Abstract
Background
Earlier tPA treatment for acute ischemic stroke increases efficacy, prompting national efforts to reduce door-to-needle times (DNTs). We utilized lean process improvement methodology to develop a streamlined IV tPA protocol.
Methods
In early 2011, a multi-disciplinary team analyzed the steps required to treat acute ischemic stroke patients with IV tPA, utilizing value stream analysis (VSA). We directly compared the tPA-treated patients in the “pre-VSA” epoch to the “post-VSA” epoch with regard to baseline characteristics, protocol metrics, and clinical outcomes.
Results
The VSA revealed several tPA protocol inefficiencies: routing of patients to room, then to CT, then back to room; serial processing of work flow; and delays in waiting for lab results. On 3/1/2011, a new protocol incorporated changes to minimize delays: routing patients directly to head CT prior to patient room, utilizing parallel process work-flow, and implementing point-of-care labs. In the pre-and post-VSA epochs, 132 and 87 patients were treated with IV tPA, respectively. Compared to pre-VSA, DNTs and percent of patients treated ≤60 minutes from hospital arrival were improved in the post-VSA epoch: 60 min vs. 39 min (p<0.0001) and 52% vs. 78% (p<0.0001), respectively, with no change in symptomatic hemorrhage rate.
Conclusions
Lean process improvement methodology can expedite time-dependent stroke care, without compromising safety.
Keywords: door-to-needle time, acute stroke protocol, tPA, thrombolytic, value stream analysis, lean manufacturing
Introduction
Clinical outcomes are improved as a function of earlier tPA delivery1, 2. Therefore, national guidelines have encouraged rapid evaluation and treatment of acute ischemic stroke patients. Stroke-specific American Heart Association “Get-With-The-Guidelines” (GWTG) were created to ensure that evidence-based practices were adopted by hospitals and incorporated into stroke patient care3. One GWTG metric is a goal time from patient arrival to treatment with IV tPA [door-to-needle time (DNT)] of ≤60 minutes. Despite the known time-dependence of tPA efficacy, only 27% of 25,000 tPA-treated patients within the GTWG database received tPA within 60 minutes from hospital arrival4.
Lean manufacturing principles were originally pioneered by Taiichi Ohno, father of the Toyota Production System, who aimed to eliminate inefficiencies within automobile production, leaving only the crucial steps that added value to the customer5. After five decades of utilization in the manufacturing sector, lean principles have been recently applied to healthcare, leading to shorter emergency department (ED) wait times and improved procurement of endovascular stents within radiology departments6, 7.
In early 2011, we assembled a multi-disciplinary team to employ a common lean tool known as Value Stream Analysis (VSA) to improve DNTs for stroke patients receiving IV tPA. The “current-state analysis” mapped out wasteful operations and those that added value. A “future-state analysis” removed wasteful steps and retained value-added steps. An “action plan” was created to implement the streamlined protocol and provide feedback for continued improvement. The protocol’s efficiency and safety metrics were compared before and after implementation.
Methods
Data Collection
Data were prospectively collected as part of the Cognitive Rehabilitation Research Group Stroke Registry developed at Washington University in 1998. This registry includes stroke patients admitted to an urban, tertiary care hospital admitting 1500 stroke patients annually. In the current study, tPA-treated stroke patients were included with the following clinical information: demographics, past medical history, admission National Institutes of Health Stroke Scale (NIHSS), DNT, onset-to-needle time (ONT), door-to-CT completion, and door-to-laboratory completion times, discharge location, 90 day modified Rankin Scale (mRS), and length of hospital stay (LOS). Symptomatic intracerebral hemorrhage (sICH) (defined as in the NINDS tPA trial as ICH within 36 hours of symptom onset that correlated with any decline in neurological status) and stroke mimics (defined as a discharge diagnosis other than ischemic stroke) were retrospectively collected from the patients’ charts.
Lean Improvement Process and Value Stream Analysis
Beginning in 2006, the hospital’s leadership made a commitment to improving the “flow of value” to patients by applying lean principles. Lean performance engineers utilized several lean tools for process improvement directed at multiple disease processes, one of which was stroke. We present the results of one of several lean strategies, known as VSA, which was utilized to expedite tPA delivery to acute stroke patients arriving in the ED.
Briefly, a lean engineer, ED and neurology physicians, ED nurses, patient care and radiology technicians, and an ED pharmacist met over the course of two days to perform a VSA which involved four steps: 1) “current-state analysis”: each step of the tPA protocol was identified as a source of lost productivity (i.e. waste) or a step that “added value”; 2) “future-state analysis”: a “lean” process was formulated to minimize any wasteful operations; 3) an “action plan” was created for rapid implementation of the new protocol; and 4) a “feedback loop” was instituted to continuously identify and eliminate waste over time (online supplement).
Statistical Analysis
The “pre-VSA” epoch (1/1/2009–2/28/2011) was compared to the “post-VSA” epoch (3/1/2011–3/1/2012) with regard to baseline characteristics, protocol metrics, and clinical outcomes. Wilcoxon Rank Sum and Fisher’s Exact tests measured differences in continuous and binary data, respectively (p<0.05 required for significance). To adjust for imbalances in baseline characteristics, analysis of covariance was performed including all imbalanced variables as covariates and pre vs. post-VSA as a fixed effect on DNTs. Kruskal-Willis analysis of variance measured differences in DNT over the four quarters post-VSA to determine if DNTs were sustained. To assess which baseline or protocol-related variables predicted DNT post-VSA, a linear regression model was created with predictors selected using a forward stepwise procedure (p≤0.20 required for entry; p≤0.05 required to be retained). Predictors considered for the model included door-to-CT time and all baseline characteristics (VSA was not included as a variable since door-to-CT was a major change within the VSA).
Results
Streamlined Protocol Resulting from the VSA (Figure)
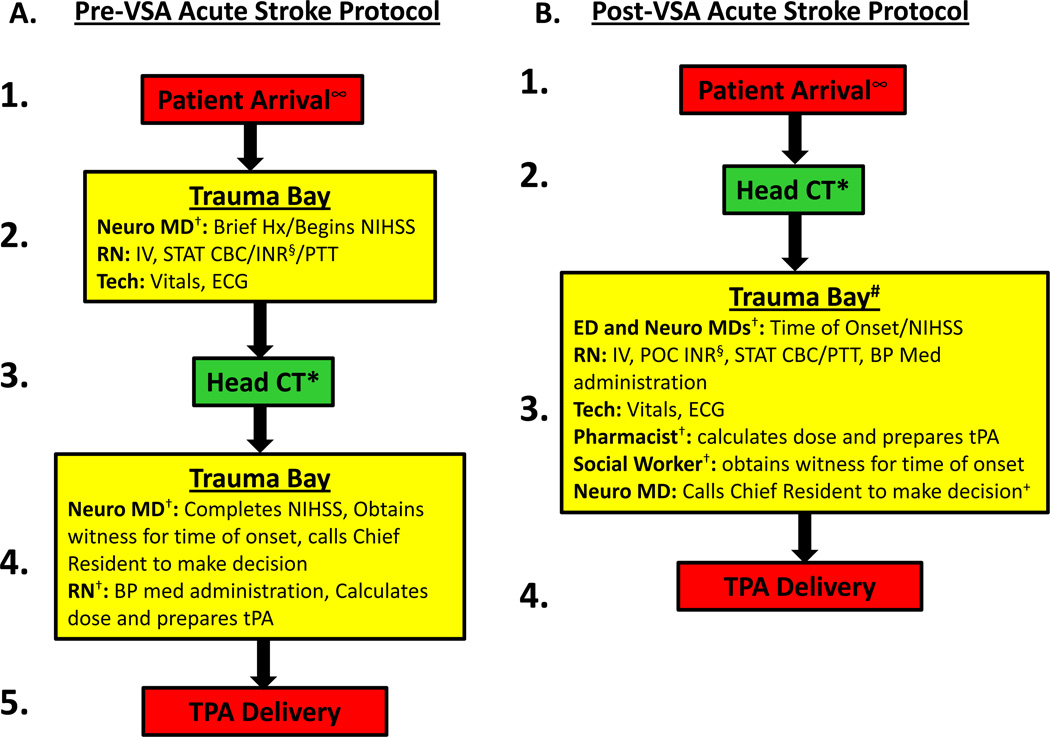
The VSA identified several barriers preventing rapid tPA treatment including: 1) inefficient patient flow, requiring a patient to be routed by EMS to the trauma bay, then to CT, then back to the room; 2) serial processing of multiple tasks and inefficient use of available staff; and 3) delays in laboratory processing in patients suspected of being on anticoagulation. After the VSA, the streamlined acute stroke protocol addressed these sources of waste identified above while retaining value-added steps (Figure): 1) patients were taken directly to the CT by EMS, and then brought to the trauma bay; 2) the ED and neurology residents were assigned distinct and parallel tasks: obtaining the brief history and performing the NIHSS; a pharmacist and social worker were added to the team to calculate and prepare tPA dose, and bring witnesses to the bedside (or via phone) for physicians to determine time of onset; and 3) point of care (POC) testing for INR was implemented.
Figure. Acute stroke protocol pre-VSA (A) and post-VSA (B).
∞ EMS pre-notified the ED triage nurse of the patient’s arrival so that treating physicians and staff were waiting and ready to evaluate the patient immediately upon arrival. The patient could not be pre-registered and prior medical records could not be investigated prior to patient arrival as EMS was not allowed to give patient identifiers via radio due to violation of patient privacy.
† Serial tasks were changed to In-parallel.
* Head CT moved to first step in the protocol.
# Transport from CT to trauma bay might be considered “time-wasteful”; however, three reasons prevented utilizing the CT scanner as the location for tPA delivery: (1) given the ED traffic and the demand for CT utilization, it was necessary to permit its use for other disease categories (such as trauma and others); (2) transport from CT scanner to trauma bay is about 30 seconds; and (3) the space and lighting for patient evaluation in the trauma bay are superior to that in the CT scanner room.
§ Point-of-Care INR sent (useful for patients suspected or known to be taking coumadin/warfarin).
+ Calling the Chief Resident might be considered “time-wasteful”; however, we ultimately decided that it “added value” by ensuring tPA administration was supervised by experienced physicians, ensuring safe and appropriate tPA delivery.
VSA=Value Stream Analysis; Neuro MD=neurology medical doctor; Hx=history; NIHSS=National Institutes of Health Stroke Scale; RN=registered nurse; Tech=patient care technician.
Patient Characteristics (Table 1)
In the pre-VSA and post-VSA epochs, 132 and 87 ischemic stroke patients were treated with IV tPA, respectively. Shorter onset-to-arrival time and arrival “off-hours” (Friday 5PM-Monday 7AM) have been associated with longer tPA delivery and did not differ between the two cohorts2, 4, 8.
Protocol metrics and clinical outcomes (Table 2, S1–S5)
Compared to pre-VSA, DNTs and percent of patients treated ≤60 minutes from hospital arrival were improved post-VSA: 60min vs. 39min (p<0.0001) and 52% vs. 78% (p<0.0001), respectively. After adjustment for imbalanced baseline characteristics, the post-VSA epoch continued to demonstrate significantly lower DNTs, (F=32.4, p<0.0001). The frequency distribution shifted to shorter DNTs post-VSA, with fewer outliers beyond 100 min (S5). DNT improvement also translated to improved ONTs (p=0.016).
To determine if improved DNTs were sustained throughout the post-VSA year and were not initially reduced due to the novelty of the protocol, the quarterly DNTs were compared and did not differ across the year, p=0.88 (S1). Moreover, the volume of tPA-treated patients did not appear to adversely affect DNTs as a consistent increase in volume was seen in the post-VSA epoch (S2).
As head CT became the first step in the protocol, door-to-CT times were lower post-VSA (p<0.0001). To determine if routing patients directly to CT contributed to lower DNTs, linear regression for prediction of DNT was performed. The final model included door-to-CT time [regression coefficient, β (standard error, SE)] = [0.76 (0.20)], p<0.0001, and onset-to-arrival time [β(SE)] = [−0.098(0.046)], p=0.035. Thus, shorter door-to-CT and longer onset-to-arrival times were associated with shorter DNTs.
To determine if the protocol’s efficiency was at the expense of patient safety, clinical outcomes were compared. There were no differences in sICH, discharge location, 90 day mRS, LOS, and stroke mimic rate between the two epochs.
Discussion
The current study examined the application of “lean” towards quality improvement for expediting tPA administration at an urban, tertiary care hospital. With key members of the stroke team present at a two-day VSA, several unnecessary and redundant operations were identified and eliminated, leading to lower DNTs with 78% of patients receiving tPA within one hour of hospital arrival. Post-VSA, the tPA frequency distribution shifted left on the time axis and became more narrowly distributed with fewer outliers, suggesting not only shorter treatment times, but also less variability in treatment times (S5). This protocol change did not compromise patient safety, as symptomatic ICH rates and clinical outcomes did not differ.
Our data suggest that removing the additional transportation step (having EMS route patients directly to head CT, Figure) played a large role in the shorter DNT post-VSA (Table 2; linear regression model showing door-to-CT times independently predicted DNTs). Another variable which predicted shorter DNTs was greater onset-to-arrival times which has been found in several large IV tPA datasets2, 4 and indicates an additional education opportunity to avoid “exhausting” the tPA window when a patient arrives early.
Table 2.
Protocol Metrics and Outcomes Pre- and Post-VSA.
| Pre-VSA 1/1/2009–2/28/2011 N=132 |
Post-VSA 3/1/2011–3/1/2012 N=87 |
P-value | |
|---|---|---|---|
| Door-to-Needle Time, min* | 60 [46,73] | 39 [28,56] | <0.0001 |
| % Patients with DNT ≤60min | 52% | 78% | <0.0001 |
| Onset-to-Needle Time, min* | 131 [105,165] | 111 [80,158] | 0.016 |
| Door-to-CT Time**, min* | 16 [10,22] | 1 [0,4] | <0.0001 |
| Door-to-CBC Time**, min* | 22 [16,29] | 24 [17,34] | 0.13 |
| Door-to-PTT Time**, min* | 34 [29,42] | 40 [31,47] | 0.14 |
| Symptomatic ICH | 3.0% | 3.4% | 1.0 |
| Favorable Discharge Location+ | 76% | 83% | 0.24 |
| 90 day mRS 0–2++ | 49% | 43% | 0.34 |
| Length of Hospital Stay, days* | 4 [3,7] | 3 [2,6] | 0.056 |
| Stroke Mimic | 6.8% | 11.5% | 0.33 |
CBC=Complete blood count
PTT=partial thromboplastin time
ICH=intracerebral hemorrhage
Reported as median [interquartile ratio].
Times represent time-to-test-completion.
Favorable discharge location=discharge to home or inpatient rehabilitation; unfavorable ischarge location=discharge to nursing home or in-hospital death
90 day mRS=Modified Rankin Score of 0–2 on telephone follow-up.
These data come from a single-center with small patient numbers, thus limiting our ability to detect potential differences in clinical outcomes. While the majority of data was prospectively collected, historical controls were used for the comparison cohort, and time-dependent variables unrelated to protocol changes could affect results. The stroke mimic rate did not significantly differ between the two cohorts, but was 11.5% post-VSA, with increased diagnoses due to conversion disorder (S3). While within the range of other studies reporting stroke mimic rates in tPA-treated patients9–11, expediting tPA may increase the number of stroke mimics treated and warrants continued monitoring of clinical outcomes and rate of sICH. Of note, both pre- and post-VSA stroke mimic rates may appear falsely low as some physicians may be reluctant to change the discharge diagnosis to stroke mimic if the patient has been treated with tPA.
Conclusion
Lean manufacturing principles were applied to expedite IV tPA delivery with dramatic reduction in DNTs and without compromising patient safety. Future studies may determine if this intervention is sustainable across various hospital settings.
Supplementary Material
Table 1.
Baseline Characteristics
| Pre-VSA 1/1/2009–2/28/2011 N=132 |
Post-VSA 3/1/2011–3/1/2012 N=87 |
P-value | |
|---|---|---|---|
| Age, years* | 70 [53,81] | 61 [50,74] | 0.023 |
| Gender, Female | 52% | 56% | 0.58 |
| Race, African-American | 45% | 68% | 0.001 |
| Initial NIHSS* | 9 [6,17] | 7 [4,14] | 0.022 |
| Onset-to-Arrival Time, min* | 62 [43,93] | 67 [38,91] | 0.75 |
| Patient Arrival “Off-hours”** | 41% | 44% | 0.78 |
| Past Medical History | |||
| Hypertension | 62% | 83% | 0.001 |
| Diabetes Mellitus | 25% | 31% | 0.35 |
| Coronary Artery Disease | 23% | 25% | 0.75 |
| Congestive Heart Failure | 15% | 15% | 1.0 |
| Prior Stroke / TIA | 20% | 29% | 0.19 |
| Tobacco Use | 25% | 37% | 0.07 |
| Dyslipidemia | 33% | 33% | 1.0 |
Reported as median [interquartile ratio].
“Off-hours”=arrival 5PM Friday-7AM Monday.
Acknowledgements
We would like to acknowledge the Washington University neurology and ED residents, Barnes-Jewish Hospital ED nurses, radiology and patient care technicians, and the St. Louis area EMS providers. We would like to acknowledge Brian Hoff, Lean Performance Engineer at Barnes-Jewish Hospital, for reviewing the manuscript for overall accuracy with regard to lean process improvement.
Sources of Funding: This study was supported by NIHK23NS069807 to AF, the Specialized Programs of Translational Research in Acute Stroke (SPOTRIAS) grant, NIH5P50NS055977, to JML/PP, and the Institute of Clinical and Translational Sciences at Washington University, UL1 TR000448.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None.
References
- 1.Hacke W, Donnan G, Fieschi C, Kaste M, von Kummer R, Broderick JP, et al. Association of outcome with early stroke treatment: Pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363:768–774. doi: 10.1016/S0140-6736(04)15692-4. [DOI] [PubMed] [Google Scholar]
- 2.Mikulik R, Kadlecova P, Czlonkowska A, Kobayashi A, Brozman M, Svigelj V, et al. Factors influencing in-hospital delay in treatment with intravenous thrombolysis. Stroke. 2012;43:1578–1583. doi: 10.1161/STROKEAHA.111.644120. [DOI] [PubMed] [Google Scholar]
- 3.Smaha LA. The American Heart Association get with the guidelines program. American Heart Journal. 2004;148:S46–S48. doi: 10.1016/j.ahj.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 4.Fonarow GC, Smith EE, Saver JL, Reeves MJ, Bhatt DL, Grau-Sepulveda MV, et al. Timeliness of tissue-type plasminogen activator therapy in acute ischemic stroke: Patient characteristics, hospital factors, and outcomes associated with door-to-needle times within 60 minutes. Circulation. 2011;123:750–758. doi: 10.1161/CIRCULATIONAHA.110.974675. [DOI] [PubMed] [Google Scholar]
- 5.Ohno T. The Toyota production system: Beyond large-scale production. Portland, OR: Productivity Press; 1988. [Google Scholar]
- 6.Ng D, Vail G, Thomas S, Schmidt N. Applying the lean principles of the Toyota production system to reduce wait times in the emergency department. Canadian Journal of Emergency Medicine. 2010;12:50–57. doi: 10.1017/s1481803500012021. [DOI] [PubMed] [Google Scholar]
- 7.Teichgraber UK, de Bucourt M. Applying value stream mapping techniques to eliminate non-value-added waste for the procurement of endovascular stents. European Journal of Radiology. 2012;81:e47–e52. doi: 10.1016/j.ejrad.2010.12.045. [DOI] [PubMed] [Google Scholar]
- 8.Saver JL, Smith EE, Fonarow GC, Reeves MJ, Zhao X, Olson DM, et al. The "golden hour" and acute brain ischemia: Presenting features and lytic therapy in >30,000 patients arriving within 60 minutes of stroke onset. Stroke. 2010;41:1431–1439. doi: 10.1161/STROKEAHA.110.583815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chernyshev OY, Martin-Schild S, Albright KC, Barreto A, Misra V, Acosta I, et al. Safety of tpa in stroke mimics and neuroimaging-negative cerebral ischemia. Neurology. 2010;74:1340–1345. doi: 10.1212/WNL.0b013e3181dad5a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winkler DT, Fluri F, Fuhr P, Wetzel SG, Lyrer PA, Ruegg S, et al. Thrombolysis in stroke mimics: Frequency, clinical characteristics, and outcome. Stroke. 2009;40:1522–1525. doi: 10.1161/STROKEAHA.108.530352. [DOI] [PubMed] [Google Scholar]
- 11.Artto V, Putaala J, Strbian D, Meretoja A, Piironen K, Liebkind R, et al. Stroke mimics and intravenous thrombolysis. Annals of Emergency Medicine. 2012;59:27–32. doi: 10.1016/j.annemergmed.2011.09.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.