Abstract
OBJECTIVE
Obese individuals with type 2 diabetes have an increased risk of cardiovascular disease. The effect of bariatric surgery on cardiovascular events in obese individuals with type 2 diabetes remains to be determined. The Swedish Obese Subjects (SOS) study is a prospective, controlled intervention study that examines the effects of bariatric surgery on hard end points. The aim of the present study was to examine the effect of bariatric surgery on cardiovascular events in the SOS study participants with type 2 diabetes.
RESEARCH DESIGN AND METHODS
All SOS study participants with type 2 diabetes at baseline were included in the analyses (n = 345 in the surgery group and n = 262 in the control group). Mean follow-up was 13.3 years (interquartile range 10.2–16.4) for all cardiovascular events.
RESULTS
Bariatric surgery was associated with a reduced myocardial infarction incidence (38 events among the 345 subjects in the surgery group vs. 43 events among the 262 subjects in the control group; log-rank P = 0.017; adjusted hazard ratio [HR] 0.56 [95% CI 0.34–0.93]; P = 0.025). No effect of bariatric surgery was observed on stroke incidence (34 events among the 345 subjects in the surgery group vs. 24 events among the 262 subjects in the control group; log-rank P = 0.852; adjusted HR 0.73 [0.41–1.30]; P = 0.29). The effect of surgery in reducing myocardial infarction incidence was stronger in individuals with higher serum total cholesterol and triglycerides at baseline (interaction P value = 0.02 for both traits). BMI (interaction P value = 0.12) was not related to the surgery outcome.
CONCLUSIONS
Bariatric surgery reduces the incidence of myocardial infarction in obese individuals with type 2 diabetes. Preoperative BMI should be integrated with metabolic parameters to maximize the benefits of bariatric surgery.
Obesity is a growing burden for Western countries with approximately one-third of the population being affected in the U.S. (1). Excess body weight is associated with increased incidence of type 2 diabetes and cardiovascular disease (2–4). To date, bariatric surgery is the most effective treatment to achieve weight loss in obese individuals (5). The Swedish Obese Subjects (SOS) study is a nonrandomized but controlled, prospective, interventional trial on the effect of bariatric surgery on mortality and morbidity compared with conventional obesity treatment (6). We recently reported that bariatric surgery was associated with a decreased incidence of cardiovascular events in the overall SOS study (7).
In individuals with type 2 diabetes, bariatric surgery results in sustained weight loss and also reduces blood glucose values (8–10). The American Diabetes Association (11), International Diabetes Federation (12), and other organizations (13,14) recommend bariatric surgery for adults with type 2 diabetes and BMI ≥35 kg/m2, especially for those whose diabetes is difficult to control with a lifestyle and pharmacological approach. However, data on the long-term benefits of bariatric surgery on hard end points in individuals with type 2 diabetes are not available. Whether the metabolic improvement results in a reduced number of cardiovascular events in obese individuals with type 2 diabetes remains to be determined. Therefore, the aim of the present report was to examine the effect of bariatric surgery on cardiovascular events in SOS study participants with type 2 diabetes at baseline.
RESEARCH DESIGN AND METHODS
Study design
The SOS study has been previously described in detail (5–7,15). In brief, the SOS study is a prospective, nonrandomized, controlled interventional trial on the effect of bariatric surgery on mortality and morbidity compared with conventional obesity treatment. A total of 4,047 obese individuals were enrolled from 1 September 1987 to 31 January 2001. Among these subjects, 2,010 underwent bariatric surgery, and a contemporaneously matched control group of 2,037 individuals was created using 18 matching variables. Written informed consent has been obtained by all study participants. All clinical investigations have been conducted according to the principles expressed in the Declaration of Helsinki. Seven local ethics review boards approved SOS study protocol. Inclusion and exclusion criteria were identical for both study groups. Inclusion criteria were between 37 and 60 years of age and BMI ≥34 kg/m2 for men and ≥38 kg/m2 for women. The exclusion criteria were earlier surgery for gastric or duodenal ulcer, earlier bariatric surgery, gastric ulcer during the past 6 months, ongoing malignancy, active malignancy during the past 5 years, myocardial infarction during the past 6 months, bulimic eating pattern, drug or alcohol abuse, psychiatric or cooperative problems contraindicating bariatric surgery, and other contraindicating conditions (such as chronic glucocorticoid or anti-inflammatory treatment) (6). Subjects with hypertension, diabetes, dyslipidemia, or myocardial infarction and stroke >6 months before recruitment were not excluded. Participants were examined at matching, at baseline, and after 0.5, 1, 2, 3, 4, 6, 8, 10, 15, and 20 years. Biochemical parameters were measured at the matching and baseline examinations and at 2-, 10-, 15-, and 20-year follow-up. Fasting blood samples were obtained in the morning after an overnight fast as previously described (6). Type 2 diabetes was defined as fasting blood glucose ≥6.1 mmol/L (when using fasting plasma glucose, the cutoff values are ≥7.0 mmol/L or 126 mg/dL) (16,17) and/or self-reported therapy with glucose-lowering medications at baseline.
The current report is based on 607 subjects with type 2 diabetes at baseline: 345 subjects underwent bariatric surgery (227 vertical-banded gastroplasty, 61 gastric banding, and 57 gastric bypass), and 262 subjects in the control group received the standard obesity and diabetes treatment at their centers of registration.
Study end points
The end points analyzed in the current report were fatal and nonfatal cardiovascular events (myocardial infarction and stroke, whichever came first) as well as myocardial infarction and cerebral stroke analyzed separately. These end points, which were predefined in the original study protocol from 1987 for the total SOS study population, are analyzed here in the SOS participants with type 2 diabetes at baseline. The following ICD-9/ICD-10 codes were used: myocardial infarction, 410/I21, I22; intracerebral bleeding, 431/I61; cerebral artery occlusion, 433, 434/I63, I65, and I66; and acute but nondefined stroke in terms of bleeding or occlusion, 436/I64. Angina pectoris, claudication, transitory ischemic attack, and subarachnoid hemorrhage were not included in the analyses. Information about the end points was obtained by cross-checking social security numbers from the SOS database with the Swedish National Patient Register, the Cause of Death Register, and the Register of the Total Population. The information in the health registries was completed until 31 December 2009. On this cutoff date of the analysis, the median follow-up time was 13.3 years (interquartile range 10.2–16.4 years).
Statistical analysis
Baseline characteristics and changes over time were described as mean ± SD. Baseline continuous variables and 2-year follow-up changes in the treatment groups were compared by using a linear regression model adjusted for age, sex, and BMI. A χ2 test was used to compare categorical variables.
Time of progression to end points was compared between the surgery and control groups with Kaplan-Meier estimates of cumulative incidence rates. Survival distributions in the two treatment groups were compared using a log-rank test. Cox proportional hazards models based on baseline data were also used to evaluate time to cardiovascular event. Hazard ratios (HRs) were also adjusted for cardiovascular disease risk factors. Continuous traits HRs have been expressed per 1-SD difference at baseline in the SOS population with type 2 diabetes.
For baseline risk factor–treatment interaction analysis, dichotomous variables could have one of two values (for example, sex: men/women), whereas for the other parameters, the interaction test was calculated using the original continuous variables. No adjustment for multiple testing was performed. The number of surgical procedures needed to prevent one myocardial infarction was calculated as the reciprocal of the absolute risk difference between subjects from the surgery and control groups, and it was estimated in a 15-year follow-up.
Statistical analyses were carried out using the Statistical Package for Social Science (version 18.0.0, SPSS, Inc., Chicago, IL). Intention-to-treat principle was applied in all calculations. Two-sided P values <0.05 were considered as statistically significant.
RESULTS
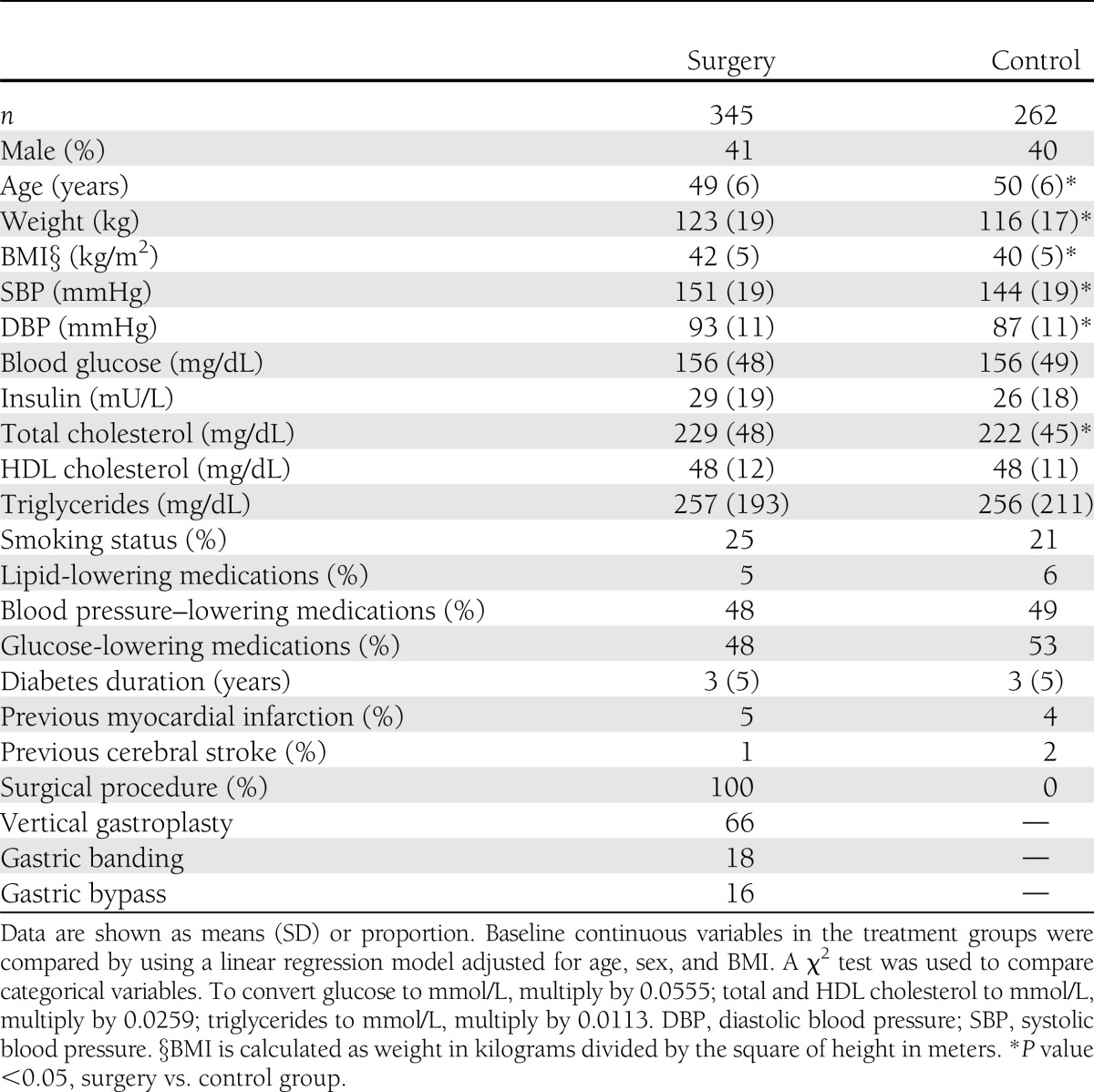
Baseline characteristics
All SOS participants with type 2 diabetes at baseline (n = 607) were included in the analysis. Characteristics of the 345 surgically treated subjects and the 262 matched controls that had type 2 diabetes at baseline are shown in Table 1. The mean age of the surgery group was lower compared with the control group. The surgery group had higher BMI, systolic blood pressure, diastolic blood pressure, and total cholesterol compared with the control group. No differences in other parameters, including blood glucose, insulin, HDL cholesterol, triglycerides, smoking status, and lipid-, glucose-, and blood pressure–lowering medications, were observed. Furthermore, at baseline, the proportion of subjects previously affected by myocardial infarction or cerebral stroke was not different between groups.
Table 1.
Characteristics of SOS study participants with type 2 diabetes at baseline
Weight and metabolic changes after bariatric surgery
At 2 years, the proportion of individuals with clinical and biochemical follow-up was 89 and 79% in the surgery and control groups, respectively. Mean 2-year follow-up changes in weight and metabolic parameters are described in Supplementary Fig. 1. Bariatric surgery was associated with a significant decrease in body weight, blood glucose, serum triglycerides, and systolic and diastolic blood pressure (P value for each trait was <0.001) and a significant increase in HDL cholesterol (P < 0.001) compared with usual care. No significant difference in total cholesterol changes between the surgery and control groups was observed.
Effect of bariatric surgery on cardiovascular disease
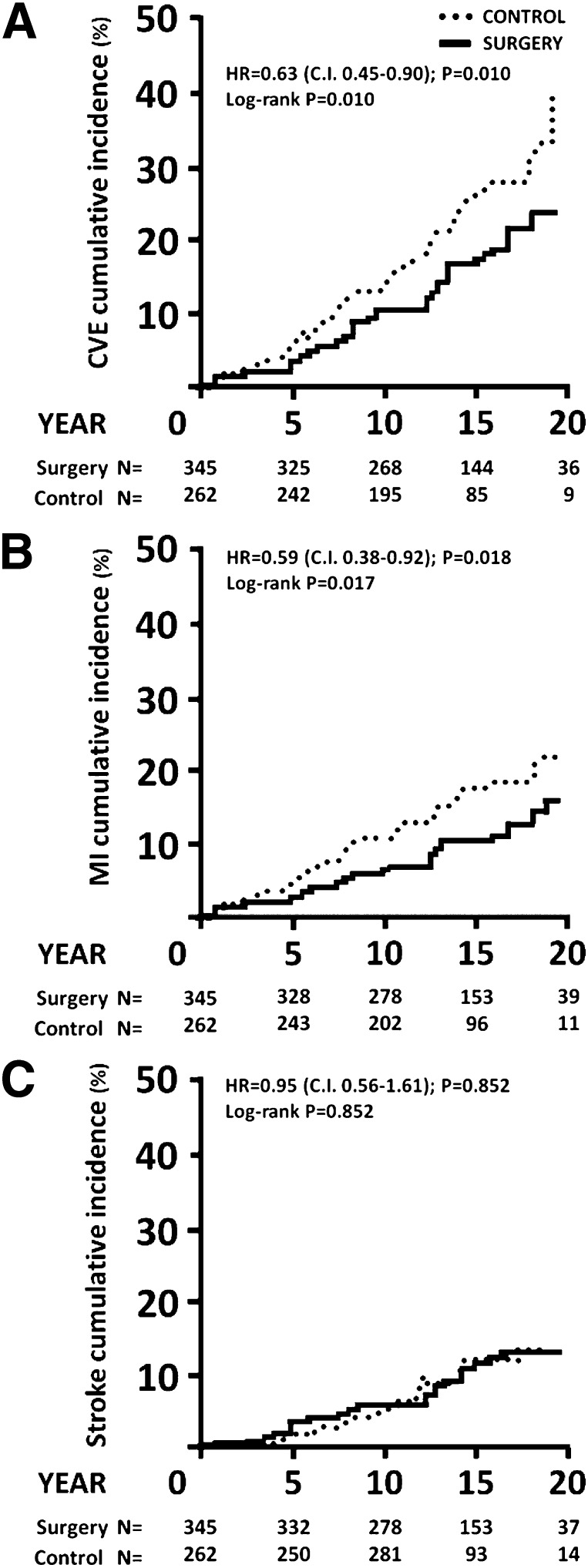
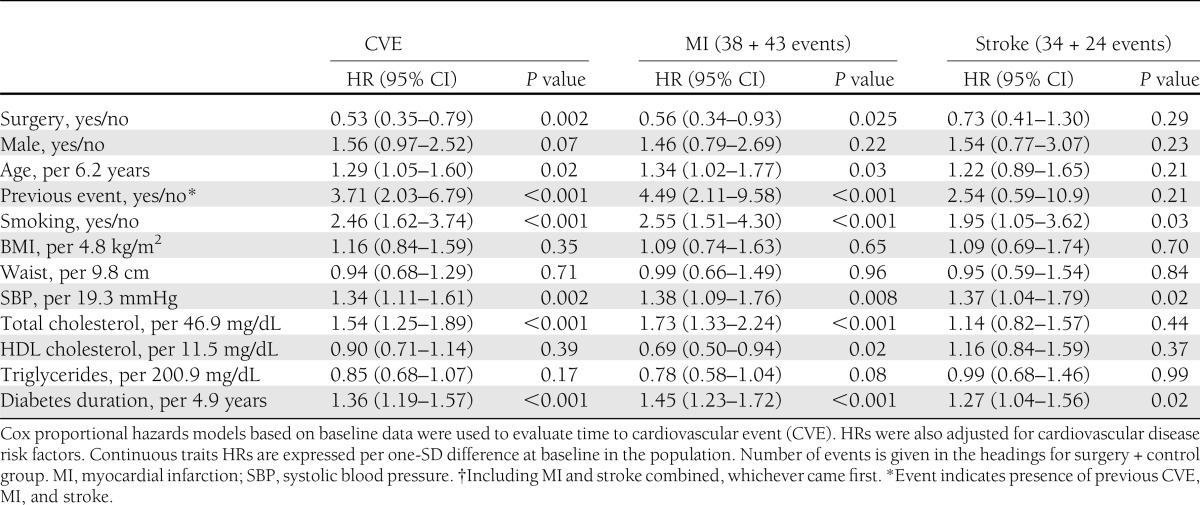
Bariatric surgery was associated with reduced incidence of fatal and nonfatal cardiovascular events. During the follow-up period, 63 first-time cardiovascular events (myocardial infarction or stroke, whichever came first) occurred among 345 individuals in the surgery group compared with 65 events among 262 individuals in the control group (log-rank P = 0.010; unadjusted HR 0.63 [95% CI 0.45–0.90]; P = 0.010) (Fig. 1A). After adjustment for baseline anthropometrical and clinical characteristics, the HR for cardiovascular events was 0.53 (0.35–0.79; P = 0.002) (Table 2). In the adjusted analyses, classical cardiovascular risk factors (age, smoking, systolic blood pressure, total cholesterol, and diabetes duration) remained significantly associated with an increased risk of cardiovascular events (Table 2). Results were virtually identical after excluding individuals in whom myocardial infarction or stroke occurred within 2 years from baseline (data not shown).
Figure 1.
Cumulative incidence of cardiovascular events in SOS subjects with diabetes at baseline. A: There were 63 cardiovascular events (CVEs) among the 345 subjects in the surgery group (median follow-up = 13.9 years [10.7–16.9 years]), compared with 65 events among the 262 subjects in the control group (median follow up = 12.2 years [9.9–15.8 years]). CVEs: myocardial infarction and stroke combined, whichever came first. B: Among the 345 subjects in the surgery group, there were 38 myocardial infarctions (MIs), compared with 43 among the 262 subjects in the control group. C: Out of 345 subjects in the surgery group, 34 had stroke, compared with 24 of the 262 subjects in the control group.
Table 2.
Multivariable Cox proportional hazards models for fatal plus nonfatal cardiovascular events in SOS subjects with diabetes at baseline
Next, the incidence of myocardial infarction and stroke were assessed separately. Bariatric surgery was associated with lower incidence of myocardial infarction. A total of 38 of the 345 individuals in the surgery group and 43 of the 262 individuals in the control group had myocardial infarction during follow-up (log-rank P = 0.017; HR = 0.59 [95% CI 0.38–0.92]; P = 0.018) (Fig. 1B). After adjustment for baseline anthropometrical and clinical characteristics, the HR for myocardial infarction was 0.56 (0.34–0.93; P = 0.025) (Table 2). No significant differences in the incidence of myocardial infarction were found between the different surgical procedures (vertical gastroplasty, gastric banding, and gastric bypass) (Supplementary Fig. 2).
A total of 34 of the 345 individuals from the surgery group and 24 of the 262 individuals from the control group had stroke during follow-up. Bariatric surgery was not associated with changes in the incidence of cerebral stroke in unadjusted (log-rank P = 0.85; HR 0.95 [95% CI 0.56–1.61]; P = 0.85) (Fig. 1C) or adjusted analyses (HR 0.73 [0.41–1.30]; P = 0.29) (Table 2).
Risk factor–treatment interaction analyses and number needed to treat
To test if baseline characteristics were related to the treatment benefit of bariatric surgery with respect to myocardial infarction, a subgroup analysis on baseline risk factor–treatment interaction was performed. Specifically, the relative treatment effect and the treatment interaction on myocardial infarction incidence were assessed after stratifying by sex, previous myocardial infarction, smoking, and glucose-lowering medication and by the median of baseline age, BMI, weight, waist, insulin, total cholesterol, triglycerides, HDL cholesterol, blood pressure, and diabetes duration (Supplementary Table 1). In diabetic subjects, the surgical treatment benefit with respect to myocardial infarction events was significantly associated with baseline serum total cholesterol and triglycerides (interaction P = 0.02 for both), with a greater relative treatment benefit in subjects with higher total cholesterol and triglycerides. In contrast, the treatment benefit of bariatric surgery was not related to other clinical and metabolic parameter traits, including BMI (Supplementary Table 1). Still, when individuals were stratified by median of BMI (40.6 kg/m2), a significant protective effect of bariatric surgery was found in individuals below the median (HR 0.43 [95% CI 0.22–0.84]; P = 0.010) (Supplementary Table 1).
Finally, the number needed to treat (NNT) was calculated to estimate the number of obese diabetic subjects needed to undergo bariatric surgery to prevent one myocardial infarction over 15 years (Supplementary Table 1). The estimated NNT in the overall diabetic SOS population was 16 (Supplementary Table 1). No significant difference in NNT after stratifying the population by baseline characteristics was found (Supplementary Table 1).
CONCLUSIONS
This is the first prospective report showing that bariatric surgery reduces the incidence of myocardial infarction in obese subjects with type 2 diabetes. In obese subjects with type 2 diabetes, bariatric surgery was associated with a lower incidence of cardiovascular events, and the beneficial effect of the surgical treatment was also present after adjustment for baseline parameters. When analyzed separately, bariatric surgery was associated with a reduced incidence of myocardial infarction, but no effect was observed on stroke incidence. We have recently shown bariatric surgery in the overall SOS study cohort was associated with reduced incidence of cardiovascular events and myocardial infarction or stroke when analyzed separately; however, the effect size on stroke was modest (7). The absence of an effect on stroke incidence in subjects with type 2 diabetes at baseline could be explained by low statistical power given that only 15% (only those with type 2 diabetes at baseline) of the overall SOS study participants were included.
Several metabolic variables (blood glucose, circulating lipids, and blood pressure) were markedly improved in the surgery group. These data are in line with previous reports on the metabolic changes after bariatric surgery (5,9,18,19). However, this is the first study to demonstrate that the beneficial effects of bariatric surgery on cardiovascular risk factors are also followed by reduced risk of myocardial infarction in individuals with type 2 diabetes. The NNT shows that 16 individuals with type 2 diabetes need to be operated to prevent one myocardial infarction over a period of 15 years. In the surgery group, no difference in the incidence of myocardial infarction was observed across the three different surgical procedures; however, our study was not statistically powered to detect such difference.
Interestingly, in our study, bariatric surgery was associated with a reduction of myocardial infarction incidence in diabetic individuals with BMI below the median (40.6 kg/m2). This result corroborates the guidelines from the American Diabetes Association (11), International Diabetes Federation (12), and other organizations (13,14) in which bariatric surgery is considered as a therapeutic option in diabetic subjects with BMI <40 kg/m2. Furthermore, the risk factor–treatment interaction analysis showed that the effect of surgery on myocardial infarction was greater in participants with higher total cholesterol and triglyceride levels, possibly suggesting that, in obese diabetic individuals, those with dyslipidemia should be prioritized. Moreover, in the previously reported analyses on the overall SOS study cohort (7), baseline fasting insulin levels rather than BMI predicted the surgery treatment benefit on cardiovascular event. Taken together, these results suggest that to maximize the benefits, metabolic parameters should be used to select obese patients for surgery (7,20).
A limitation of the current report is that the SOS study intervention was not randomized for ethical reasons due to high postoperative mortality in the 1980s. Another limitation of our study is that we performed a post hoc analysis, selecting only individuals with type 2 diabetes at baseline. However, myocardial infarction and stroke were predefined secondary end points for the overall SOS population in the original study protocol. Ideally, longitudinal, randomized, controlled trials (e.g., obese diabetic individuals selected by circulating triglyceride levels) should be performed to confirm these results. However, a long follow-up is needed to document effects on hard cardiovascular end points, and confirmatory data are not likely to be available for many years.
In conclusion, this is the first prospective study showing that bariatric surgery is associated with reduced incidence of myocardial infarction in obese individuals with type 2 diabetes. It also provides support for the recommendations of the international guidelines regarding bariatric surgery in obese individuals with type 2 diabetes.
Supplementary Material
Acknowledgments
This study was supported by grants from the Swedish Research Council (K2012-55X-22082-01-3, K2010-55X-11285-13, and K2008-65x-20753-01-4), the Swedish Foundation for Strategic Research to Sahlgrenska Centre for Cardiovascular and Metabolic Research, and the Swedish federal government under the LUA/ALF agreement.
No potential conflicts of interest relevant to this article were reported.
S.R. designed and conducted the analyses, interpreted the data, contributed to discussion, and wrote, reviewed, and edited the manuscript. C.M. performed the analyses, contributed to discussion, and wrote and reviewed the manuscript. M.A.B. performed the analyses, contributed to discussion, and reviewed the manuscript. C.P., K.S., P.J., and P.-A.S. contributed to discussion and reviewed the manuscript. M.P. contributed to the analyses and reviewed the manuscript. L.S. contributed to discussion and wrote, reviewed, and edited the manuscript. L.M.S.C. interpreted the data, contributed to discussion, and wrote, reviewed, and edited the manuscript. All the authors read and approved the final version of the manuscript. S.R. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank the staff members at 480 primary health care centers and 25 surgical departments in Sweden that participated in the study. Gerd Bergmark, Christina Torefalk, and Lisbeth Eriksson (Sahlgrenska Academy, University of Gothenburg) are acknowledged for invaluable administrative support.
Footnotes
Clinical trial reg. no. NCT01479452, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-0193/-/DC1.
See accompanying commentary, p. 2424.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA 2010;303:235–241 [DOI] [PubMed] [Google Scholar]
- 2.Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA 1999;282:1523–1529 [DOI] [PubMed] [Google Scholar]
- 3.Whitlock G, Lewington S, Sherliker P, et al. Prospective Studies Collaboration Body-mass index and cause-specific mortality in 900,000 adults: collaborative analyses of 57 prospective studies. Lancet 2009;373:1083–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mazzone T, Chait A, Plutzky J. Cardiovascular disease risk in type 2 diabetes mellitus: insights from mechanistic studies. Lancet 2008;371:1800–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sjöström L, Lindroos AK, Peltonen M, et al. Swedish Obese Subjects Study Scientific Group Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004;351:2683–2693 [DOI] [PubMed] [Google Scholar]
- 6.Sjöström L, Larsson B, Backman L, et al. Swedish obese subjects (SOS). Recruitment for an intervention study and a selected description of the obese state. Int J Obes Relat Metab Disord 1992;16:465–479 [PubMed] [Google Scholar]
- 7.Sjöström L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA 2012;307:56–65 [DOI] [PubMed] [Google Scholar]
- 8.Dixon JB, O’Brien PE, Playfair J, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA 2008;299:316–323 [DOI] [PubMed] [Google Scholar]
- 9.Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med 2009;122:248–256.e5 [DOI] [PubMed]
- 10.Sjöström CD, Lystig T, Lindroos AK. Impact of weight change, secular trends and ageing on cardiovascular risk factors: 10-year experiences from the SOS study. Int J Obes (Lond) 2011;35:1413–1420 [DOI] [PubMed] [Google Scholar]
- 11.American Diabetes Association Standards of medical care in diabetes—2011. Diabetes Care 2011;34(Suppl. 1):S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dixon JB, Zimmet P, Alberti KG, Rubino F, International Diabetes Federation Taskforce on Epidemiology and Prevention Bariatric surgery: an IDF statement for obese type 2 diabetes. Diabet Med 2011;28:628–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubino F, Kaplan LM, Schauer PR, Cummings DE, Diabetes Surgery Summit Delegates The Diabetes Surgery Summit consensus conference: recommendations for the evaluation and use of gastrointestinal surgery to treat type 2 diabetes mellitus. Ann Surg 2010;251:399–405 [DOI] [PubMed] [Google Scholar]
- 14.Runkel N, Colombo-Benkmann M, Hüttl TP, et al. Evidence-based German guidelines for surgery for obesity. Int J Colorectal Dis 2011;26:397–404 [DOI] [PubMed] [Google Scholar]
- 15.Sjöström L, Narbro K, Sjöström CD, et al. Swedish Obese Subjects Study Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 2007;357:741–752 [DOI] [PubMed] [Google Scholar]
- 16.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2011;34(Suppl. 1):S62–S69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications. Report of a WHO Consultation. Part 1: Diagnosis and Classification of Diabetes Mellitus. Geneva, World Health Org., 1999 (Rep. no. 99.2)
- 18.Vogel JA, Franklin BA, Zalesin KC, et al. Reduction in predicted coronary heart disease risk after substantial weight reduction after bariatric surgery. Am J Cardiol 2007;99:222–226 [DOI] [PubMed] [Google Scholar]
- 19.Iaconelli A, Panunzi S, De Gaetano A, et al. Effects of bilio-pancreatic diversion on diabetic complications: a 10-year follow-up. Diabetes Care 2011;34:561–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma AM, Kushner RF. A proposed clinical staging system for obesity. Int J Obes (Lond) 2009;33:289–295 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.