Abstract
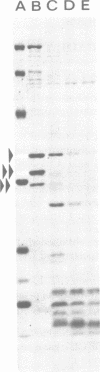
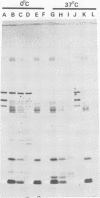
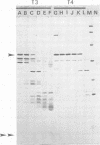
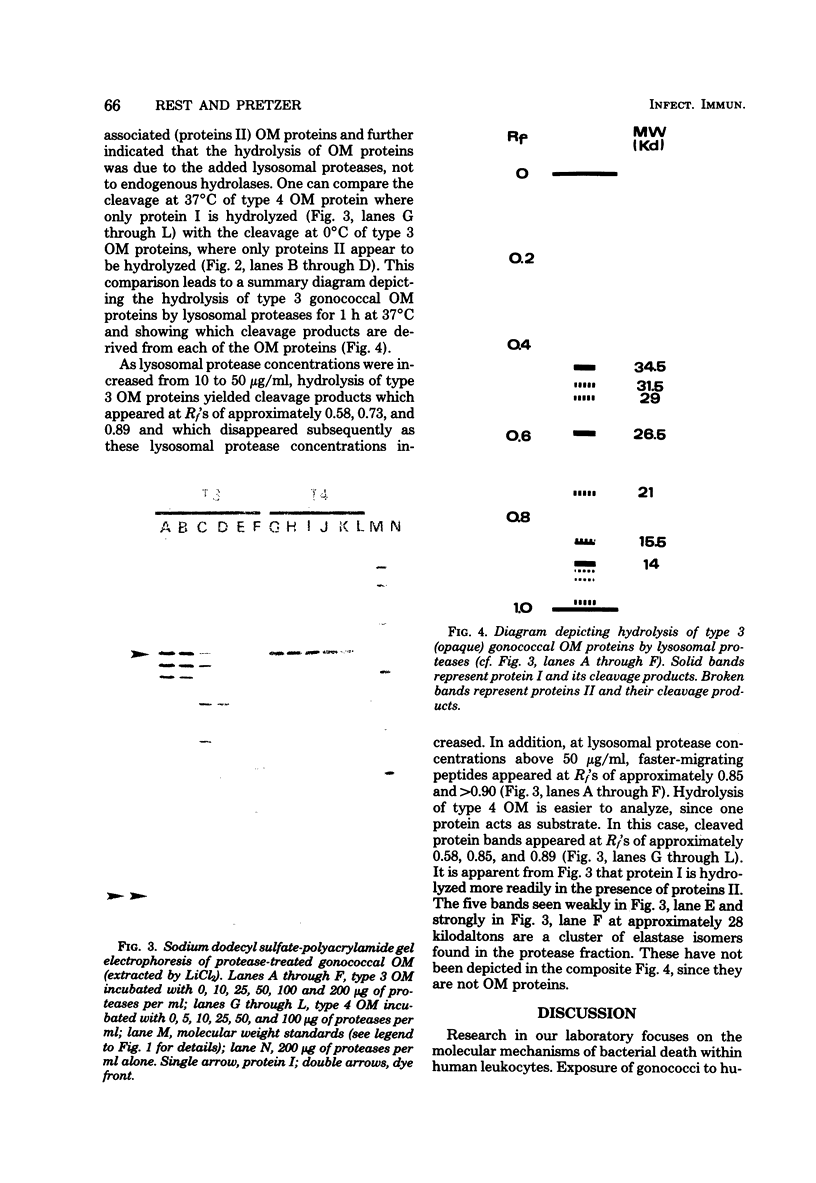
Interest in the molecular mechanisms of leukocyte bactericidal activity led us to study the effects of human neutrophil lysosomal proteases on the outer membrane (OM) proteins of Neisseria gonorrhoeae. A protease fraction containing cathepsin G and elastase activity was partially purified by gel filtration chromatography of acetate extracts of purified neutrophil granules. OM was obtained from gonococci by French press-Sarkosyl or by LiCl2 extraction. The principal (protein I) and opacity-associated (proteins II) OM proteins of N. gonorrhoeae were hydrolyzed by lysosomal proteases; proteins II were more susceptible to hydrolysis than protein I. Treatment of whole gonococci, with subsequent purification of OM, or direct treatment of purified OM led to identical hydrolysis of OM proteins by lysosomal proteases as indicated by sodium dodecyl sulfate-polyacrylamide gel patterns. Similarly, hydrolysis of purified OM proteins was identical whether OM was treated with unfractionated granule extract or with the partially purified lysosomal proteases, indicating that the observed hydrolysis by unfractionated lysosomal contents was due solely to the lysosomal protease fraction. Hydrolysis of OM proteins was dependent upon the concentration of proteases, time, and temperature. Hydrolysis of proteins II was observed with as little as 1 microgram of proteases per ml for 1 h at 37 degrees C. OM incubated alone or with heat-inactivated proteases showed no hydrolytic activity. The addition of 25 mM Na+, K+, Mg2+, or Ca2+ to incubation mixtures containing proteases and OM did not alter hydrolytic activity as indicated by sodium dodecyl sulfate-polyacrylamide gel patterns.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blake M. S., Gotschlich E. C., Swanson J. Effects of proteolytic enzymes on the outer membrane proteins of Neisseria gonorrhoeae. Infect Immun. 1981 Jul;33(1):212–222. doi: 10.1128/iai.33.1.212-222.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondin J., Janoff A. The role of lysosomal elastase in the digestion of Escherichia coli proteins by human polymorphonuclear leukocytes: experiments with living leukocytes. J Clin Invest. 1976 Oct;58(4):971–979. doi: 10.1172/JCI108551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck P., Rest R. F. Effects of human neutrophil granule extracts on macromolecular synthesis in Neisseria gonorrhoeae. Infect Immun. 1981 Aug;33(2):426–433. doi: 10.1128/iai.33.2.426-433.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K. C., Buchanan T. M. Hydrolases from Neisseria gonorrhoeae. The study of gonocosin, an aminopeptidase-P, a proline iminopeptidase, and an asparaginase. J Biol Chem. 1980 Feb 25;255(4):1704–1710. [PubMed] [Google Scholar]
- Densen P., Mandell G. L. Gonococcal interactions with polymorphonuclear neutrophils: importance of the phagosome for bactericidal activity. J Clin Invest. 1978 Dec;62(6):1161–1171. doi: 10.1172/JCI109235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilworth J. A., Hendley J. O., Mandell G. L. Attachment and ingestion of gonococci human neutrophils. Infect Immun. 1975 Mar;11(3):512–516. doi: 10.1128/iai.11.3.512-516.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip C., Fletcher G., Wulff J. L., Earhart C. F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973 Sep;115(3):717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail G., Sawyer W. D., Wegener W. S. Effect of hydrogen peroxidase and superoxide radical on viability of Neisseria gonorrhoeae and related bacteria. Proc Soc Exp Biol Med. 1977 Jun;155(2):264–269. doi: 10.3181/00379727-155-39786. [DOI] [PubMed] [Google Scholar]
- Janoff A., Blondin J. The role of elastase in the digestion of E. coli proteins by human polymorphonuclear leukocytes. I. Experiments in vitro. Proc Soc Exp Biol Med. 1974 Apr;145(4):1427–1430. doi: 10.3181/00379727-145-38027. [DOI] [PubMed] [Google Scholar]
- KELLOGG D. S., Jr, PEACOCK W. L., Jr, DEACON W. E., BROWN L., PIRKLE D. I. NEISSERIA GONORRHOEAE. I. VIRULENCE GENETICALLY LINKED TO CLONAL VARIATION. J Bacteriol. 1963 Jun;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg D. S., Jr, Cohen I. R., Norins L. C., Schroeter A. L., Reising G. Neisseria gonorrhoeae. II. Colonial variation and pathogenicity during 35 months in vitro. J Bacteriol. 1968 Sep;96(3):596–605. doi: 10.1128/jb.96.3.596-605.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G. J., Swanson J. Studies on gonococcus infection. XV. Identification of surface proteins of Neisseria gonorrhoeae correlated with leukocyte association. Infect Immun. 1978 Aug;21(2):575–584. doi: 10.1128/iai.21.2.575-584.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Novotny P., Short J. A., Hughes M., Miler J. J., Syrett C., Turner W. H., Harris J. R., MacLennan I. P. Studies on the mechanism of pathogenicity of Neisseria gonorrhoeae. J Med Microbiol. 1977 Aug;10(3):347–365. doi: 10.1099/00222615-10-3-347. [DOI] [PubMed] [Google Scholar]
- Odeberg H., Olsson I. Microbicidal mechanisms of human granulocytes: synergistic effects of granulocyte elastase and myeloperoxidase or chymotrypsin-like cationic protein. Infect Immun. 1976 Dec;14(6):1276–1283. doi: 10.1128/iai.14.6.1276-1283.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson I., Venge P. Cationic proteins of human granulocytes. II. Separation of the cationic proteins of the granules of leukemic myeloid cells. Blood. 1974 Aug;44(2):235–246. [PubMed] [Google Scholar]
- Ovcinnikov N. M., Delektorskij V. V. Electron microscope studies of gonococci in the urethral secretions of patients with gonorrhoea. Br J Vener Dis. 1971 Dec;47(6):419–439. doi: 10.1136/sti.47.6.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rest R. F. Killing of Neisseria gonorrhoeae by human polymorphonuclear neutrophil granule extracts. Infect Immun. 1979 Aug;25(2):574–579. doi: 10.1128/iai.25.2.574-579.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R. S., Fulbright R. S., Eads M. E., Sawyer W. D. Ethylenediaminetetraacetic acid-sensitive antiphagocytic activity of Neisseria gonorrhoeae. Infect Immun. 1977 Mar;15(3):817–827. doi: 10.1128/iai.15.3.817-827.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XII. Colony color and opacity varienats of gonococci. Infect Immun. 1978 Jan;19(1):320–331. doi: 10.1128/iai.19.1.320-331.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XIV. Cell wall protein differences among color/opacity colony variants of Neisseria gonorrhoeae. Infect Immun. 1978 Jul;21(1):292–302. doi: 10.1128/iai.21.1.292-302.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thongthai C., Sawyer W. D. Studies on the virulence of Neisseria gonorrhoeae. I. Relation of colonial morphology and resistance to phagocytosis by polymorphonuclear leukocytes. Infect Immun. 1973 Mar;7(3):373–379. doi: 10.1128/iai.7.3.373-379.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne K. J., Oliver R. C., Barrett A. J. Lysis and killing of bacteria by lysosomal proteinases. Infect Immun. 1976 Aug;14(2):555–563. doi: 10.1128/iai.14.2.555-563.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser L., Blout E. R. The use of p-nitrophenyl N-tert-butyloxycarbonyl-L-alaninate as substrate for elastase. Biochim Biophys Acta. 1972 Apr 7;268(1):257–260. doi: 10.1016/0005-2744(72)90223-9. [DOI] [PubMed] [Google Scholar]
- Walstad D. L., Guymon L. F., Sparling P. F. Altered outer membrane protein in different colonial types of Neisseria gonorrhoeae. J Bacteriol. 1977 Mar;129(3):1623–1627. doi: 10.1128/jb.129.3.1623-1627.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]