Abstract
The N-Methyl-D-Aspartate Receptor (NMDAR) hypofunction model of schizophrenia is based on the ability of NMDAR antagonists to produce many symptoms of the disease. Recent work in rat shows that NMDAR antagonist works synergistically with dopamine to produce delta frequency bursting in the thalamus. This finding, together with other results in the literature, suggests a mechanism for the sudden onset of schizophrenia. Among the thalamic nuclei most activated by NMDAR antagonist is the nucleus reuniens. This nucleus excites the CA1 region of the hippocampus. Experiments indicate that such activation can lead to excitation of dopaminergic cells of the VTA by a polysynaptic pathway. The resulting elevation of dopamine in the thalamus will enhance thalamic bursting, thereby creating a loop with the potential for positive feedback. We show through computer simulations that, in individuals with susceptibility to schizophrenia (e.g., because of partially compromised NMDAR function), an event that stimulates the dopamine system, such as stress, can cause the system to reach the threshold for thalamic bursting. When this occurs, positive feedback in the loop will cause all components to become highly active and to remain active after the triggering stimulus is removed. This is a physiologically specific hypothesis for the sudden and lasting transition that underlies the psychotic break in schizophrenia. Furthermore, the model provides an explanation for the observed selective activation of the CA1 hippocampal region in schizophrenia. The model also predicts an increase of basal activity in the dopamine system and thalamus; the relevant evidence is reviewed.
Keywords: CA1, delta oscillations, dopamine, N-Methyl-D-Aspartate receptor antagonist, nucleus reuniens, thalamic reticular nucleus
1. Introduction
The N-Methyl-D-Aspartate Receptor (NMDAR) hypofunction hypothesis of schizophrenia originated with the finding that NMDAR antagonists can induce the positive, negative, and cognitive symptoms of the disease in humans (1–6). NMDAR function is reduced in the disease, as measured by the amplitude of the NMDAR-dependent signal, the mismatch negativity (2, 7), and by the binding of a noncompetitive NMDAR antagonist (8).
The NMDA hypofunction theory has been linked to other major theories of the disease. The dopamine hyperfunction theory (see below in 4.3) can now be related to the NMDAR hypofunction model because NMDAR antagonist produces dopamine release (see below in 2.3). Another theory of schizophrenia, the GABA hypofunction theory, emerged from the study of postmortem tissue. It was found that there is a widespread decrease in parvalbumin and glutamic acid decarboxylase (GAD), the enzyme that synthesizes GABA in fast-spiking interneurons (9–11). These findings can now be linked to the NMDAR hypofunction theory because administration of NMDAR antagonist to rats can reproduce these effects (12, 13) and result in functional deficits in inhibition (14). Thus, major changes in the dopamine and GABA system may be downstream effects of NMDAR hypofunction.
There have been substantial efforts to understand the cellular and network mechanisms that underlie these effects of NMDAR antagonist. Although NMDARs throughout the brain are affected by the antagonists, those in the thalamus appear to have special importance (see 2.1). NMDAR antagonists, working synergistically with dopamine, cause delta frequency bursting in the nucleus reticularis (nRT), a pacemaker of the thalamocortical system (15). Such delta frequency activity is also seen in thalamic relay cells, which, in turn, drive the cortex (16, 17). Such a thalamocortical abnormality could underlie the enhanced delta frequency EEG power in the awake state observed in schizophrenia (as summarized in a meta-analysis (18)).
With this new perspective about thalamic function in mind, we reviewed previous work on the effect of NMDAR antagonist. We found evidence that the nucleus reuniens of the thalamus, a nucleus that innervates the hippocampus, is very strongly excited by NMDAR antagonist. This carries special significance because of other recent work establishing the importance of hippocampal activation in stimulating the release of dopamine (19–21). Moreover, the connection of the hippocampus to the dopamine system has been strongly implicated in animal models of schizophrenia (22–26). If NMDAR inhibition in the thalamus leads to activation of the thalamus and thereby to the activation of hippocampus and dopamine system, there will be dopamine release in the thalamus. As noted above, dopamine works synergistically with NMDAR antagonist to promote bursting in the thalamus. Taken together, these results suggest the possibility of a positive feedback loop. In the next section, we review the evidence for such a loop. In Section 3, we develop a model in which positive feedback in the loop is related to the psychotic break in schizophrenia. Section 4 deals with the question of whether the thalamus, hippocampus, and VTA are activated in schizophrenia, as predicted by our model. Limitations and further tests of the model are described in Section 5.
2. Evidence for activation of thalamus, hippocampus, and VTA in response to NMDAR antagonists
2.1 The action of NMDAR antagonist in the rat thalamus
Early work showed that systemic administration of NMDAR antagonist to rats produces cellular damage (27) and activation of immediate early genes in limbic cortex (28). These cortical changes could be prevented by systemic neuroleptics (29, 30) and thus could be relevant to schizophrenia.
Further investigation showed that the cortical damage is not due to direct action of NMDAR antagonist on cortex, but instead depends on action in the thalamus. Specifically, it was shown that the cortical damage produced by systemic NMDAR antagonists could be reproduced by microinjection of NMDAR antagonist into the thalamus, but not by microinjection into the cortex itself (31). Furthermore, injection of the GABAA agonist, muscimol, into the thalamus (this would inhibit the thalamus) blocked the effect of systemic NMDAR antagonist on cortex (32). Evidently, systemic NMDAR antagonist stimulates electrical activity in the thalamus which spreads to cortex.
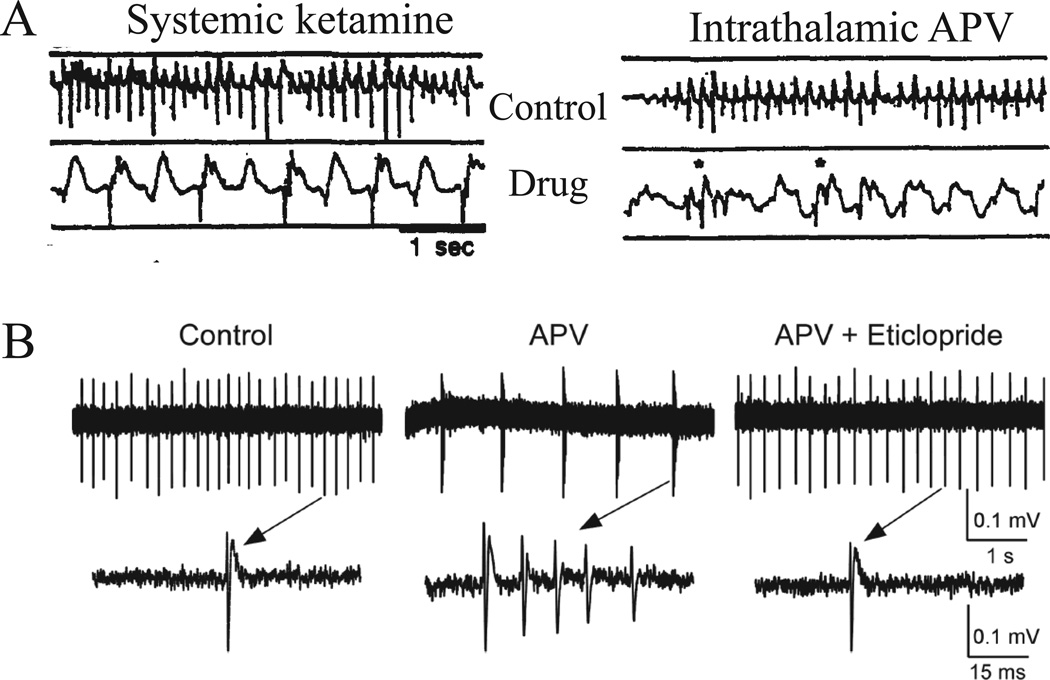
Progress has been made in elucidating the type of electrical activity that spreads from thalamus to cortex. Early work showed that subanesthetic doses of ketamine in awake cats produce synchronized delta-wave activity in the cortex and thalamus (17). The cortical delta is selectively increased in the association areas of cortex (as compared to sensory cortices). Moreover, it was shown that isolating the thalamus from other subcortical or cortical regions did not prevent delta activity in the thalamus (17). This suggests that thalamus may be a major driver of delta activity. Later, Buzsaki (16) confirmed this by showing that injection of NMDAR antagonist into the thalamus drives delta frequency oscillations in the cortical EEG (Fig. 1A).
Figure 1.
NMDAR blockers cause delta frequency oscillations in the cortex by an effect on thalamus. A. The effect of systemic injections of NMDAR antagonist on cortical EEG can be reproduced by intrathalamic microinjection of antagonist. Extracted with permission from Fig. 6 in (16). B. Application of NMDAR blocker (APV) to cells in the thalamic reticular nucleus in vitro causes a switch from regular firing to bursting at delta frequency (receptor activation is by ambient glutamate). Blockade of D2 dopamine receptors by eticlopride reverses the effect (receptor activation is by ambient dopamine). Extracted with permission from Fig. 6 in (15).
The ability to induce delta frequency EEG with NMDAR antagonist provides further support for the NMDAR hypofunction model of schizophrenia because this antagonist action mimics the elevated EEG delta power in schizophrenia (18). This elevated delta is found in the awake state and is localized primarily in frontal and central regions.
Recent work has elucidated the mechanisms by which NMDAR antagonist produces delta frequency bursting in the thalamus (15). Studies were done in slices of the nucleus reticularis of the thalamus, a group of inhibitory cells that is bidirectionally connected with relay cells of thalamic nuclei and that acts as a pacemaker for thalamocortical rhythms (33, 34). Recordings from these cells showed that application of NMDAR antagonist causes them to burst at delta frequency (Fig. 1B). Further experiments provided insight into the biophysical mechanisms by which NMDAR antagonist produces bursting. It was found that NMDAR antagonist produces a hyperpolarization of resting potential. This hyperpolarization deinactivates T-type calcium channels, which then generate calcium spikes (33–35). Each calcium spike generates a burst of action potentials, followed by a long recovery period until the next calcium spike. The mechanism by which NMDAR antagonist produces hyperpolarization (which is not seen in cortical neurons) depends on the particular properties of the NMDARs in the thalamus. Cells in this region contain an unusual form of NMDAR subunit (NR2C or NR2C-like) that, unlike NR2A and NR2B, have little Mg2+ block. It is known that the ambient extracellular glutamate concentration is high enough to partially activate all forms of NMDARs. However, NR2A/B-containing receptors do not generate current in response to ambient glutamate because they are strongly blocked by Mg2+ ions near resting potential. In thalamic neurons, however, activation of NMDARs by ambient glutamate depolarizes resting potential because the channels are not blocked by Mg2+. This explains why NMDAR antagonists hyperpolarize thalamic cells (15).
An additional finding with important implications for schizophrenia is that dopamine has a synergistic role in generating the delta frequency bursting induced by NMDAR antagonist (15). An antagonist of D2-type dopamine receptors, the type of drug that effectively treats schizophrenia, blocks the bursting (Fig. 1B). Antagonist was effective because there is sufficient ambient dopamine to activate D2 receptors and hyperpolarize the nRT cells. Thus, both dopamine and NMDAR antagonist hyperpolarize nRT cells and act synergistically to take the cell to the threshold hyperpolarization required for delta frequency bursting. The extrapolation of rat data to humans may be reasonable, given that the thalamic activation produced by NMDAR antagonist can also be observed in humans using BOLD signals (36).
2.2 The connection between activation of the thalamic nucleus reuniens and CA1 activation
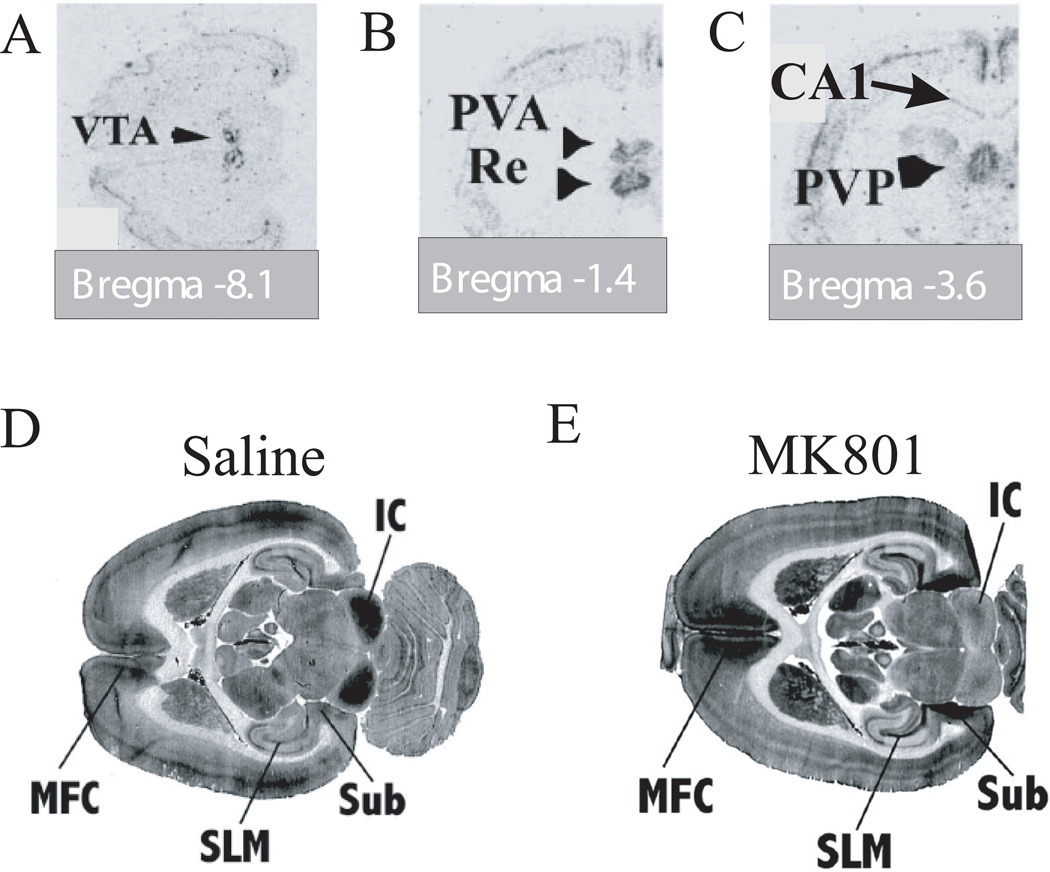
Several activity-dependent markers have been used to study the effect of NMDAR antagonists on the activity in different thalamic nuclei. These methods utilize either activation of the immediate early gene, c-Fos (28), or energy utilization, as measured by [14C]-2-deoxyglucose (2-DG) uptake (37). These in vivo studies confirm that the nRT is activated by systemic NMDAR antagonists. These studies also show that relay thalamic nuclei are activated, but in heterogeneous way. There is particularly strong activation of midline nuclei (28), including the nucleus reuniens (Fig. 2B). This nucleus is of special interest in schizophrenia because it innervates the hippocampus (38, 39), a region implicated in the disease (see below in 4.2). The nucleus reuniens, like other thalamic relay nuclei, is bidirectionally connected with the nRT (38). Thus, the observed activation of the nucleus reuniens by NMDAR antagonist is likely to be at least partly due inputs from the nRT.
Figure 2.
Activation of relevant brain structures by MK801 in vivo. c-Fos expression in ventral tegmental area (VTA) (A), nucleus reuniens (Re) (B), and CA1 hippocampal region (C) 4 hours after drug application. Nucleus reticularis was also activated (not shown). There was no c-Fos expression in CA3 (C). Extracted with permission from Fig. 1 and 2 in (28). D, E. The synaptic uptake of 2-DG 4 min after saline (D) and MK801 administration (E) shows strong increase of synaptic activity in medial prefrontal cortex (MPC), in stratum lacunosum-moleculare of CA1 (SLM), and in the subiculum (Sub). Inferior colliculus (IC) activity is decreased by MK801. Extracted with permission from Fig. 3 in (37).
There are direct and indirect pathways from the nucleus reuniens to the hippocampus, and both converge on the CA1 region, specifically in the dendritic region most distal from the cell body called the stratum lacunosum-moleculare (SLM). The indirect pathway goes via nucleus reuniens input to the entorhinal cortex. Information is then carried from layer 3 of this cortical region to the SLM, where it converges with the direct pathway (39). As shown in Fig. 2D, E, NMDAR antagonist produces a robust increase in the 2-DG signal that is specific for the SLM input to CA1 (37).
A number of physiological studies indicate that the nucleus reuniens’ input to CA1 is excitatory. In vivo stimulation of nucleus reuniens causes short-latency monosynaptic excitatory field potentials in the SLM of mid and posterior CA1 region (40–42). Microinjection of NMDA into the nucleus reuniens leads to epileptic activity in the hippocampus (43). Injection even a few millimeters away does not have this effect. Elegant studies of absence epilepsy independently demonstrate that the nucleus reuniens conveys low-frequency oscillations from thalamus to the cortex and hippocampus (44, 45). The input from entorhinal cortex to CA1 is also excitatory (reviewed in (46)) and sufficient to drive place cells in CA1 (47).
Given this evidence that both direct and indirect inputs from the nucleus reuniens are active and excitatory, it would be expected that NMDAR antagonist would increase activity in CA1. Several lines of evidence indicate that this is the case. Activation due to NMDAR blocker was observed in CA1 (c-Fos, Fig. 2C) (28) and in the subiculum, a target of CA1 output (2-DG, Fig. 2D, E) (37). Furthermore, the firing rate of pyramidal cells in rat CA1 is increased by NMDAR blocker (Dr. M.W. Jones, personal communication). An important result of the c-Fos and 2-DG studies is that activation of CA3 was not observed (Fig 2C, E) (28, 37). Thus, the enhanced activity in CA1 is not due to CA3 input. Taken together, the data support the idea that hippocampal activation occurs specifically in CA1 and that it arises by input through the SLM that depends directly and indirectly on the nucleus reuniens. However, the data do not exclude other contributions to CA1 excitation, such as local disinhibition in hippocampus due to NMDAR blockade (4, 14, 48), as will be discussed later (3.1).
2.3 Excitation of the hippocampus leads to dopamine release
Systemic application of NMDAR antagonist leads to activation of the dopamine system. This has been observed by an increased c-Fos induction in the VTA (Fig. 2A) (28), by the enhancement of the spiking of dopaminergic cells (49–51), and by the enhancement of dopamine release in target structures (28, 52). This systemic effect can not be reproduced by local microinjection of NMDAR blockers into VTA (51, 53). Moreover, disconnection of the midbrain from the forebrain decreased the effect of systemic MK801 (51). This data suggests that the activation of the VTA by systemic NMDAR antagonist is not due to local action of the antagonist in VTA.
There is now substantial evidence that activation of the hippocampus can lead to activation of dopaminergic cells via a polysynaptic pathway, resulting in dopamine release in the striatum, cortex, and hippocampus (for a review, see (21)). For instance, it has been shown that exciting the CA1/subiculum causes activation of dopamine neurons in VTA and dopamine release in target structures (19, 20). Given this pathway, it seems likely that the activation of CA1 by NMDAR antagonist effects in the thalamus will lead to dopamine release (for a test of this prediction, see Section 5).
2.4 Closing the loop; dopamine promotes thalamic bursting
The activation of dopaminergic cells will lead to dopamine release in the thalamus (for details on dopaminergic innervation of thalamus see section S2 in Supplement 1). Electrophysiological studies have shown that dopamine action in the nRT and relay nuclei is hyperpolarization by D2 action (15, 54). As mentioned previously, this hyperpolarization works synergistically with the hyperpolarization produced by NMDAR antagonist to reach the threshold for bursting in nRT (15). This threshold occurs because a sufficient hyperpolarization is needed to remove inactivation from T-type calcium channels (34, 35). The resulting bursting will produce further activation of the hippocampus, further dopamine release, and thus stronger thalamic bursting. Thus, the loop formed by the thalamus, hippocampus, and VTA has the potential for positive feedback.
3. The psychotic break: a transition to positive feedback caused by stress
3.1 Predisposition for schizophrenia and the effect of stress
We do not think that positive feedback in the thalamo-hippocampal-VTA loop occurs under normal conditions. In this section, we consider the conditions that create the possibility of such feedback in schizophrenia. We define these conditions as predispositions because none of them alone or in combination can produce the rapid onset and persistence of psychotic state; rather, they make the system vulnerable to triggers that cause the system to go into positive feedback and thereby create the psychotic break.
As noted above, there is a threshold level of hyperpolarization necessary to produce bursting of thalamic neurons. From this perspective, a predisposition to schizophrenia could arise from any factor that hyperpolarizes thalamic cells. NMDAR antagonist can act directly on thalamic cells to produce such hyperpolarization (15). Furthermore, indirect actions could also produce hyperpolarization. For instance, NMDAR antagonist may decrease activity of hippocampal interneurons and thereby increase the spiking rate of pyramidal cells (4, 21, 48, 55). This, in turn, would activate the VTA; the resulting release of dopamine would hyperpolarize thalamic cells. Some local disinhibition in dopaminergic areas may also contribute to this effect (56). Another view of predisposing factors comes from the analysis of the effects of prenatal/neonatal damage to the hippocampus as an animal model of schizophrenia (22, 23, 26). This damages hippocampal interneurons (55, 57) and leads to enhanced hippocampal activity, increased firing of dopamine cells (22, 23) and behavioral sensitization of dopaminergic system to amphetamine challenge (24–26, 58).
We now turn to the question of what may trigger the psychotic break. It is well established that the break can be triggered by psychosocial stress in late adolescence (reviewed in (59–61)). Work in animal models has shown that social stress increases the firing of dopamine cells and causes dopamine release in target structures (62). One mechanism for this is via direct activation of the VTA by corticotropine-releasing factor (63, 64). In the presence of predisposing factors, the stress-induced release of dopamine may cause sufficient hyperpolarization of thalamic cells to produce bursting and positive feedback. This may similarly be the case during the acute effects of drugs of abuse, which can trigger schizophrenia in individuals with a genetic predisposition to the disease (65). Cannabinoids, amphetamines, opioids, alcohol, and nicotine can enhance dopamine release (24, 25, 66–70).
3.2 Computer simulations of the psychotic break
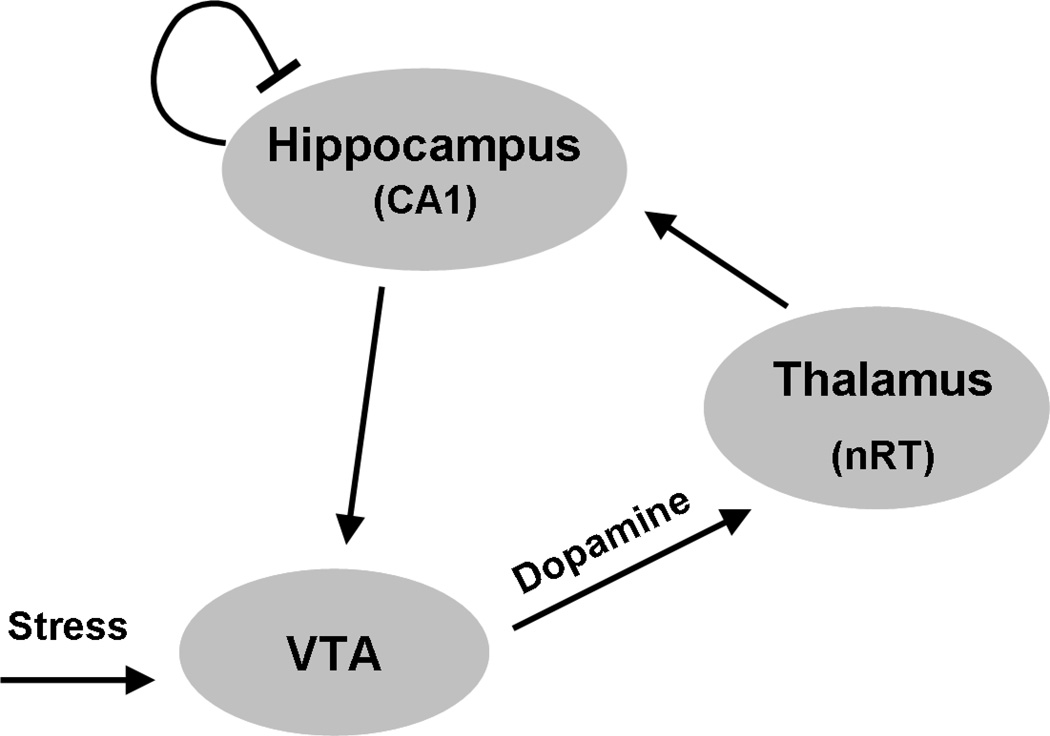
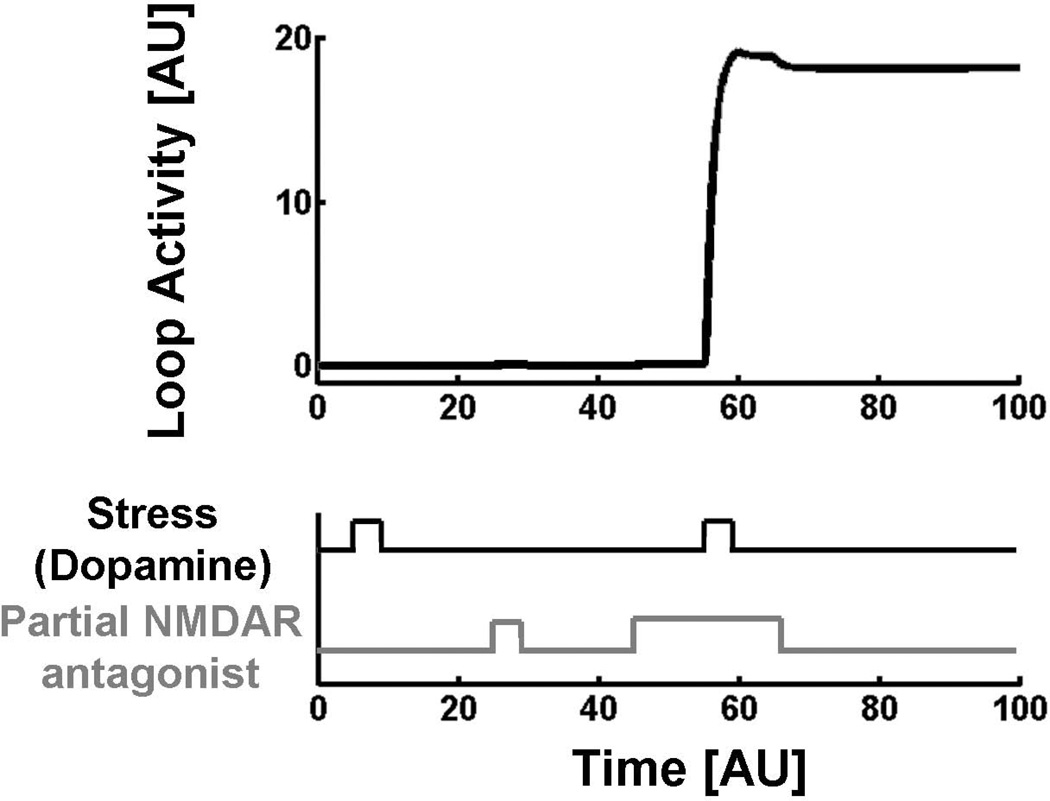
The bistable nature of the thalamo-hippocampal-VTA loop can be understood intuitively, but can also be demonstrated in the formal model (Fig. 3). The equations underlying the model are described in detail in Section S1 of Supplement 1. The results of the simulations are shown in Fig. 4. If only NMDAR block is present or only stress is present, loop activity stays very low. However, if there is a background of NMDAR block (which models a predisposition to schizophrenia), then the same stress trigger will throw the loop into positive feedback, producing maximal loop activity. This activity persists after stress is removed, thus demonstrating the bistable properties of the system under conditions of NMDAR block.
Figure 3.
Hippocampus (CA1), thalamus, and VTA form a bistable network in which each region stimulates the next region in the loop (arrows). Hippocampal firing is regulated by a feedback inhibition from interneurons. The nRT and relay cells of the thalamus are assumed to have similar bursting patterns.
Figure 4.
Computer simulations of loop activity in response to predisposition to schizophrenia (NMDAR hypofunction), stress, or the combination. Hyperactivity in the loop is reached when stress occurs in the presence of predisposition. Positive feedback maintains the activity even after the stress is removed, demonstrating bistability in the presence of predisposition. [AU] – Arbitrary units.
4. Relationship of the model to findings in schizophrenia
According to the model, the disease occurs when the thalamus, hippocampus, and VTA participate in a positive feedback loop in which all of these structures become hyperactive under basal conditions (i.e., without a task).
4.1 Evidence that the thalamus is involved in schizophrenia
Although the role of the thalamus in schizophrenia has been the subject of many metabolic imaging studies, most of these have measured task-dependent processes and have observed decreases in the resulting activation (71, 72). This may be due to higher basal activity in thalamus of patients (73). This is supported by studies that specifically measured activity under basal condition. These studies showed that patients with schizophrenia have higher basal activity than controls ((74–76), but see (77)). An increase in thalamic activity was observed in patients during auditory hallucinations (78).
4.2 Evidence that the hippocampus and, specifically, CA1 is hyperactive
Functional imaging (PET, SPECT) suggests that the hippocampus is hyperactive in the resting state in schizophrenia as compared to controls (74, 78–80) and less responsive to the task (79) or ketamine challenge (80). A recent study using high-resolution imaging of blood volume also shows elevated hippocampal activity. This study is notable in having sufficient resolution to study different hippocampal fields. The results show over-activity of CA1, but not CA3 and DG (81). These authors found that activity changed in some other brain regions, but only the CA1 abnormality correlated with symptoms’ severity (especially delusions). Moreover, the CA1 signal was predictive of development of psychosis in a prodromal group (81). Our model provides a simple explanation for why CA1, but not other hippocampal regions (e.g., CA3), is activated in schizophrenia: only the CA1 region is directly targeted by inputs from thalamus.
It is of interest that the importance of CA1 in schizophrenia resonates with findings on epileptic psychosis showing that the less severe the CA1 neuronal loss is, the higher the probability of psychosis is; when CA1 is destroyed, psychosis does not occur (82). Thus, epileptic psychosis also appears to be caused by abnormal signal generated by CA1.
4.3 Evidence that the dopaminergic system is overactive in schizophrenia
The initial suggestion for dopamine hyperfunction in schizophrenia was that the power of antipsychotic action of neuroleptics correlates with their ability to antagonize the D2 type of DA receptors. It was later shown that the dopamine synthesis and dopamine release due to amphetamine challenge are increased in patients with schizophrenia (reviewed in (83)). Finally, it was shown that baseline dopamine release is stronger in patients, directly confirming dopamine hyperfunction in schizophrenia (6, 84).
According to our model, the enhanced activity of the thalamus results from elevated dopamine levels. Consistent with this, multiple lines of evidence point to a thalamic dopamine abnormality in schizophrenia. Earlier postmortem analysis showed increased dopamine content in thalamus (85). Later in vivo PET studies found decreased D2-type dopamine receptor ligand binding in thalamus (86–88), suggesting that D2-type receptors may be already occupied by dopamine. The authors caution, however, that other interpretations (like decreased D2 receptor density) are possible. Most recent PET studies demonstrated increased thalamic dopamine synthesis (89) that correlated with the Positive and Negative Syndrome Scale (PANSS) scores of patients. Similarly, dopamine transporter binding was increased in the thalamus, and the increase correlated with PANSS scores (90). These data, therefore, not only point to a hyperdopaminergic state in thalamus in schizophrenia, but also suggest its relevance to the symptomology of the disease.
5. Limitations and additional experiments
We now discuss several limitations of the current model and point to areas of research that could answer outstanding questions. Although it has been shown that NMDAR antagonist can cause delta frequency bursting in the nRT (Fig.1B) and delta bursting in the thalamus (16, 17), there has been no systematic study of the firing patterns produced by NMDAR antagonist in various thalamic relay nuclei in rat. It remains to be determined whether the bursting in relay nuclei is produced by nRT–dependent processes or by the direct action of NMDAR antagonist. Based on c-Fos and 2-DG methods, it is clear that the overall activation of different relay nuclei is highly heterogeneous. It would be important to observe firing patterns in different thalamic nuclei. Understanding this heterogeneity may shed light on why the cortex is heterogeneously affected by NMDAR antagonist: notably, some human cortical areas are activated, whereas other show no change or even a decrease in metabolism (72, 75, 76, 78, 81). Furthermore, low-frequency EEG oscillations are not uniformly enhanced in schizophrenia but appear localized to particular frontal and temporal regions (18, 91). The specific symptoms of schizophrenia are likely to depend on which parts of the thalamocortical system are affected. According to the theory of (91, 92), region of cortex in which the low frequency oscillations occur lose function and account for the negative symptoms of the disease; because of loss of lateral inhibition, neighboring regions become overactive and account for the positive symptoms of the disease.
Another area of uncertainty regards the reason for the age dependence of the response to NMDAR antagonist, both in humans and animal models. The psychotic response to NMDAR blocker does not occur until late adolescence (reviewed in (93, 94)). The psychotic break in schizophrenia generally also occurs in late adolescence, and it is possible that common developmental mechanisms are involved. In animal studies, it was shown that NMDAR blocker-induced cortical damage is also age dependent, with onset at ~P45 (29, 93). This may be partially due to the development of vulnerability of cortical GABAergic interneurons (95). Some NMDAR blocker-dependent developmental markers are specific to thalamic relay nuclei (94). However, D2 and NMDAR antagonist-dependent nRT bursting was observed as early as P17 (15). This suggests that there may be age dependence in the response of relay cells to NMDAR antagonist or to the input from nRT. Further research will be required to clarify this important issue.
Our bistable model successfully captures some issues related to neuroleptic treatment, but not others. The model correctly accounts for the ability of D2-type dopamine antagonists to interfere with the loop and thereby exert therapeutic action. The model predicts that the effect of neuroleptic treatment should be essentially immediate. However, there are both immediate (96) and slower effects (97). The latter is not accounted for by our model. Because our model has bistability, it further predicts that, once having interfered with the positive feedback, neuroleptic could be removed without immediate restoration of the psychosis. The literature provides some support for this prediction: after stabilizing a patient with neuroleptic treatment, withdrawal of drug often does not cause immediate relapse. Instead, relapse can sometimes take weeks or months to occur (98) and may again be caused by another trigger such as stress. In these cases, schizophrenia can be appropriately described as a bistable system, with sharp and stable upward transition to the psychotic state and neuroleptic-induced downward transition to a more normal state. However, in other patients, relapse may be fast. There are thus several complexities of the disease that are not accounted for by our model.
The basic framework of the model can be further tested in several ways. As discussed in Section 4, the model predicts that, in psychotic state, all components of the loop should show basal hyperactivity in patients as compared to control. In particular, we would expect that thalamic nucleus reuniens (medioventral nucleus) and limbic portions of the nRT should be more active in patients as compared to controls. High-resolution studies may be able to test this prediction. The model also makes predictions that can be experimentally tested in animals. For instance, we expect that 1) strong bursting activity in nRT and nucleus reuniens in vivo should occur with systemic application of NMDAR antagonist and be reduced or blocked by intrathalamic D2 antagonist; 2) activation of the hippocampus by NMDAR antagonist should be greatly reduced by D2 antagonist or inactivation of nucleus reuniens; 3) NMDAR antagonist should fail to produce dopamine release after lesion of the hippocampus or blockade of its activity; 4) direct activation of nucleus reuniens into sustained bursting by electrical or optogenetic methods should lead to hippocampal activation and dopamine release.
Supplementary Material
Acknowledgements
This work was supported by NIH/NIMH Conte Center Grant 5P50 MH060450 and NIH/NIMH R01MH086518 grant. The authors greatly appreciate personal communication of yet unpublished results from Dr. Matthew W. Jones and the comments on the manuscripts from Drs. Joseph Coyle and Robert W. Greene.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Olney JW, Newcomer JW, Farber NB. NMDA receptor hypofunction model of schizophrenia. J Psychiatr Res. 1999;33(6):523–533. doi: 10.1016/s0022-3956(99)00029-1. [DOI] [PubMed] [Google Scholar]
- 2.Umbricht D, et al. Ketamine-induced deficits in auditory and visual context-dependent processing in healthy volunteers: implications for models of cognitive deficits in schizophrenia. Arch Gen Psychiatry. 2000;57(12):1139–1147. doi: 10.1001/archpsyc.57.12.1139. [DOI] [PubMed] [Google Scholar]
- 3.Coyle JT. Glutamate and schizophrenia: beyond the dopamine hypothesis. Cell Mol Neurobiol. 2006;26(4–6):365–384. doi: 10.1007/s10571-006-9062-8. [DOI] [PubMed] [Google Scholar]
- 4.Lisman JE, et al. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31(5):234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abi-Saab WM, D'Souza DC, Moghaddam B, Krystal JH. The NMDA antagonist model for schizophrenia: promise and pitfalls. Pharmacopsychiatry. 1998;31(Suppl 2):104–109. doi: 10.1055/s-2007-979354. [DOI] [PubMed] [Google Scholar]
- 6.Laruelle M, Kegeles LS, Abi-Dargham A. Glutamate, dopamine, and schizophrenia: from pathophysiology to treatment. Ann N Y Acad Sci. 2003;1003:138–158. doi: 10.1196/annals.1300.063. [DOI] [PubMed] [Google Scholar]
- 7.Javitt DC, Steinschneider M, Schroeder CE, Arezzo JC. Role of cortical N-methyl-D-aspartate receptors in auditory sensory memory and mismatch negativity generation: implications for schizophrenia. Proc Natl Acad Sci U S A. 1996;93(21):11962–11967. doi: 10.1073/pnas.93.21.11962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pilowsky LS, et al. First in vivo evidence of an NMDA receptor deficit in medication-free schizophrenic patients. Mol Psychiatry. 2006;11(2):118–119. doi: 10.1038/sj.mp.4001751. [DOI] [PubMed] [Google Scholar]
- 9.Benes FM. Altered glutamatergic and GABAergic mechanisms in the cingulate cortex of the schizophrenic brain. Arch Gen Psychiatry. 1995;52(12):1015–1018. doi: 10.1001/archpsyc.1995.03950240033007. discussion 1019–1024. [DOI] [PubMed] [Google Scholar]
- 10.Benes FM, et al. Regulation of the GABA cell phenotype in hippocampus of schizophrenics and bipolars. Proc Natl Acad Sci U S A. 2007;104(24):10164–10169. doi: 10.1073/pnas.0703806104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6(4):312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 12.Behrens MM, et al. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science. 2007;318(5856):1645–1647. doi: 10.1126/science.1148045. [DOI] [PubMed] [Google Scholar]
- 13.Cochran SM, Fujimura M, Morris BJ, Pratt JA. Acute and delayed effects of phencyclidine upon mRNA levels of markers of glutamatergic and GABAergic neurotransmitter function in the rat brain. Synapse. 2002;46(3):206–214. doi: 10.1002/syn.10126. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Behrens MM, Lisman JE. Prolonged exposure to NMDAR antagonist suppresses inhibitory synaptic transmission in prefrontal cortex. J Neurophysiol. 2008;100(2):959–965. doi: 10.1152/jn.00079.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Llinas RR, Lisman JE. Inhibition of NMDARs in the nucleus reticularis of the thalamus produces delta frequency bursting. Frontiers in Neural Circuits. 2009;3:20. doi: 10.3389/neuro.04.020.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buzsaki G. The thalamic clock: emergent network properties. Neuroscience. 1991;41(2–3):351–364. doi: 10.1016/0306-4522(91)90332-i. [DOI] [PubMed] [Google Scholar]
- 17.Miyasaka M, Domino EF. Neural mechanisms of ketamine-induced anesthesia. Int J Neuropharmacol. 1968;7(6):557–573. doi: 10.1016/0028-3908(68)90067-1. [DOI] [PubMed] [Google Scholar]
- 18.Boutros NN, et al. The status of spectral EEG abnormality as a diagnostic test for schizophrenia. Schizophr Res. 2008;99(1–3):225–237. doi: 10.1016/j.schres.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Floresco SB, Todd CL, Grace AA. Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons. J Neurosci. 2001;21(13):4915–4922. doi: 10.1523/JNEUROSCI.21-13-04915.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Legault M, Rompre PP, Wise RA. Chemical stimulation of the ventral hippocampus elevates nucleus accumbens dopamine by activating dopaminergic neurons of the ventral tegmental area. J Neurosci. 2000;20(4):1635–1642. doi: 10.1523/JNEUROSCI.20-04-01635.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46(5):703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Lodge DJ, Grace AA. Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J Neurosci. 2007;27(42):11424–11430. doi: 10.1523/JNEUROSCI.2847-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lodge DJ, Grace AA. Hippocampal dysfunction and disruption of dopamine system regulation in an animal model of schizophrenia. Neurotox Res. 2008;14(2–3):97–104. doi: 10.1007/BF03033801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lodge DJ, Grace AA. Amphetamine activation of hippocampal drive of mesolimbic dopamine neurons: a mechanism of behavioral sensitization. J Neurosci. 2008;28(31):7876–7882. doi: 10.1523/JNEUROSCI.1582-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lodge DJ, Grace AA. Gestational methylazoxymethanol acetate administration: a developmental disruption model of schizophrenia. Behav Brain Res. 2009;204(2):306–312. doi: 10.1016/j.bbr.2009.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tseng KY, Chambers RA, Lipska BK. The neonatal ventral hippocampal lesion as a heuristic neurodevelopmental model of schizophrenia. Behav Brain Res. 2009;204(2):295–305. doi: 10.1016/j.bbr.2008.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olney JW, Labruyere J, Price MT. Pathological changes induced in cerebrocortical neurons by phencyclidine and related drugs. Science. 1989;244(4910):1360–1362. doi: 10.1126/science.2660263. [DOI] [PubMed] [Google Scholar]
- 28.Vaisanen J, Ihalainen J, Tanila H, Castren E. Effects of NMDA-receptor antagonist treatment on c-fos expression in rat brain areas implicated in schizophrenia. Cell Mol Neurobiol. 2004;24(6):769–780. doi: 10.1007/s10571-004-6918-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakki R, Nickolenko J, Chang J, Sagar SM, Sharp FR. Haloperidol prevents ketamine- and phencyclidine-induced HSP70 protein expression but not microglial activation. Exp Neurol. 1996;137(2):234–241. doi: 10.1006/exnr.1996.0022. [DOI] [PubMed] [Google Scholar]
- 30.Kargieman L, Santana N, Mengod G, Celada P, Artigas F. Antipsychotic drugs reverse the disruption in prefrontal cortex function produced by NMDA receptor blockade with phencyclidine. Proc Natl Acad Sci U S A. 2007;104(37):14843–14848. doi: 10.1073/pnas.0704848104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tomitaka S, Tomitaka M, Tolliver BK, Sharp FR. Bilateral blockade of NMDA receptors in anterior thalamus by dizocilpine (MK-801) injures pyramidal neurons in rat retrosplenial cortex. Eur J Neurosci. 2000;12(4):1420–1430. doi: 10.1046/j.1460-9568.2000.00018.x. [DOI] [PubMed] [Google Scholar]
- 32.Farber NB, Jiang X, Dikranian K, Nemmers B. Muscimol prevents NMDA antagonist neurotoxicity by activating GABAA receptors in several brain regions. Brain Res. 2003;993(1–2):90–100. doi: 10.1016/j.brainres.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 33.Jones EG. Synchrony in the interconnected circuitry of the thalamus and cerebral cortex. Ann N Y Acad Sci. 2009;1157:10–23. doi: 10.1111/j.1749-6632.2009.04534.x. [DOI] [PubMed] [Google Scholar]
- 34.Llinas RR, Steriade M. Bursting of thalamic neurons and states of vigilance. J Neurophysiol. 2006;95(6):3297–3308. doi: 10.1152/jn.00166.2006. [DOI] [PubMed] [Google Scholar]
- 35.Llinas R, Jahnsen H. Electrophysiology of mammalian thalamic neurones in vitro. Nature. 1982;297(5865):406–408. doi: 10.1038/297406a0. [DOI] [PubMed] [Google Scholar]
- 36.Deakin JF, et al. Glutamate and the neural basis of the subjective effects of ketamine: a pharmaco-magnetic resonance imaging study. Arch Gen Psychiatry. 2008;65(2):154–164. doi: 10.1001/archgenpsychiatry.2007.37. [DOI] [PubMed] [Google Scholar]
- 37.Duncan GE, Miyamoto S, Leipzig JN, Lieberman JA. Comparison of brain metabolic activity patterns induced by ketamine, MK-801 and amphetamine in rats: support for NMDA receptor involvement in responses to subanesthetic dose of ketamine. Brain Res. 1999;843(1–2):171–183. doi: 10.1016/s0006-8993(99)01776-x. [DOI] [PubMed] [Google Scholar]
- 38.Cavdar S, et al. The pathways connecting the hippocampal formation, the thalamic reuniens nucleus and the thalamic reticular nucleus in the rat. J Anat. 2008;212(3):249–256. doi: 10.1111/j.1469-7580.2008.00858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McKenna JT, Vertes RP. Afferent projections to nucleus reuniens of the thalamus. J Comp Neurol. 2004;480(2):115–142. doi: 10.1002/cne.20342. [DOI] [PubMed] [Google Scholar]
- 40.Dolleman-Van der Weel MJ, Lopes da Silva FH, Witter MP. Nucleus reuniens thalami modulates activity in hippocampal field CA1 through excitatory and inhibitory mechanisms. J Neurosci. 1997;17(14):5640–5650. doi: 10.1523/JNEUROSCI.17-14-05640.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morales GJ, Ramcharan EJ, Sundararaman N, Morgera SD, Vertes RP. Analysis of the actions of nucleus reuniens and the entorhinal cortex on EEG and evoked population behavior of the hippocampus. Conf Proc IEEE Eng Med Biol Soc. 2007;2007:2480–2484. doi: 10.1109/IEMBS.2007.4352831. [DOI] [PubMed] [Google Scholar]
- 42.Bertram EH, Zhang DX. Thalamic excitation of hippocampal CA1 neurons: a comparison with the effects of CA3 stimulation. Neuroscience. 1999;92(1):15–26. doi: 10.1016/s0306-4522(98)00712-x. [DOI] [PubMed] [Google Scholar]
- 43.Hirayasu Y, Wada JA. N-methyl-D-aspartate injection into the massa intermedia facilitates development of limbic kindling in rats. Epilepsia. 1992;33(6):965–970. doi: 10.1111/j.1528-1157.1992.tb01745.x. [DOI] [PubMed] [Google Scholar]
- 44.Hosford DA, et al. Neural network of structures in which GABAB receptors regulate absence seizures in the lethargic (lh/lh) mouse model. J Neurosci. 1995;15(11):7367–7376. doi: 10.1523/JNEUROSCI.15-11-07367.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang X, et al. The circuitry of atypical absence seizures in GABA(B)R1a transgenic mice. Pharmacol Biochem Behav. 2009;94(1):124–130. doi: 10.1016/j.pbb.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 46.Otmakhova NA, Lisman JE. Dopamine Selectively Inhibits the Direct Cortical Pathway to the CA1 Hippocampal Region. J Neurosci. 1999;19(4):1437–1445. doi: 10.1523/JNEUROSCI.19-04-01437.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leutgeb S, Leutgeb JK, Treves A, Moser MB, Moser EI. Distinct ensemble codes in hippocampal areas CA3 and CA1. Science. 2004;305(5688):1295–1298. doi: 10.1126/science.1100265. [DOI] [PubMed] [Google Scholar]
- 48.Grunze HC, et al. NMDA-dependent modulation of CA1 local circuit inhibition. J Neurosci. 1996;16(6):2034–2043. doi: 10.1523/JNEUROSCI.16-06-02034.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bennett S, Gronier B. Effects of antipsychotic treatments and D-serine supplementation on the electrophysiological activation of midbrain dopamine neurons induced by the noncompetitive NMDA antagonist MK 801. Synapse. 2007;61(8):679–688. doi: 10.1002/syn.20413. [DOI] [PubMed] [Google Scholar]
- 50.Connelly ST, Shepard PD. Competitive NMDA receptor antagonists differentially affect dopamine cell firing pattern. Synapse. 1997;25(3):234–242. doi: 10.1002/(SICI)1098-2396(199703)25:3<234::AID-SYN2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 51.Zhang J, Chiodo LA, Freeman AS. Electrophysiological effects of MK-801 on rat nigrostriatal and mesoaccumbal dopaminergic neurons. Brain Res. 1992;590(1–2):153–163. doi: 10.1016/0006-8993(92)91091-r. [DOI] [PubMed] [Google Scholar]
- 52.Verma A, Moghaddam B. NMDA receptor antagonists impair prefrontal cortex function as assessed via spatial delayed alternation performance in rats: modulation by dopamine. J Neurosci. 1996;16(1):373–379. doi: 10.1523/JNEUROSCI.16-01-00373.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kretschmer BD. Modulation of the mesolimbic dopamine system by glutamate: role of NMDA receptors. J Neurochem. 1999;73(2):839–848. doi: 10.1046/j.1471-4159.1999.0730839.x. [DOI] [PubMed] [Google Scholar]
- 54.Lavin A, Grace AA. Dopamine modulates the responsivity of mediodorsal thalamic cells recorded in vitro. J Neurosci. 1998;18(24):10566–10578. doi: 10.1523/JNEUROSCI.18-24-10566.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lodge DJ, Behrens MM, Grace AA. A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. J Neurosci. 2009;29(8):2344–2354. doi: 10.1523/JNEUROSCI.5419-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bontempi B, Sharp FR. Systemic morphine-induced Fos protein in the rat striatum and nucleus accumbens is regulated by mu opioid receptors in the substantia nigra and ventral tegmental area. J Neurosci. 1997;17(21):8596–8612. doi: 10.1523/JNEUROSCI.17-21-08596.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Francois J, et al. Selective reorganization of GABAergic transmission in neonatal ventral hippocampal-lesioned rats. Int J Neuropsychopharmacol. 2009;12(8):1097–1110. doi: 10.1017/S1461145709009985. [DOI] [PubMed] [Google Scholar]
- 58.Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology (Berl) 2001;156(2–3):194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- 59.Corcoran C, et al. The stress cascade and schizophrenia: etiology and onset. Schizophr Bull. 2003;29(4):671–692. doi: 10.1093/oxfordjournals.schbul.a007038. [DOI] [PubMed] [Google Scholar]
- 60.Howes OD, et al. Pathways to schizophrenia: the impact of environmental factors. Int J Neuropsychopharmacol. 2004;7(Suppl 1):S7–S13. doi: 10.1017/S1461145704004122. [DOI] [PubMed] [Google Scholar]
- 61.Phillips LJ, et al. Stress, the hippocampus and the hypothalamic-pituitary-adrenal axis: implications for the development of psychotic disorders. Aust N Z J Psychiatry. 2006;40(9):725–741. doi: 10.1080/j.1440-1614.2006.01877.x. [DOI] [PubMed] [Google Scholar]
- 62.Anstrom KK, Miczek KA, Budygin EA. Increased phasic dopamine signaling in the mesolimbic pathway during social defeat in rats. Neuroscience. 2009;161(1):3–12. doi: 10.1016/j.neuroscience.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wanat MJ, Hopf FW, Stuber GD, Phillips PE, Bonci A. Corticotropin-releasing factor increases mouse ventral tegmental area dopamine neuron firing through a protein kinase C-dependent enhancement of Ih. J Physiol. 2008;586(8):2157–2170. doi: 10.1113/jphysiol.2007.150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ungless MA, et al. Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons. Neuron. 2003;39(3):401–407. doi: 10.1016/s0896-6273(03)00461-6. [DOI] [PubMed] [Google Scholar]
- 65.Schuckit MA. Comorbidity between substance use disorders and psychiatric conditions. Addiction. 2006;101(Suppl 1):76–88. doi: 10.1111/j.1360-0443.2006.01592.x. [DOI] [PubMed] [Google Scholar]
- 66.Carlezon WA, Jr, Thomas MJ. Biological substrates of reward and aversion: a nucleus accumbens activity hypothesis. Neuropharmacology. 2009;56(Suppl 1):122–132. doi: 10.1016/j.neuropharm.2008.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cowen MS, Lawrence AJ. The role of opioid-dopamine interactions in the induction and maintenance of ethanol consumption. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23(7):1171–1212. doi: 10.1016/s0278-5846(99)00060-3. [DOI] [PubMed] [Google Scholar]
- 68.Gardner EL. Endocannabinoid signaling system and brain reward: emphasis on dopamine. Pharmacol Biochem Behav. 2005;81(2):263–284. doi: 10.1016/j.pbb.2005.01.032. [DOI] [PubMed] [Google Scholar]
- 69.Self DW. Regulation of drug-taking and -seeking behaviors by neuroadaptations in the mesolimbic dopamine system. Neuropharmacology. 2004;47(Suppl 1):242–255. doi: 10.1016/j.neuropharm.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 70.Wise RA. Roles for nigrostriatal--not just mesocorticolimbic--dopamine in reward and addiction. Trends Neurosci. 2009;32(10):517–524. doi: 10.1016/j.tins.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Buehlmann E, et al. Hippocampus abnormalities in at risk mental states for psychosis? A cross-sectional high resolution region of interest magnetic resonance imaging study. J Psychiatr Res. 2009 doi: 10.1016/j.jpsychires.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 72.Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66(8):811–822. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fukumoto-Motoshita M, et al. Hyperfrontality in patients with schizophrenia during saccade and antisaccade tasks: a study with fMRI. Psychiatry Clin Neurosci. 2009;63(2):209–217. doi: 10.1111/j.1440-1819.2009.01941.x. [DOI] [PubMed] [Google Scholar]
- 74.Malaspina D, et al. Resting neural activity distinguishes subgroups of schizophrenia patients. Biol Psychiatry. 2004;56(12):931–937. doi: 10.1016/j.biopsych.2004.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ben-Shachar D, et al. Cerebral glucose utilization and platelet mitochondrial complex I activity in schizophrenia: A FDG-PET study. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(4):807–813. doi: 10.1016/j.pnpbp.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 76.Soyka M, Koch W, Moller HJ, Ruther T, Tatsch K. Hypermetabolic pattern in frontal cortex and other brain regions in unmedicated schizophrenia patients. Results from a FDG-PET study. Eur Arch Psychiatry Clin Neurosci. 2005;255(5):308–312. doi: 10.1007/s00406-005-0563-0. [DOI] [PubMed] [Google Scholar]
- 77.Potkin SG, et al. A PET study of the pathophysiology of negative symptoms in schizophrenia. Positron emission tomography. Am J Psychiatry. 2002;159(2):227–237. doi: 10.1176/appi.ajp.159.2.227. [DOI] [PubMed] [Google Scholar]
- 78.Silbersweig DA, et al. A functional neuroanatomy of hallucinations in schizophrenia. Nature. 1995;378(6553):176–179. doi: 10.1038/378176a0. [DOI] [PubMed] [Google Scholar]
- 79.Heckers S, et al. Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nat Neurosci. 1998;1(4):318–323. doi: 10.1038/1137. [DOI] [PubMed] [Google Scholar]
- 80.Medoff DR, Holcomb HH, Lahti AC, Tamminga CA. Probing the human hippocampus using rCBF: contrasts in schizophrenia. Hippocampus. 2001;11(5):543–550. doi: 10.1002/hipo.1070. [DOI] [PubMed] [Google Scholar]
- 81.Schobel SA, et al. Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Arch Gen Psychiatry. 2009;66(9):938–946. doi: 10.1001/archgenpsychiatry.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Suckling J, et al. Temporal lobe epilepsy with and without psychosis: exploration of hippocampal pathology including that in subpopulations of neurons defined by their content of immunoreactive calcium-binding proteins. Acta Neuropathol. 2000;99(5):547–554. doi: 10.1007/s004010051159. [DOI] [PubMed] [Google Scholar]
- 83.Carlsson A, et al. Interactions between monoamines, glutamate, and GABA in schizophrenia: new evidence. Annu Rev Pharmacol Toxicol. 2001;41:237–260. doi: 10.1146/annurev.pharmtox.41.1.237. [DOI] [PubMed] [Google Scholar]
- 84.Abi-Dargham A, et al. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci U S A. 2000;97(14):8104–8109. doi: 10.1073/pnas.97.14.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Oke AF, Putz C, Adams RN, Bird ED. Neuroleptic treatment is an unlikely cause of elevated dopamine in thalamus of schizophrenic subjects. Psychiatry Res. 1992;45(4):203–208. doi: 10.1016/0925-4927(92)90015-v. [DOI] [PubMed] [Google Scholar]
- 86.Buchsbaum MS, et al. D2/D3 dopamine receptor binding with [F-18]fallypride in thalamus and cortex of patients with schizophrenia. Schizophr Res. 2006;85(1–3):232–244. doi: 10.1016/j.schres.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 87.Talvik M, et al. Dopamine D2 receptor binding in drug-naive patients with schizophrenia examined with raclopride-C11 and positron emission tomography. Psychiatry Res. 2006;148(2–3):165–173. doi: 10.1016/j.pscychresns.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 88.Kessler RM, et al. Dopamine D2 receptor levels in striatum, thalamus, substantia nigra, limbic regions, and cortex in schizophrenic subjects. Biol Psychiatry. 2009;65(12):1024–1031. doi: 10.1016/j.biopsych.2008.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nozaki S, et al. Regional dopamine synthesis in patients with schizophrenia using L-[beta-11C]DOPA PET. Schizophr Res. 2009;108(1–3):78–84. doi: 10.1016/j.schres.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 90.Arakawa R, et al. Increase in thalamic binding of [(11)C]PE2I in patients with schizophrenia: a positron emission tomography study of dopamine transporter. J Psychiatr Res. 2009;43(15):1219–1223. doi: 10.1016/j.jpsychires.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 91.Llinas R, Urbano FJ, Leznik E, Ramirez RR, van Marle HJ. Rhythmic and dysrhythmic thalamocortical dynamics: GABA systems and the edge effect. Trends Neurosci. 2005;28(6):325–333. doi: 10.1016/j.tins.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 92.Llinas RR, Ribary U, Jeanmonod D, Kronberg E, Mitra PP. Thalamocortical dysrhythmia: A neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc Natl Acad Sci U S A. 1999;96(26):15222–15227. doi: 10.1073/pnas.96.26.15222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Farber NB, et al. Age-specific neurotoxicity in the rat associated with NMDA receptor blockade: potential relevance to schizophrenia? Biol Psychiatry. 1995;38(12):788–796. doi: 10.1016/0006-3223(95)00046-1. [DOI] [PubMed] [Google Scholar]
- 94.Takebayashi H, Yamamoto N, Umino A, Nishikawa T. Developmentally regulated and thalamus-selective induction of leiomodin2 gene by a schizophrenomimetic, phencyclidine, in the rat. Int J Neuropsychopharmacol. 2009;12(8):1111–1126. doi: 10.1017/S1461145709009997. [DOI] [PubMed] [Google Scholar]
- 95.Lema Tome CM, et al. Decline in age-dependent, MK801-induced injury coincides with developmental switch in parvalbumin expression: somatosensory and motor cortex. Dev Psychobiol. 2008;50(7):665–679. doi: 10.1002/dev.20325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophr Bull. 2009;35(3):549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Grace AA, Bunney BS, Moore H, Todd CL. Dopamine-cell depolarization block as a model for the therapeutic actions of antipsychotic drugs. Trends Neurosci. 1997;20(1):31–37. doi: 10.1016/S0166-2236(96)10064-3. [DOI] [PubMed] [Google Scholar]
- 98.Viguera AC, Baldessarini RJ, Hegarty JD, van Kammen DP, Tohen M. Clinical risk following abrupt and gradual withdrawal of maintenance neuroleptic treatment. Arch Gen Psychiatry. 1997;54(1):49–55. doi: 10.1001/archpsyc.1997.01830130055011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.