Abstract
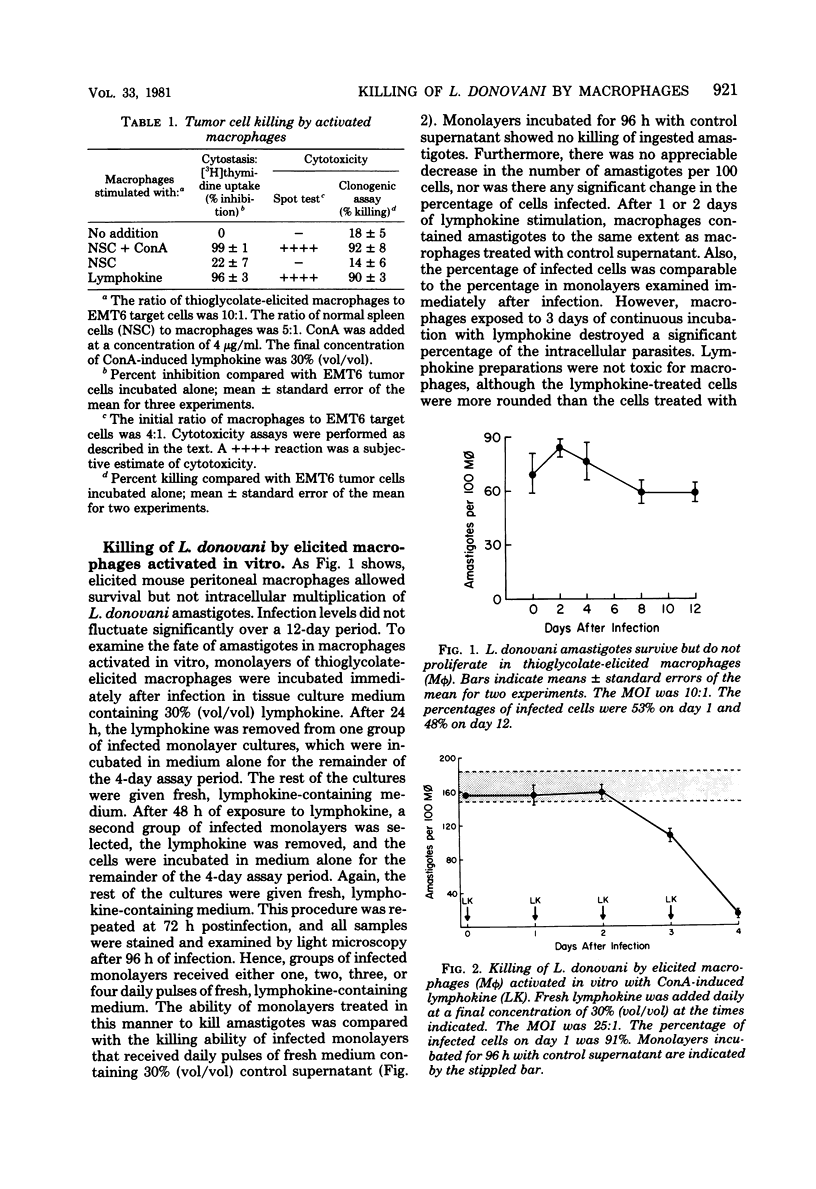
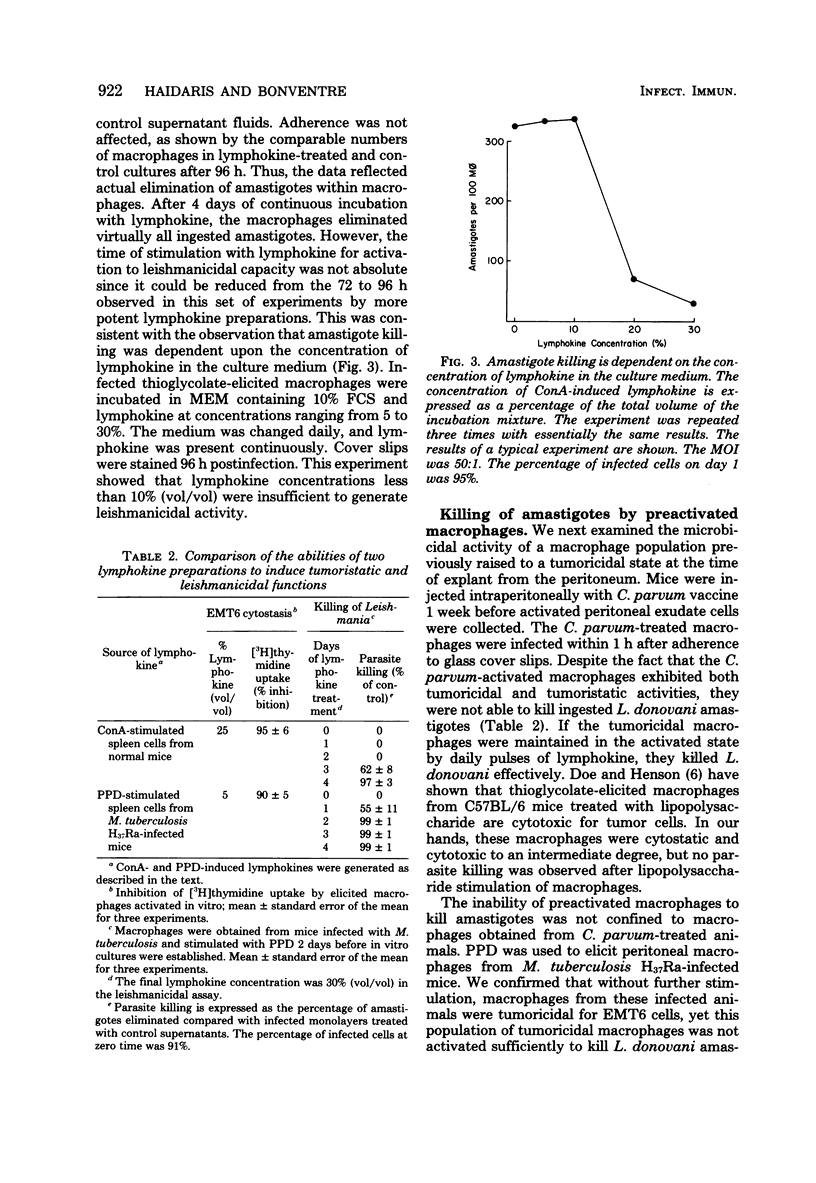
Tissue macrophages are the obligatory host cells for Leishmania donovani, the causative agent of visceral leishmaniasis. In this study we sought to determine whether activated macrophages, as defined by the functional criterion of tumor cell cytotoxicity, were also able to exert a microbicidal effect on ingested L. donovani amastigotes. We found that mouse peritoneal macrophages activated by a variety of means exerted a cytotoxic effect on tumor cell targets but were not able to kill L. donovani amastigotes unless the infected macrophages were exposed continually to an activating stimulus. Corynebacterium parvum, Mycobacterium tuberculosis H37Ra, and lymphokine-activated peritoneal macrophages from C57BL/6J mice were cytotoxic for EMT6 tumor cell targets. However, L. donovani Sudan strain 1S amastigotes were not killed by these macrophages unless the activated state was maintained by daily addition of lymphokine to the infected monolayers for several days postinfection. The killing of amastigotes was dependent on the time of exposure to lymphokine, as well as on the concentration of lymphokine added to the culture.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O. Effector mechanisms of cytolytically activated macrophages. I. Secretion of neutral proteases and effect of protease inhibitors. J Immunol. 1980 Jan;124(1):286–292. [PubMed] [Google Scholar]
- Berman J. D., Dwyer D. M., Wyler D. J. Multiplication of Leishmania in human macrophages in vitro. Infect Immun. 1979 Oct;26(1):375–379. doi: 10.1128/iai.26.1.375-379.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun R., Berens R. L., Krassner S. M. Inhibition of Leishmania donovani transformation by hamster spleen homogenates and active human lymphocytes. Nature. 1976 Aug 19;262(5570):689–691. doi: 10.1038/262689a0. [DOI] [PubMed] [Google Scholar]
- Buchmüller Y., Mauel J. Studies on the mechanisms of macrophage activation. II. Parasite destruction in macrophages activated by supernates from concanavalin A-stimulated lymphocytes. J Exp Med. 1979 Aug 1;150(2):359–370. doi: 10.1084/jem.150.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K. P., Dwyer D. M. Multiplication of a human parasite (Leishmania donovani) in phagolysosomes of hamster macrophages in vitro. Science. 1976 Aug 20;193(4254):678–680. doi: 10.1126/science.948742. [DOI] [PubMed] [Google Scholar]
- Doe W. F., Henson P. M. Macrophage stimulation by bacterial lipopolysaccharides. I. Cytolytic effect on tumor target cells. J Exp Med. 1978 Aug 1;148(2):544–556. doi: 10.1084/jem.148.2.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Lambert L. H., Jr, Remington J. S. Control of carcinogenesis: a possible role for the activated macrophage. Science. 1972 Sep 15;177(4053):998–1000. doi: 10.1126/science.177.4053.998. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Chapman H. A., Jr, Weinberg J. B. Macrophage tumor killing: influence of the local environment. Science. 1977 Jul 15;197(4300):279–282. doi: 10.1126/science.327547. [DOI] [PubMed] [Google Scholar]
- Jones T. C., Len L., Hirsch J. G. Assessment in vitro of immunity against Toxoplasma gondii. J Exp Med. 1975 Feb 1;141(2):466–482. doi: 10.1084/jem.141.2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lainson R., Shaw J. J. Epidemiology and ecology of leishmaniasis in Latin-America. Nature. 1978 Jun 22;273(5664):595–600. doi: 10.1038/273595a0. [DOI] [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauel J., Behin R., Biroum-Noerjasin Quantitative release of live microorganisms from infected macrophages by sodium dodecyl sulphate. Nat New Biol. 1973 Jul 18;244(133):93–94. doi: 10.1038/newbio244093a0. [DOI] [PubMed] [Google Scholar]
- Mauel J., Buchmüller Y., Behin R. Studies on the mechanisms of macrophage activation. I. Destruction of intracellular Leishmania enriettii in macrophages activated by cocultivation with stimulated lymphocytes. J Exp Med. 1978 Aug 1;148(2):393–407. doi: 10.1084/jem.148.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacy C. A., Leonard E. J., Meltzer M. S. Macrophages in resistance to rickettsial infections: characterization of lymphokines that induce rickettsiacidal activity in macrophages. J Immunol. 1981 Jan;126(1):204–207. [PubMed] [Google Scholar]
- Nogueira N., Cohn Z. A. Trypanosoma cruzi: in vitro induction of macrophage microbicidal activity. J Exp Med. 1978 Jul 1;148(1):288–300. doi: 10.1084/jem.148.1.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirahata T., Shimizu K., Suzuki N. Effects of immune lymphocyte products and serum antibody on the multiplication of Toxoplasma in murine peritoneal macrophages. Z Parasitenkd. 1976 Mar 31;49(1):11–23. doi: 10.1007/BF00445014. [DOI] [PubMed] [Google Scholar]
- Walker W. S. Functional heterogeneity of macrophages in the induction and expression of acquired immunity. J Reticuloendothel Soc. 1976 Jul;20(1):57–65. [PubMed] [Google Scholar]
- Wing E. J., Gardner I. D., Ryning F. W., Remington J. S. Dissociation of effector functions in populations of activated macrophages. Nature. 1977 Aug 18;268(5621):642–644. doi: 10.1038/268642a0. [DOI] [PubMed] [Google Scholar]



