Obstructive sleep apnea (OSA) has serious cardiovascular consequences, and increases the risk of stroke.1,2 OSA is common in stroke patients3,4 and is associated with impaired quality of life, reduced cognitive function, and excessive fatigue,5-7 conditions which are common in stroke victims and that may delay post-stroke recovery.8-10 It is possible that treating OSA could improve clinical recovery in stroke patients.
Continuous positive airway pressure (CPAP) is the ‘gold standard’ treatment for OSA; however, there have been few studies of CPAP use in patients post stroke, and the ability for CPAP to definitively improve outcomes has yet to be established in this population. Part of the motivation for this review stems from our experience with a clinical trial that closed due to futility.1 As a “post-mortem” on the trial’s closure, we scrutinized the literature, summarizing outcome data in the area and considering recruitment experience in similar trials. Finally, we comment on possible study design characteristics that might make future trials more successful.
CPAP Treatment in Stroke Survivors
Number of studies
A PUBMED search up to November, 2011 using the terms stroke OR transient ischemic attack AND apnea AND continuous positive airway pressure revealed 17 published studies (See Online Supplement, Table 1). Studies were heterogeneous with regards to timing of treatment onset, follow-up assessment timing, and outcomes studied, making a meta-analysis inappropriate; therefore, we conducted a systematic qualitative review of the literature.
Nine studies were observational,11-19 three of which examined the same cohort of patients over time.16-18 Six studies randomized patients to CPAP versus treatment as usual (TAU),20-26 and one study randomized patients to CPAP or sham CPAP.27 On average, studies followed a small number of patients: observational studies included a median of 22 patients who had been prescribed CPAP treatment and randomized controlled trials (RCTS) included a median of 50 patients who were randomized to CPAP or control.
Timing of CPAP Intervention
It is conceivable that findings may be influenced by the timing of the CPAP intervention vis-a-vis the stroke onset. All studies were initiated in acute hospital stroke units or rehabilitation units. There was variability in the time between stroke and study enrollment, with patients recruited between 48 hours – 2 months post stroke. Studies also varied widely in length, with follow-up periods ranging from 1 night to 7 years.14,16
Outcomes
Table 1 (Online Supplement) includes a detailed review of duration, main outcomes and findings from each of the studies included in this review.
Recruitment, Acceptability and Adherence
Eight studies investigated treatment adherence and/or acceptability as a primary outcome, with the duration of trials ranging from 1 night to 60 months.11-15,19,20,23 Common theme across studies was that CPAP recruitment was challenging and adherence at follow-up was poor. In short-term observational studies (1 night – 2 months), 50-100% of subjects prescribed CPAP were described as adherent throughout the study period.12-15 However, observational studies with longer-term follow-up periods (18 months – 7 years) reported significantly higher attrition, with adherence at follow-up ranging from 8 – 29%.11,16-18
Recruitment into randomized studies appeared particularly challenging; investigators reported screening hundreds of potential participants, but enrolling relatively low percentages. Of participants who were considered for inclusion into an RCT, 70% had at least one exclusionary criteria.20-22,26,27 When approached, 55% of patients without an exclusionary criteria refused to participate.20-22,26,27 Of the participants randomized to CPAP intervention, for which adherence data was available, (N = 165), 48% of those assigned to CPAP were adherent at the last assessment point. (See Online Supplement, Tables 2 and 3).
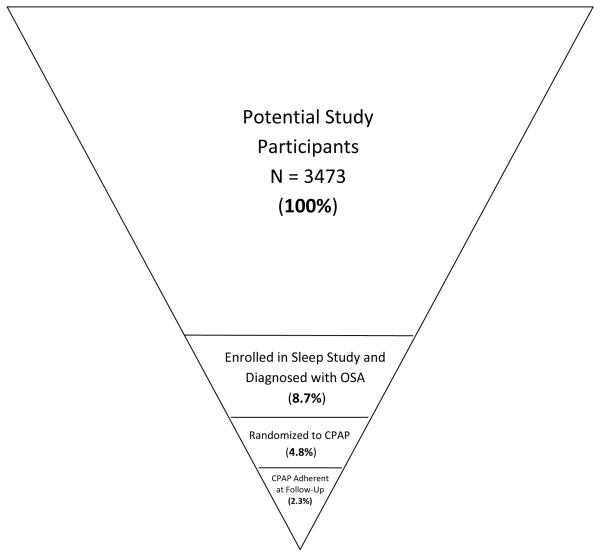
Randomized studies ranged from 1-3 months in duration and the total percentage of participants who underwent screening and were available for follow-up was typically 4 – 13%.20-24,26 Of the 3,473 participants screened in the randomized clinical trial (RCT) studies, only 2.3% were CPAP adherent at the last assessment point (See Figure 1). Figure 1 illustrates the difficulty associated with isolating a patient population that is eligible, willing to participate in a study, and compliant with the rigors of this type of treatment study.
Figure 1.
Recruitment and Retention in Randomized Studies. Studies were included in the summary if they reported data for each time point. Notably, the 8.7% of stroke patients enrolled in a sleep study and diagnosed with OSA reflects the difficulty of recruiting patients into randomized studies, not the prevalence of OSA in this population.
OSA = obstructive sleep apnea; CPAP = continuous positive airway pressure.
The literature offers little guidance as to “why” the adherence is so poor. The most commonly cited factors associated with poor adherence were post-stroke related neurologic impairments, patient dependency and lack of an available caregiver to assist with CPAP use.13-15,17,22,24 Mask discomfort, subjective sleep disturbance and claustrophobia were also listed by several investigators as reasons for non-adherence.14,15,22 In some studies, increased pre-treatment sleepiness was associated with better adherence;14,17 however, this finding was not consistently found.15 Finally, one study reported that higher depressive symptoms were associated with worse CPAP adherence.22
Cardiovascular Events after Stroke
Three studies investigated whether CPAP use was associated with reductions in subsequent cardiovascular events and mortality. One observational study found that in stroke patients with OSA, CPAP non-adherence was associated with a significant increase in new vascular events in comparison to those who adhered to CPAP or did not have OSA.16,17 Additionally, patients who were non-adherent to CPAP had an increased risk of mortality at 5-year follow-up compared to the other groups 18 One RCT found no differences in vascular events between patients randomized to CPAP versus TAU after 3-months.20 Contrastingly, another RCT found that CPAP versus TAU was associated with a longer time to next cardiovascular event (14.9 versus 7.9 months); however, cardiovascular mortality was similar between groups at 2-year follow-up.25
Neurological and Psychological Recovery from Stroke
Four studies investigated whether CPAP treatment was associated with improved neurological recovery from stroke, improvements in activities of daily living (ADL), or reductions in sleepiness and depression. In two studies, CPAP compared to TAU was not associated with significant gains in cognitive functioning (assessed with the Mini-Mental Status Exam or Addenbrooke’s Cognitive Examination).22,24 One study reported that CPAP treatment compared to TAU over one-month was associated with improvements in stroke-related impairments (Canadian Neurologic Scale), but not in the 6-minute walk test, attention or executive functioning.26 One study found that CPAP treatment over one-month compared to TAU was associated with better stroke-related recovery (assessed by the National Institute of Health Stroke Scale (NIHSS)).21 Finally, one-month post stroke, CPAP compared to TAU was associated with greater neurological improvement, assessed by the Rankin and Canadian scales, but these differences were indiscernible at 24-month follow-up.25 Three studies comparing CPAP to TAU failed to find improvements in ADLs assessed with the Barthel Index.22,24,25
CPAP compared to TAU was not associated with significant changes in sleepiness or subjective health status in one study.22 Similarly, no difference in sleepiness or depressive symptoms were observed in patients randomized to CPAP or sham CPAP over three months.27 In contrast, one study found that compared to TAU, CPAP treatment over one month was associated with an improvement in depressive symptoms.24 Another investigation found that CPAP treatment over one-month compared to TAU was associated with improvements in sleepiness and the affective component of depression.26
Discussion
This review suggests that the benefits of CPAP treatment in stroke patients are far from clear. There is some encouraging preliminary evidence that CPAP use for longer periods of time (1.5, 2 and 7-years) may be associated with delayed onset of new cardiovascular incidents post-stroke;16,17,25 however, none of the studies examining cardiovascular outcomes utilized an active control group. The question also remains-- was higher patient impairment at study onset associated with both non-adherence and negative health consequences in the observational studies?
Disappointingly, no studies found that CPAP was associated with improvements in ADLs over 1-3 month follow-up periods and it is unsettled whether CPAP use improves neurological recovery post stroke.22,24,27 It may be that it is inappropriate to use brief and/or broad measures of neurological recovery as the marker of treatment efficacy (e.g., mental status exams). For instance, visual field cuts or hemiparesis may never respond to CPAP, whereas one could speculate that executive function may improve. Lack of consistent significant findings regarding cognitive tests may reflect differing levels of sensitivity of the tests across studies; more nuanced neuropsychological testing might reveal aspects of functioning that CPAP affects.
The evidence regarding CPAP’s ability to improve sleepiness and depression after stroke was also mixed. Given the heterogeneity of stroke patients and the complicated milieu of comorbid medical and psychological problems affecting the population, it may be that specific patient subsets are more likely to show improvements in sleepiness/depression with CPAP use. This same logic likely pertains to cardiovascular and neuropsychological changes.
Enrollment and patient retention was a major challenge. Of the over 3,000 patients approached to participate in RCTs investigating the effects of CPAP for OSA post-stroke, less than 3% were adherent to CPAP at study conclusion (see Figure 1). Stroke related impairment, mask discomfort, sleep disturbances and claustrophobia were common reasons for non-adherence. Additionally, in our trial we found many stroke patients refused initial treatment because they were too overwhelmed by the stroke and recovery activities.
Given the inconsistent findings, the potential selection bias of patients electing to comply with CPAP (e.g., potentially less serious stroke impairments, fewer communication barriers) and the known impact of placebo on outcomes, it is still unclear whether CPAP is associated with improved stroke-related cardiovascular, psychological and neuropsychological outcomes.
Power
An issue that limited many studies was lack of available power. For example, Bravata, et al (2010) noted a trend in their data, which suggested that the vascular event rate was lower in participants using CPAP compared to controls; they also noted that increasing CPAP adherence was associated with a lower event rate (p=0.08). 20 Similarly, Parra, et al (2011) reported that cardiovascular mortality rates were lower in the CPAP arm than the control group (p = 0.16).25 However, both sets of authors commented that they were underpowered to detect a significant change in the outcome variable. Small sample sizes and associated power limitations cloud the interpretation of many of the null findings reported in the literature. Unfortunately, the need for increased sample sizes is made difficult by issues of recruitment and retention.
Future Directions
This review raises many questions about the state of the research and how to proceed with future trials. A vast number of patients were screened across studies, yielding relatively low enrollment and completion numbers. Such recruitment challenges are typical for RCTs in stroke patients.28 For instance, in a meta-analysis of acute stroke trials the recruitment rate across North America was only 0.57 patients per month.29
Strategic Enrollment
Given the low enrollment, sample heterogeneity makes it difficult to observe effects. In small trials, imbalance in key covariates between treatment arms (e.g., severity of stroke) could confound treatment effects. A standard approach to ensure balanced randomization arms is to stratify randomization on key covariates. However this is only feasible if the number of strata is small, which may not be the case in stroke studies where many patient factors could influence outcomes. A possibly better alternative for sleep treatment trials in stroke patients would be to use covariate-adaptive randomization,30 where a participant’s treatment allocation is based on the covariate distributions observed thus far.
Stroke patients are often hesitant to participate in a sleep study. Given there is so much reluctance, it may be possible that one can screen, preliminarily, for the existence of pre-existing OSA, by querying the stroke patient’s spouse or bed partner.
CPAP may not be the appropriate treatment for everyone with OSA after stroke. If a target population that can manage the device could be identified, the field could then begin to study the characteristics that define people who have the highest likelihood of benefiting. Even if the target population is restricted on clinical grounds, there may be so much variability in baseline and in changes in outcome variables that it is difficult to show statistically significant differences versus TAU or sham CPAP. Since it is not clear that patients with certain stroke subtype or location are more or less likely to benefit from OSA intervention, it may not yet be possible to focus on a population that has the best chance of responding to the intervention. Thus, large sample sizes with high treatment adherence at study completion are necessary.
Even in studies with relatively liberal inclusionary criteria, patients with significant neurological deficits such as neglect or language impairment may not be able to adhere to the therapy or be able to consent for such studies. Furthermore, the degree of such patients’ communication impairments would make it difficult to obtain any but the coarsest of characterizations of quality of life and functioning.
Multicenter Enrollment
Given the limitations of expected tolerability and patient availability, no single center is likely to recruit a large enough sample. Although a number of design modifications may be able to increase yield, future studies will need access to a large number of stroke survivors. Given our experience and those reported in various published studies, one may assume that only 5-10% of stroke patients may be willing to be in such a study. The rest would be ineligible due to factors such as severity of stroke or seriously confounding other illnesses/conditions (e.g. dementia) or would be unwilling to enroll in a study that was interpreted as having questionable benefit.
Diagnosis
There is a difference of opinion in the field whether a full polysomnogram (PSG) is necessary to diagnose OSA or whether a more abbreviated sleep study may be adequate. A full PSG provides precise information on sleep characteristics, which might answer some research questions, but the full PSG imposes greater response burden on patients. We have seen patients who were feeling so overwhelmed by their stroke and so tired from rehabilitation activities that they refused a PSG because of their fear that it would leave them all the more exhausted.
Treatment Comparison
Interestingly, the type of comparison treatment selected for the contrast with CPAP may affect enrollment. While a placebo-CPAP intervention may be the optimal theoretical placebo comparison in terms of treatment expectancy, informing potential stroke patients that they may be randomized to a placebo-CPAP intervention may just be “too much” for them to consider, given the patient burden of CPAP treatment. In comparison, studies that merely randomized patients to receive either CPAP or standard stroke rehabilitation may be more acceptable to patients. Alternatively, OSA treatment devices such as dental retainers may be better tolerated and could potentially serve as an alternative treatment.
Treatment Choices
There may be subtle but important problems in fitting CPAP masks to stroke patients. It is conceivable that stroke patients will have problems with mouth leaks if nasal or nasal pillow CPAP masks are used, due to frequent facial hemiparesis after a stroke. In such cases, the use of a full-face CPAP mask that covers nose and mouth or a total facemask that covers the entire face (eyes, nose, mouth) may be more efficacious. It also makes sense that simpler systems requiring less complex motor skills would be easier for stroke patients to utilize and there is some evidence that a 1-piece head frame system versus traditional strap headgear is easier for patients to use.31
It is unclear if CPAP or bilevel continuous airway pressure (BPAP) would be more effective or tolerable for stroke survivors with hemiplegia who often have weakness of the diaphragm and accessory muscle of respiration, including the abdominal muscles, which results in a restrictive respiratory pattern, hypoventilation and often hypoxia.32,33 The sensation of not being able to easily exhale is a common complaint of new CPAP users that, if not addressed, often results in CPAP intolerance. Some OSA patients tolerate a BPAP set-up better, since it gives the sensation that exhalation is easier. Evaluation of respiratory function post stroke is not routine. However, in the setting of OSA and stroke, evaluation of respiratory muscle strength and respiratory function may be useful to guide the clinician in the choice of CPAP or BPAP to treat OSA in this population; however, there is no prior research to guide recommendations.
Adherence
Adherence with CPAP is a challenge and appears to be particularly difficult for stroke patients. First off, the patient needs to be able to perceive a benefit to treatment. Stroke patients may be just too overwhelmed by their strokes to consider short- or long-term benefits of CPAP. In addition, problems with mask fitting or the ability to manage the device is a likely barrier for many patients.
Adherence of another kind—staff and caregiver compliance with CPAP—can also be a challenge on a rehabilitation unit. Nursing staff on such units may be relatively unfamiliar and resistant to CPAP apparatus at the bedside. Careful in-service training is necessary to insure that the prescribed CPAP is actually offered to the patient each night. Staff hesitations in the arenas of time constraints, lack of perceived benefits and/or concerns about patient burden may translate into poorer compliance on the part of the patient. Of equal importance is the education that must be provided to caregivers once the stroke patient is discharged from the hospital. Given the widespread impairments associated with many strokes, it may be unrealistic to expect that patients will continue CPAP use on their own after discharge. For this reason, it will be important to educate caregivers about CPAP and to understand what types of support they need to maximize their compliance with CPAP administration.
Conclusions
Stroke is a heterogeneous disease extending across various ages, comorbidities and stroke severity. Perhaps at the far extreme of severity, CPAP may do little to improve functional outcomes and quality of life. However, at this point, we do not know whether stroke survivors with differing levels of impairment (mild, severe) may benefit differentially from treatment. In the future, studies of recovery will need to be powered to recognize this heterogeneity as well as the heterogeneity of the patient’s other health conditions that may affect cognitive and quality of life indicators. It is possible for instance that certain types of strokes may be associated with greater or lesser benefits from CPAP. It is also uncertain if there might be an optimal time in terms of stroke onset for providing CPAP. As a practical matter, severe cognitive or sensorimotor impairments may preclude managing CPAP on one’s own, so ability to even attempt the treatment may improve after initial stroke recovery. Similarly, it is not established how long treatment should continue, although CPAP is typically prescribed for chronic treatment.
OSA is common in stroke patients, and CPAP use entails substantial subject burden; however, the question remains unanswered whether stroke patients will in fact even utilize CPAP once they are out of the hospital. This leads to a Catch-22: until it is clearer that CPAP can be shown to enhance outcomes after stroke, it is unlikely that patients will be motivated to try this intervention. Yet it is difficult to show benefit if researchers or clinicians cannot get sufficient numbers of stroke patients to wear CPAP long enough to show improvements. If anything, findings from this review demonstrate the importance of controlled comparisons. Since so many questions remain unanswered, multi-site studies with large numbers of patients may be the only way to find out if CPAP helps these patients.
Supplementary Material
Acknowledgments
Sources of Funding This work was supported by grants HL091848 (JED) and Canadian Institute of Health Research (LMT)
Footnotes
Burden of obstructive sleep apnea in stroke (BOSAST), NCT00952211, HL091848
Conflicts of Interest/Disclosures Ms. Tomfohr was the recipient of a Canadian Institute of Health Research (CIHR) doctoral grant linked to the BOSAST trial. Dr. Hemmen is a co-investigator for the STOP IT trial grant funded by the National Institute of Health (NIH), he is also involved with Genentech in a study of tPA use in patients who awaken after stroke. Dr. Hemmen has served as an expert witness in cases related to stroke, and is on the advisory board of Genentech and Boehringer Ingelheim. Dr. Ancoli-Israel is a consultant for Astra Zeneca, Ferring Pharmaceuticals Inc., GlaxoSmithKline, Hypnocore, Johnson & Johnson, Merck, NeuroVigil, Inc., Pfizer, Philips, Purdue Pharma LP, and Sanofi-Aventis. Dr. Loredo has served as an expert witness in legal cases associated with sleep disorders. Dr.’s Hemmen, Natarajan, Ancoli-Israel, Loredo, Heaton, Bardwell, and Lee were co-investigator’s on the BOSAST trial, funded by NIH. Dr. Dimsdale was the principal investigator on the BOSAST trial.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- (1).McNicholas WT, Javaheri S. Pathophysiologic mechanisms of cardiovascular disease in obstructive sleep apnea. Sleep Med Clin. 2007;2:539–547. [Google Scholar]
- (2).Lopez-Jimenez F, Kuniyoshi FHS, Gami A, Somers VK. Obstructive Sleep Apnea. Chest. 2008;133:793–804. doi: 10.1378/chest.07-0800. [DOI] [PubMed] [Google Scholar]
- (3).Johnson KG, Johnson DC. Frequency of sleep apnea in stroke and TIA patients: a meta-analysis. J Clin Sleep Med. 2010;6:131–137. [PMC free article] [PubMed] [Google Scholar]
- (4).Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. J Am Coll Cardiol. 2008;52:686–717. doi: 10.1016/j.jacc.2008.05.002. [DOI] [PubMed] [Google Scholar]
- (5).Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- (6).Jackson ML, Howard ME, Barnes M. Cognition and daytime functioning in sleep-related breathing disorders. Prog Brain Res. 2011;190:53–68. doi: 10.1016/B978-0-444-53817-8.00003-7. [DOI] [PubMed] [Google Scholar]
- (7).Chervin RD. Sleepiness, fatigue, tiredness, and lack of energy in obstructive sleep apnea. Chest. 2000;118:372–379. doi: 10.1378/chest.118.2.372. [DOI] [PubMed] [Google Scholar]
- (8).Christensen D, Johnsen SP, Watt T, Harder I, Kirkevold M, Andersen G. Dimensions of post-stroke fatigue: a two-year follow-up study. Cerebrovas Dis. 2008;26:134–141. doi: 10.1159/000139660. [DOI] [PubMed] [Google Scholar]
- (9).Oksala N, Jokinen H, Melkas S, Oksala A, Pohjasvaara T, Hietanen M, et al. Cognitive impairment predicts poststroke death in long-term follow-up. J Neurol Neurosurg Ps. 2009;80:1230–1235. doi: 10.1136/jnnp.2009.174573. [DOI] [PubMed] [Google Scholar]
- (10).Pasquini M, Leys D, Rousseaux M, Pasquier F, Hénon H. Influence of cognitive impairment on the institutionalisation rate 3 years after a stroke. J Neurol Neurosurg Ps. 2007;78:56–59. doi: 10.1136/jnnp.2006.102533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Bassetti CL, Milanova M, Gugger M. Sleep-disordered breathing and acute ischemic stroke: Diagnosis, risk factors, treatment, evolution, and long-term clinical outcomes. Stroke. 2006;37:967–972. doi: 10.1161/01.STR.0000208215.49243.c3. [DOI] [PubMed] [Google Scholar]
- (12).Disler P, Hansford A, Skelton J, Wright P, Kerr J, O’Reilly J, et al. Diagnosis and treatment of obstructive sleep apnea in a stroke rehabilitation unit: a feasibility study. Am J Physical Med Rehab. 2002;81:622–625. doi: 10.1097/00002060-200208000-00011. [DOI] [PubMed] [Google Scholar]
- (13).Palombini L, Guilleminault C. Stroke and treatment with nasal CPAP. Eur J Neurol. 2006;13:198–200. doi: 10.1111/j.1468-1331.2006.01169.x. [DOI] [PubMed] [Google Scholar]
- (14).Scala R, Turkington PM, Wanklyn P, Bamford J, Elliott MW. Acceptance, effectiveness and safety of continuous positive airway pressure in acute stroke: a pilot study. Respir Med. 2009;103:59–66. doi: 10.1016/j.rmed.2008.08.002. [DOI] [PubMed] [Google Scholar]
- (15).Wessendorf T, Wang Y, Thilmann A, Sorgenfrei U, Konietzko N, Teschler H. Treatment of obstructive sleep apnoea with nasal continuous positive airway pressure in stroke. Eur Resp J. 2001;18:623–629. doi: 10.1183/09031936.01.00057201. [DOI] [PubMed] [Google Scholar]
- (16).Martínez-García M, Campos-Rodríguez F, Soler-Cataluña J, Catalán-Serra P, Román-Sánchez P, Montserrat J. Increased incidence of non-fatal cardiovascular events in stroke patients with sleep apnoea. Effect of CPAP treatment: A 7-year follow-up study. Eur Resp J. 2011 doi: 10.1183/09031936.00011311. epub. [DOI] [PubMed] [Google Scholar]
- (17).Martínez-García MÁ, Galiano-Blancart R, Román-Sánchez P, Soler-Cataluña JJ, Cabero-Salt L, Salcedo-Maiques E. Continuous positive airway pressure treatment in sleep apnea prevents new vascular events after ischemic stroke. Chest. 2005;128:2123–2129. doi: 10.1378/chest.128.4.2123. [DOI] [PubMed] [Google Scholar]
- (18).Martínez-García MÁ, Soler-Cataluna JJ, Ejarque-Martinez L, Soriano Y, Roman-Sanchez P, Illa FB, et al. Continuous positive airway pressure treatment reduces mortality in patients with ischemic stroke and obstructive sleep apnea: a 5-year follow-up study. Am J Respir Crit Care Med. 2009;180:36–41. doi: 10.1164/rccm.200808-1341OC. [DOI] [PubMed] [Google Scholar]
- (19).Broadley SA, Jorgensen L, Cheek A, Salonikis S, Taylor J, Thompson PD, et al. Early investigation and treatment of obstructive sleep apnoea after acute stroke. J Clin Neurosci. 2007;14:328–333. doi: 10.1016/j.jocn.2006.01.017. [DOI] [PubMed] [Google Scholar]
- (20).Bravata DM, Concato J, Fried T, Ranjbar N, Sadarangani T, McClain V, et al. Auto-titrating continuous positive airway pressure for patients with acute transient ischemic attack: A randomized feasibility trial. Strok. 2010;41:1464–1470. doi: 10.1161/STROKEAHA.109.566745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Bravata DM, Concato J, Fried T, Ranjbar N, Sadarangani T, McClain V, et al. Continuous positive airway pressure: evaluation of a novel therapy for patients with acute ischemic stroke. Sleep. 2011;34:1271–1277. doi: 10.5665/SLEEP.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Hsu CY, Vennelle M, Li HY, Engleman HM, Dennis MS, Douglas NJ. Sleep-disordered breathing after stroke: a randomised controlled trial of continuous positive airway pressure. J Neurol Neurosurg Ps. 2006;77:1143–1149. doi: 10.1136/jnnp.2005.086686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Hui DSC, Choy DKL, Wong LKS, Ko FWS, Li TST, Woo J, et al. Prevalence of sleep-disordered breathing and continuous positive airway pressure compliance: Results in Chinese patients with first-ever ischemic stroke. Chest. 2002;122:852–860. doi: 10.1378/chest.122.3.852. [DOI] [PubMed] [Google Scholar]
- (24).Sandberg O, Franklin K, Bucht G, Eriksson S, Gustafson Y. Nasal continuous positive airway pressure in stroke patients with sleep apnoea: a randomized treatment study. Eur Resp J. 2001;18:630–634. doi: 10.1183/09031936.01.00070301. [DOI] [PubMed] [Google Scholar]
- (25).Parra O, Sánchez-Armengol Á , Bonnin M, Arboix A, Campos-Rodríguez F, Pérez-Ronchel J, et al. Early treatment of obstructive apnoea and stroke outcome: a randomised controlled trial. Eur Resp J. 2011;37:1128–1136. doi: 10.1183/09031936.00034410. [DOI] [PubMed] [Google Scholar]
- (26).Ryan CM, Bayley M, Green R, Murray BJ, Bradley TD. Influence of continuous positive airway pressure on outcomes of rehabilitation in stroke patients with obstructive sleep apnea. Stroke. 2011;42:1062–1067. doi: 10.1161/STROKEAHA.110.597468. [DOI] [PubMed] [Google Scholar]
- (27).Brown DL, Chervin RD, Kalbfleisch JD, Zupancic MJ, Migda EM, Svatikova A, et al. Sleep Apnea Treatment After Stroke (SATS) Trial: Is It Feasible? J Stroke Cerebrovas. 2011 doi: 10.1016/j.jstrokecerebrovasdis.2011.06.010. epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Hobson RW, II, Brott TG, Roubin GS, Silver FL, Barnett HJM. Carotid artery stenting. Meeting the recruitment challenge of a clinical trial. Stroke. 2005;36:1314–1315. doi: 10.1161/01.STR.0000165919.26944.05. [DOI] [PubMed] [Google Scholar]
- (29).Elkins JS, Khatabi T, Fung L, Rootenberg J, Johnston SC. Recruiting Subjects for Acute Stroke Trials. Stroke. 2006;37:123–128. doi: 10.1161/01.STR.0000195149.44390.aa. [DOI] [PubMed] [Google Scholar]
- (30).Lachin JM, Matts JP, Wei L. Randomization in clinical trials: conclusions and recommendations. Control Clin Trials. 1988;9:365–374. doi: 10.1016/0197-2456(88)90049-9. [DOI] [PubMed] [Google Scholar]
- (31).Brown DL, Concannon M, Kaye AB, Zupancic M, Lisabeth LD. Comparison of two headgear systems for sleep apnea treatment of stroke patients. Cerebrovas Dis. 2009;27:183–186. doi: 10.1159/000185610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Lanini B, Bianchi R, Romagnoli I, Coli C, Binazzi B, Gigliotti F, et al. Chest wall kinematics in patients with hemiplegia. Am J Respir Crit Care Med. 2003;168:109–113. doi: 10.1164/rccm.200207-745OC. [DOI] [PubMed] [Google Scholar]
- (33).Similowski T, Catala M, Rancurel G, Derenne JP. Impairment of central motor conduction to the diaphragm in stroke. Am J Respir Crit Care Med. 1996;154:436–441. doi: 10.1164/ajrccm.154.2.8756819. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.