Abstract
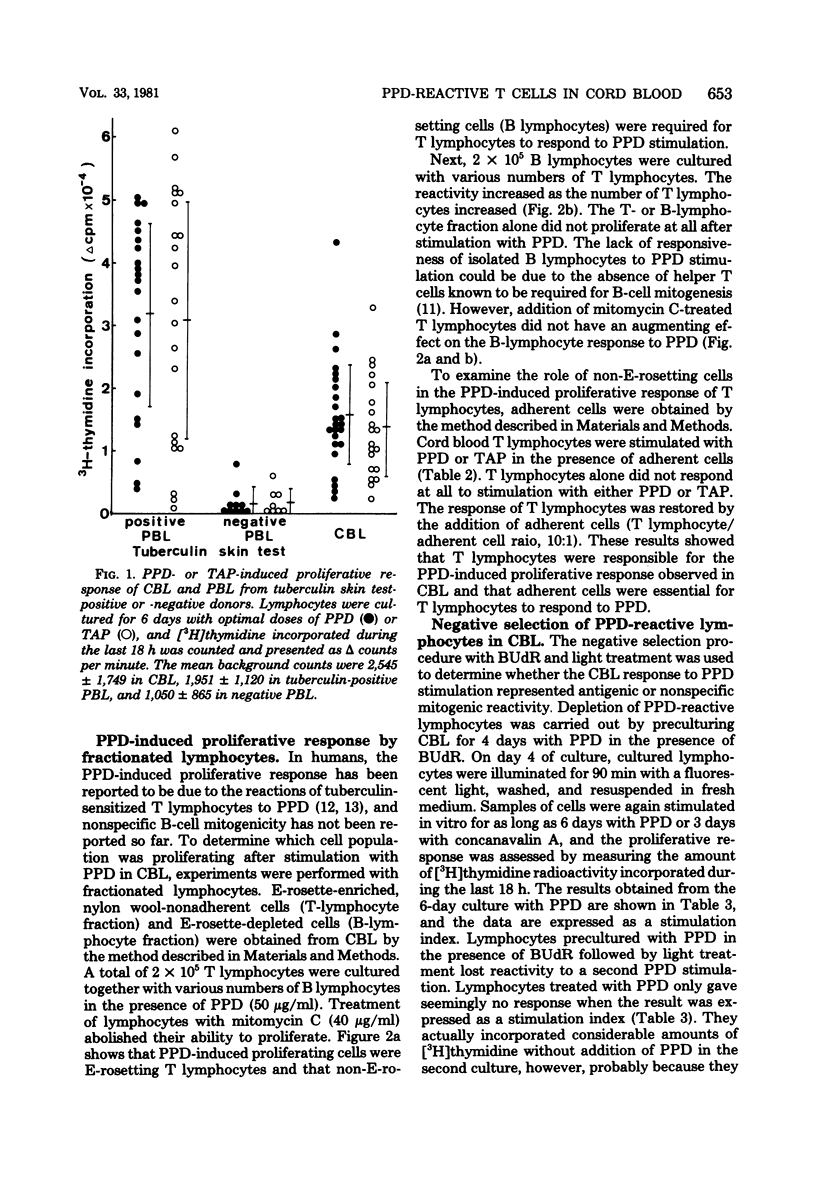
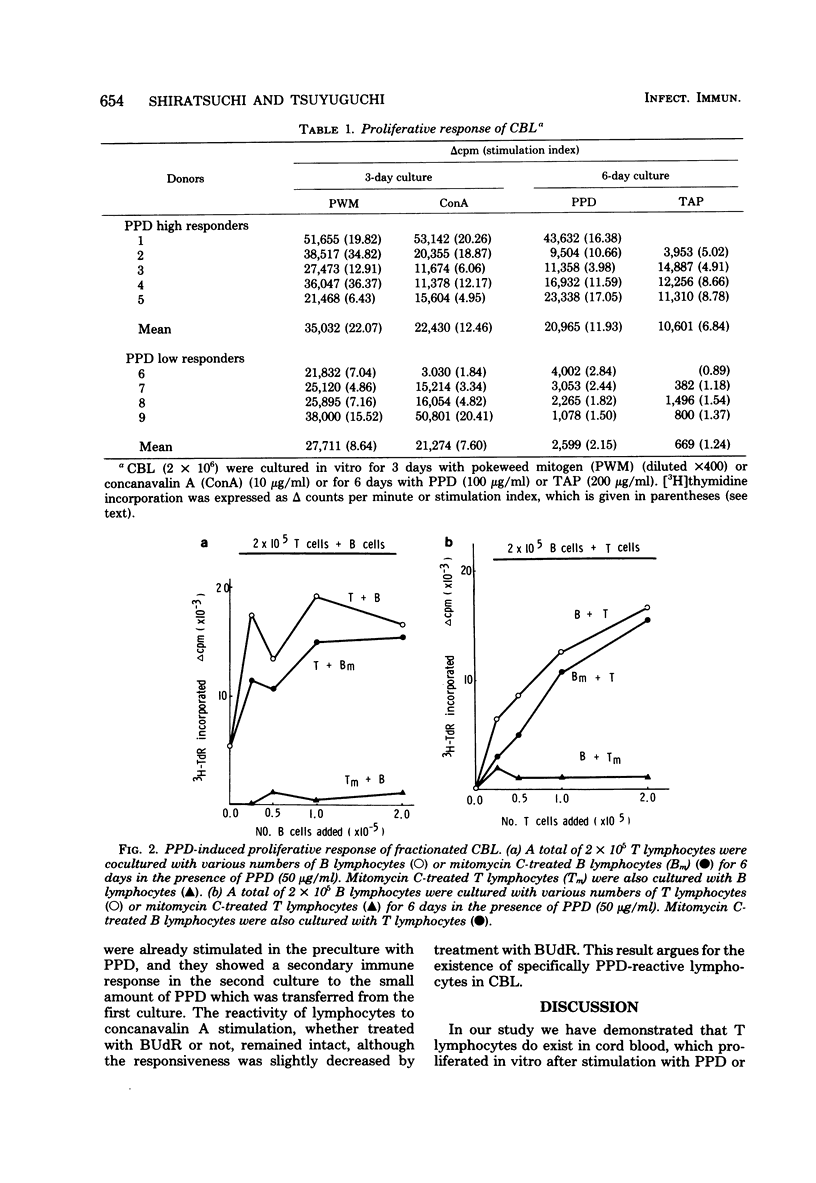
Lymphocytes obtained from cord blood of newborn babies who were born of healthy mothers were studied in vitro for their responsiveness to purified protein derivative (PPD) of tuberculin. Cord blood lymphocytes proliferated in vitro by stimulation with PPD, despite wide variations in the results. Studies with fractionated lymphocytes revealed that PPD-responding cells belonged to E-rosetting, nylon wool-nonadherent T lymphocytes. Non-E-rosetting B lymphocytes alone did not proliferate at all after stimulation with PPD. In addition, bromodeoxyuridine and light treatment of in vitro PPD-stimulated lymphocytes eliminated the responsiveness to PPD. These results suggest that T lymphocytes do exist in cord blood and respond in vitro to stimulation with PPD. A possible role for PPD-reactive T lymphocytes in cell-mediated protective immunity in newborns is discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Lernhardt W., Melchers F. The purified protein derivative of turberculin, a B-cell mitogen that distinguishes in its action resting, small B cells from activated B-cell blasts. J Exp Med. 1979 Dec 1;150(6):1339–1350. doi: 10.1084/jem.150.6.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma I., Kimura H., Niinaka T., Aoki T., Yamamura Y. Chemical and immunological studies on mycobacterial polysaccharides. 1. Purification and properties of polysaccharides from human tubercle bacilli. J Bacteriol. 1968 Feb;95(2):263–271. doi: 10.1128/jb.95.2.263-271.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergholtz B. O., Thorsby E. Macrophage/T-lymphocyte interaction in the immune response to PPD in humans. Scand J Immunol. 1979;9(6):511–516. doi: 10.1111/j.1365-3083.1979.tb03279.x. [DOI] [PubMed] [Google Scholar]
- Blomgren H. Role of B cells in the expression of the PPD response of human lymphocytes in vitro. Scand J Immunol. 1975 Sep;4(5-6):499–510. doi: 10.1111/j.1365-3083.1975.tb02655.x. [DOI] [PubMed] [Google Scholar]
- Ellner J. J., Schacter B. Z., Bhe F. T. Tuberculin response of lymphocytes from human skin test nonreactors; evidence for in vitro primary sensitization of T lymphocytes. Cell Immunol. 1979 Jun;45(1):213–220. doi: 10.1016/0008-8749(79)90379-4. [DOI] [PubMed] [Google Scholar]
- Fauci A. S., Pratt K. R. Activation of human B lymphocytes. I. Direct plaque-forming cell assay for the measurement of polyclonal activation and antigenic stimulation of human B lymphocytes. J Exp Med. 1976 Sep 1;144(3):674–684. doi: 10.1084/jem.144.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granberg C., Hirvonen T., Toivanen P. Cell-mediated lympholysis by human maternal and neonatal lymphocytes: mother's reactivity against neonatal cells and vice versa. J Immunol. 1979 Dec;123(6):2563–2567. [PubMed] [Google Scholar]
- Hammarström L., Bird A. G., Smith C. I. Mitogenic activation of human lymphocytes: a protein A plaque assay evaluation of polyclonal B-cell activators. Scand J Immunol. 1980;11(1):1–13. doi: 10.1111/j.1365-3083.1980.tb00202.x. [DOI] [PubMed] [Google Scholar]
- Hayward A. R., Lawton A. R. Induction of plasma cell differentiation of human fetal lymphocytes: evidence for functional immaturity of T and B cells. J Immunol. 1977 Oct;119(4):1213–1217. [PubMed] [Google Scholar]
- Hensen E. J., Elferink B. G. Primary sensitisation and restimulation of human lymphocytes with soluble antigen in vitro. Nature. 1979 Jan 18;277(5693):223–225. doi: 10.1038/277223a0. [DOI] [PubMed] [Google Scholar]
- Hirano T., Kuritani T., Kishimoto T., Yamamura Y. In vitro immune response of human peripheral lymphocytes. I. The mechanism(s) involved in T cell helper functions in the pokeweed mitogen-induced differentiation and proliferation of B cells. J Immunol. 1977 Oct;119(4):1235–1241. [PubMed] [Google Scholar]
- Jensen B., Kurpisz M., Rubin B. Antigen specific lymphocyte activity in vitro by peripheral blood leucocytes from Mantoux positive and negative human beings. I. Comparison of quantitative and qualitative differences in the PPD-specific lymphoproliferative response of lymphocytes from the two kinds of donors. Clin Exp Immunol. 1977 Feb;27(2):303–312. [PMC free article] [PubMed] [Google Scholar]
- Kasakura S. Blastogenesis of lymphocytes induced by PPD-primed X-irradiated autologous lymphocytes. J Immunol. 1969 Nov;103(5):1078–1084. [PubMed] [Google Scholar]
- Lipsky P. E., Rosenthal A. S. The induction and regulation of guinea pig B-lymphocyte proliferation in vitro. J Immunol. 1976 Nov;117(5 Pt 1):1594–1602. [PubMed] [Google Scholar]
- Moriya N., Nagaoki T., Okuda N., Taniguchi N. Suppression of adult B cell differentiation in pokeweed mitogen-stimulated cultures by Fc(IgG) receptor-negative T cells from cord blood. J Immunol. 1979 Oct;123(4):1795–1798. [PubMed] [Google Scholar]
- Newman W., Stoner G. L., Bloom B. R. Primary in vitro sensitisation of human T cells. Nature. 1977 Sep 8;269(5624):151–153. doi: 10.1038/269151a0. [DOI] [PubMed] [Google Scholar]
- Nilsson B. S. The response of lymphocytes from tuberculin-positive or negative humans to various doses of PPD-tuberculin in vitro. Cell Immunol. 1972 Mar;3(3):493–500. doi: 10.1016/0008-8749(72)90254-7. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Tishon A., Moretta L. Active thymus derived suppressor lymphocytes in human cord blood. Nature. 1977 Sep 22;269(5626):333–335. doi: 10.1038/269333a0. [DOI] [PubMed] [Google Scholar]
- Ringdén O., Rynnel-Dagö B., Kunori T., Freijd A., Möller E. Antibody production and DNA synthesis of human lymphocyte subpopulations induced by PPD tuberculin. Clin Exp Immunol. 1979 Jun;36(3):528–535. [PMC free article] [PubMed] [Google Scholar]
- Rodey G. E., Luehrman L. K., Thomas D. W. In vitro primary immunization of human peripheral blood lymphocytes to KLH: evidence for HLA-D region restriction. J Immunol. 1979 Nov;123(5):2250–2254. [PubMed] [Google Scholar]
- Ruuskanen O., Pittard W. B., 3rd, Miller K., Pierce G., Sorensen R. U., Polmar S. H. Staphylococcus aureus Cowan I-induced immunoglobulin production in human cord blood lymphocytes. J Immunol. 1980 Jul;125(1):411–413. [PubMed] [Google Scholar]
- Schlesinger J. J., Covelli H. D. Evidence for transmission of lymphocyte responses to tuberculin by breast-feeding. Lancet. 1977 Sep 10;2(8037):529–532. doi: 10.1016/s0140-6736(77)90665-1. [DOI] [PubMed] [Google Scholar]
- Sultzer B. M., Nilsson B. S. PPD tuberculin--a B-cell mitogen. Nat New Biol. 1972 Dec 13;240(102):198–200. doi: 10.1038/newbio240198a0. [DOI] [PubMed] [Google Scholar]
- Tsuyuguchi I., Shiratsuchi H., Teraoka O., Hirano T. Increase in T cells bearing IgG Fc receptors in peripheral blood of patients with tuberculosis by in vitro stimulation with purified protein derivative. Am Rev Respir Dis. 1980 Jun;121(6):951–957. doi: 10.1164/arrd.1980.121.6.951. [DOI] [PubMed] [Google Scholar]
- Turunen O. Significance of serum factors in the stimulation of human umbilical cord and fetal lymphocytes by soluble protein A from Staphylococcus aureus. Eur J Immunol. 1979 Aug;9(8):597–601. doi: 10.1002/eji.1830090805. [DOI] [PubMed] [Google Scholar]
- Yamamura Y., Onoue K., Azuma I. Biology of the mycobacterioses. Chemical and immunological studies on peptides and polysaccharides from tubercle bacilli. Ann N Y Acad Sci. 1968 Sep 5;154(1):88–97. doi: 10.1111/j.1749-6632.1968.tb16698.x. [DOI] [PubMed] [Google Scholar]
- Yamazaki S., Koyama K., Someya S., Azuma I., Yamamura Y. Studies on the allergenicity of various tuberculoprotein derivatives and the adjuvanticity of wax D fractions of Mycobacterium tuberculosis. Am Rev Respir Dis. 1969 Nov;100(5):691–698. doi: 10.1164/arrd.1969.100.5.691. [DOI] [PubMed] [Google Scholar]


