. 1999;46(4):539–548. doi: 10.1002/(sici)1097-4636(19990915)46:4<539::aid-jbm12>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 12.Hayes AJ, MacPherson S, Morrison H, Dowthwaite G, Archer CW. Anat Embryol. 2001;203(6):469–479. doi: 10.1007/s004290100178. [DOI] [PubMed] [Google Scholar]
- 13.Nugent AE, Reiter DA, Fishbein KW, McBurney DL, Murray T, Bartusik D, Ramaswamy S, Spencer RG, Horton WE. Tissue Eng, Part A. 2010;16(7):2183–2196. doi: 10.1089/ten.tea.2009.0717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Canal T, Peppas NA. J Biomed Mater Res. 1989;23(10):1183–1193. doi: 10.1002/jbm.820231007. [DOI] [PubMed] [Google Scholar]
- 15.Lu S, Anseth KS. Macromolecules. 2000;33(7):2509–2515. [Google Scholar]
- 16.Cruise GM, Scharp DS, Hubbell JA. Biomaterials. 1998;19(14):1287–1294. doi: 10.1016/s0142-9612(98)00025-8. [DOI] [PubMed] [Google Scholar]
- 17.Merrill EW, Dennison KA, Sung C. Biomaterials. 1993;14(15):1117–1126. doi: 10.1016/0142-9612(93)90154-t. [DOI] [PubMed] [Google Scholar]
- 18.Strehin IA, Elisseeff JH. Characterizing ECM Production by Cells Encapsulated in Hydrogels. In: Even-Ram S, Artym V, editors. Extracellular Matrix Protocols. Vol. 522. Humana Press; Totowa, NJ: 2009. pp. 349–362. [DOI] [PubMed] [Google Scholar]
- 19.Elisseeff J, McIntosh W, Anseth K, Riley S, Ragan P, Langer R. J Biomed Mater Res. 2000;51(2):164–171. doi: 10.1002/(sici)1097-4636(200008)51:2<164::aid-jbm4>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 20.Roberts JJ, Nicodemus GD, Giunta S, Bryant SJ. J Biomed Mater Res, Part A. 2011;97A(3):281–291. doi: 10.1002/jbm.a.33057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hwang N, Varghese S, Li H, Elisseeff J. Cell Tissue Res. 2011;344(3):499–509. doi: 10.1007/s00441-011-1153-2. [DOI] [PubMed] [Google Scholar]
- 22.Hwang NS, Varghese S, Lee HJ, Theprungsirikul P, Canver A, Sharma B, Elisseeff J. FEBS Lett. 2007;581(22):4172–4178. doi: 10.1016/j.febslet.2007.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Atkins EDT, Meader D, Scott JE. Int J Biol Macromol. 1980;2:318–319. [Google Scholar]
- 24.Bryant SJ, Chowdhury TT, Lee DA, Bader DL, Anseth KS. Ann Biomed Eng. 2004;32(3):407–417. doi: 10.1023/b:abme.0000017535.00602.ca. [DOI] [PubMed] [Google Scholar]
- 25.Lin S, Sangaj N, Razafiarison T, Zhang C, Varghese S. Pharm Res. 2011;28(6):1422–1430. doi: 10.1007/s11095-011-0378-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frean SP, Abraham LA, Lees P. Res Vet Sci. 1999;67(2):183–190. doi: 10.1053/rvsc.1999.0328. [DOI] [PubMed] [Google Scholar]
- 27.Chiu LH, Chen SC, Wu KC, Yang CB, Fang CL, Lai WFT, Tsai YH. J Cell Physiol. 2011;226(8):1981–1988. doi: 10.1002/jcp.22530. [DOI] [PubMed] [Google Scholar]
- 28.Kawasaki K, Ochi M, Uchio Y, Adachi N, Matsusaki M. J Cell Physiol. 1999;179(2):142–148. doi: 10.1002/(SICI)1097-4652(199905)179:2<142::AID-JCP4>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen LH, Kudva AK, Saxena NS, Roy K. Biomaterials. 2011;32(29):6946–6952. doi: 10.1016/j.biomaterials.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen LH, Kudva AK, Guckert NL, Linse KD, Roy K. Biomaterials. 2011;32(5):1327–1338. doi: 10.1016/j.biomaterials.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 31.Klein TJ, Rizzi SC, Schrobback K, Reichert JC, Jeon JE, Crawford RW, Hutmacher DW. Soft Matter. 2010;6(20):5175–5183. [Google Scholar]
- 32.Shane Anderson A, Loeser RF. Best Pract Res Cl Rh. 2010;24(1):15–26. doi: 10.1016/j.berh.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin JA, Ellerbroek SM, Buckwalter JA. J Orthop Res. 1997;15(4):491–498. doi: 10.1002/jor.1100150403. [DOI] [PubMed] [Google Scholar]
- 34.Blaney Davidson E, Scharstuhl A, Vitters E, van der Kraan P, van den Berg W. Arthritis Res Ther. 2005;7(6):R1338–R1347. doi: 10.1186/ar1833. [DOI] [PMC free article] [PubMed] [Google Scholar]