Abstract
Information about object-associated manipulations is lateralized to left parietal regions, while information about the visual form of tools is represented bilaterally in ventral occipito-temporal cortex. It is unknown how lateralization of motor-relevant information in left hemisphere dorsal regions may affect the visual processing of manipulable objects. We used a lateralized masked priming paradigm to test for a Right Visual Field (RVF) advantage in tool processing. Target stimuli were tools and animals, and briefly presented primes were identical to, or scrambled versions of the targets. In Experiment 1, primes were presented either to the left or right of the centrally presented target, while in Experiment 2 primes were presented in one of 8 locations arranged radially around the target. In both experiments there was a RVF advantage in priming effects for tool but not for animal targets. Control experiments showed that participants were at chance for matching the identity of the lateralized primes in a picture-word matching experiment, and also ruled out a general RVF speed-of-processing advantage for tool images. These results indicate that the overrepresentation of tool knowledge in the left hemisphere affects visual object recognition, and suggest that interaction between the dorsal and ventral streams occurs during object categorization.
Introduction
An important issue is how basic principles of organization of the primate visual system contribute to determining the cortical organization of object knowledge. The first cortical stage of visual processing in the primate brain processes information from the contralateral visual field: early visual regions in the left hemisphere process stimuli presented to the right visual field across both eyes (RVF), and early visual regions in the right hemisphere process stimuli presented to the left visual field across both eyes (LVF; e.g., Engel, Glover, and Wandell, 1997; Fellemen and Van Essen, 1991). Independently of this property of the visual system, some domains of information are lateralized to the left or right hemispheres. For example language is predominantly left lateralized in the majority of individuals (e.g., Gazzaniga and Smylie, 1984; Knecht, Dräger, Deppe, Bobe, Lohmann, Flöel, et al., 2000). Investigations of hemispheric biases in visual processing of different classes of stimuli have found visual field advantages contralateral to the hemisphere that differentially represents that domain. For instance, there is a strong RVF advantage for recognition of printed words (e.g., Bub and Lewine, 1988; Finkbeiner, Almeida, and Caramazza, 2006; Hunter and Brysbaert, 2008). Knowledge about how to manipulate objects is also strongly left lateralized in the majority of individuals (Johnson-Frey, 2004; Kroliczak and Frey, 2009). The goal of the current investigation is to test the hypothesis that the overrepresentation of tool knowledge in the left hemisphere leads to a RVF advantage for visual processing of tool stimuli.
A second principle of organization within the primate visual system that is relevant to understanding hemispheric lateralization of tools is the division of labor between the dorsal and ventral visual pathways. The dorsal visual pathway projects from V1 to dorsal occipital cortex and posterior parietal cortex, while the ventral stream projects from V1 to ventral and lateral occipito-temporal regions, terminating in anterior temporal cortex (Goodale and Milner, 1992; Goodale, Milner, Jakobsen, and Carey, 1991; Ungerleider and Mishkin, 1982; see also Goodale, Kroliczak, and Westwood, 2005; Ungerleider, 1995). The classic understanding of the division of labor between these two streams is that the dorsal stream is critical for extracting visuomotor and spatial information relevant to action, while the ventral stream extracts object identity across variations in size, orientation, luminance, and distance.
fMRI studies indicate that tool knowledge is strongly left lateralized in the dorsal stream but not in the ventral stream. Specifically, viewing tools compared to a baseline category such as animals, elicits differential BOLD responses in the left ventral premotor cortex, the left inferior parietal lobule, and the left middle temporal gyrus, while the same contrast leads to differential activation bilaterally in the medial fusiform gyrus in the ventral stream (Chao and Martin, 2000; Mahon, Milleville, Negri, Rumiati, Caramazza, and Martin, 2007; Nopponey, Price, Penny, and Friston, 2006; for reviews see Lewis, 2006; Martin, 2007).
Other functional imaging studies have further decomposed the function of the brain regions that comprise the tool network. For instance, Kellenbach, Brett, and Patterson (2003) identified regions in left posterior parietal cortex, left ventral premotor cortex, and the left posterior middle temporal gyrus that were activated when participants made decisions about the motor movements associated with object use (see also Boronat, Buxbaum, Coslett, Tang, Saffran, Kimberg, et al., 2005). Canessa, Borgo, Cappa, Perani, Falini, Buccino and colleagues (2008) replicated the findings of Kellenbach and colleagues (2003) and Boronat and colleagues (2005) in left parietal cortex, and also found that judgments about object function lead to differential activation near the left temporal pole (see also Anzellotti, Mahon, Schwarzbach, and Caramazza, 2011 for relevant findings). Those functional imaging data converge with the patterns of impairments observed in brain damaged patients: brain damage affecting left hemisphere parietal structures can lead to impairments for knowledge of how to manipulate tools (Johnson-Frey, 2004; Mahon et al., 2007; Tranel, Damasio, and Damasio, 1997). In contrast, neurological diseases that lead to deterioration of anterior temporal cortices (e.g., semantic dementia, Alzheimer’s disease, herpes simplex encephalitis) can be associated with impaired knowledge of object function and spared knowledge of how to manipulate objects (e.g., Negri, Lunardelli, Reverberi, Gigli, and Rumiati, 2007b; Sirigu, Duhamel, and Poncet, 1991; but see Hodges, Spatt, and Patterson, 1999). An interesting and currently debated issue is whether the left lateralized organization of visuomotor knowledge of tools and their action based properties follows the lateralization of language representations, irrespective of handedness (e.g., see Kroliczak, Piper, and Frey, 2011; Kroliczak and Frey, 2009; for review see Roby-Brami, Hermsdörfer, Roy, and Jacobs, 2011).
There is some indication that the overrepresentation of tools in left dorsal stream regions has behavioral consequences. In a recent bilateral visual field presentation experiment, Verma and Brysbaert (2011) reported that participants were 17 ms faster to recognize tools when the to-be-recognized tool was presented to the RVF; importantly, the RVF advantage was not found in an object/non-object categorization experiment that presented images in the identical visual field locations (see also Hunter and Brysbaert, 2008 for RVF effects with linguistic stimuli). Handy, Grafton, Shroff, Ketay, and Gazzaniga (2003) used a combination of ERP (Experiments 1 and 2) and fMRI (Experiment 3) to investigate spatial attention for tools; the right visual field, along with the lower visual field, was found to dominantly capture participants’ attention (as measured with the contralateral P1 response at lateral occipital recording sites in the ERP experiments). Handy and colleagues argued that the differential P1 activity observed when tools were presented in the lower right visual quadrant reflected the extraction of visuomotor information that implicitly biases attention toward that location. The results from their fMRI experiment suggest that the RVF effects were mediated by left-lateralized parietal and pre-motor brain regions that process action-related properties of tools.
Thus, on the basis of previous research there is some indication that tools may enjoy a RVF advantage for visual analysis. Here we sought to directly evaluate this issue by asking participants to categorize centrally presented images of tools and animals that were immediately preceded by lateralized primes. In Experiment 1, the primes were presented to the left or to the right of the target position. In Experiment 2, in order to more fully characterize visual field biases, primes were presented in 8 positions around the target location. In both experiments, the primes were presented briefly and a visual mask was presented immediately after the primes in order to reduce top down influences that are known to affect object identification (for discussion see e.g., Di Lollo, Enns, and Rensink, 2000; VanRullen and Koch, 2003). The goal was not to render prime stimuli ‘invisible’ or ‘unconscious’ but to limit the contribution of possible strategic factors that may operate when primes are fully visible.
Beyond testing the neurocognitive hypothesis that left lateralization of tool knowledge may affect visual object processing, this set of experiments also permits an evaluation of an important theory about how tool concepts are represented. The embodied cognition hypothesis of tool recognition argues that the ability to recognize tools depends on simulation of motor-relevant information about how to manipulate those objects (Gallese and Lakoff, 2005; Kiefer and Pulvermüller, in press; Pulvermüller, 2005; Simmons and Barsalou, 2003). A number of different types of evidence have been marshaled in support of that hypothesis; however, common to all of the data argued to support the embodied cognition hypothesis as it relates to tool recognition is the observation that motor relevant information is automatically activated in the course of visually processing of tools (e.g., see Chao and Martin, 2000; for reviews see Barsalou, 2008; Gallese and Lakoff, 2005; Martin, 2007; for theoretical precedent see Allport, 1985). We have argued (e.g., Mahon and Caramazza, 2008) that those data are ambiguous, in that they could indicate either that 1) visual recognition of a tool concept includes (representationally, or constitutively) motor-relevant information about how to manipulate objects (i.e., embodied hypothesis of tool recognition), or 2) that high-level visual representations subserving tool recognition have privileged connectivity with parietal structures that represent their associated manipulations, and that the action-based information is retrieved subsequent to, or contingent on, visual recognition (for relevant findings, see Mahon et al., 2007).
Transcranial magnetic stimulation (TMS) studies have sought to evaluate the involvement of motor representations during conceptual processing. For instance, Pulvermüller, Hauk, Nikulin, and Ilmoniemi (2005) found that stimulating somatotopic-specific sites (i.e., arm and leg sites) along motor cortex selectively affected lexical decisions for arm (e.g., pick) and leg (e.g., kick) related words (respectively). Similarly, after stimulating motor cortex and measuring motor-evoked potentials (MEPs) in distal hand muscles, Oliveri, Finocchiaro, Shapiro, Gangitano, Caramazza, and Pascual-Leone (2004) found a selective increase in MEPs when participants passively read action verbs. Functional neuroimaging experiments also provide converging evidence that the motor system is involved, in a somatotopic-specific way, when observing (Buccino, Binkofski, Fink, Fadiga, Fogassi, Gallese, et al., 2001) or passively reading (Hauk, Johnsrude, and Pulvermüller, 2004) mouth-, arm-, and foot-related actions (for review see Barsalou, 2008; Pulvermüller, 2005). Those studies show, decisively, that there is an association between motor system activation and the processing action-related stimuli. However, it remains an open issue as to whether motor information forms a part (constitutively) of the conceptual representations of action words, or whether spreading activation among conceptual and motor representations could explain the observed associations (e.g., Mahon and Caramazza, 2008).
The available neuropsychological data argue against strong forms of the embodied cognition hypothesis: patient evidence indicates that damage to parietal regions can lead to impairments for using objects while naming and categorizing the same objects can be spared (for reviews and discussion, see Chatterjee, 2010; Johnson-Frey, 2004; Mahon and Caramazza, 2005; Negri, Rumiati, Zadini, Ukmar, Mahon, and Caramazza, 2007a; but see Pazzaglia, Pizzamiglio, Pes, and Aglioti, 2008). If those patient data are taken at face value, then the hypothesis of privileged connectivity between the processes subserving high-level visual recognition of tools and knowledge of how to manipulate tools becomes more likely. Thus, if in the priming experiments reported below, we were to observe a RVF bias for tools then those data would suggest that the bias is driven, at least in part, by interactions between brain regions that support visuomotor processing (dorsal stream) and brain regions that are known to be involved in visual object recognition and categorization (ventral stream).
Experiment 1
Methods
Participants
Seventeen University of Rochester undergraduate students (2 male) participated in the experiment in exchange for payment (age: 19 to 25 years, M = 20.3 years, SD = 1.7 years). Sixteen of the participants were strongly right-handed, as established with the Edinburgh Handedness Questionnaire (average handedness coefficient for right-handed participants = .94; average handedness coefficient for the left-handed participant = −.69). The participants all had normal or corrected-to normal vision, and gave written informed consent in accordance with the University of Rochester participant review board.
Materials
60 items (30 tools, 30 animals; see Supplemental Table 3) were used as stimuli for both primes and targets. The items were matched on lexical frequency (Celex; tools, M = 20.2, SD = 37.2; animals, M = 22.8, SD = 30.6; t < 1), and concept familiarity (MRC Psycholinguist Database; tools, M = 519.3, SD = 62.4; animals, M = 519.8, SD = 33.9; t < 1). Seventy percent additive noise was overlaid on the prime and target stimuli to facilitate the impact of the primes on the targets, as well as reduce their visibility (for precedent on this procedure, see Almeida, Mahon, Nakayama, and Caramazza, 2008; Almeida, Mahon, and Caramazza, 2010). Primes were always identical to target images; for the scrambled baseline, the identity prime was the image that was scrambled, in order to preserve all low level visual information between the intact and scrambled conditions.
Testing Apparatus
The experiment was run on a desktop computer monitor (1920×1080 pixels; temporal resolution = 60Hz; viewing distance = 60 cm) using E Prime 2.0 software (Psychology Software Tools, Pittsburgh, PA) and a serial response box with ms precision (Psychology Software Tools, Pittsburgh, PA). Target stimuli were presented centrally, and subtended ~ 5° of visual angle; prime and mask stimuli were the same size as target stimuli and the outside edges of the targets were ~ .28 ° from the inside edges of prime and mask stimuli.
Design
The design of Experiment 1 was a 2 (prime location; left, right) by 2 (category of target; animal, tool) by 2 (prime condition; identity, scrambled) within-subjects design. Each replication of the design was distributed over 3 blocks (80 trials/block, block duration ~6 minutes). Of the 80 trials within a block, half were animal and half tool targets, half identity primes and half scrambled primes, and half LVF primes and half RVF primes. Trial order within a block was random. One participant completed the design 9 times, and the remainder of participants completed the design 10 times (40,560 trials across all participants).
Procedure
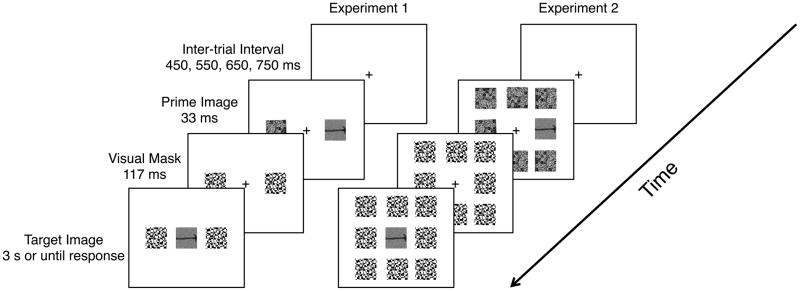
The experimenter first named each stimulus to the participant to ensure proper categorization in the experimental task; the experiment began immediately afterwards. Figure 1 shows a schematic of the detailed trial structure. On each trial, a fixation cross appeared for a jittered amount of time (450, 550, 650, or 750 milliseconds (ms); 27, 33, 39, 45 monitor refreshes); then, the prime, either a tool or animal, or a scrambled image of a tool or animal was presented to the left or the right of the fixation cross for approximately 33 ms (2 monitor refreshes). When a prime (either intact or scrambled) was presented on either the left or the right, a scrambled image (within category) was presented in the opposite prime location. Bilateral stimulation was used to render it more difficult to determine on which side of the screen a prime had been presented. The result was that in the scrambled-prime condition, scrambled primes were presented bilaterally, while in the identity-prime condition, an intact prime image (identical to the to-be-seen target) was presented on one side while a scrambled image was presented for the same duration on the opposite side. Immediately after the offset of the prime a black and white high contrast pattern mask was presented in the same spatial location as the prime, and a different mask was presented simultaneously in the same location as the scrambled within-category image. The mask was on the screen for approximately 117 ms (7 monitor refreshes). Immediately upon the offset of the mask, the target image was presented in the center of the screen for 3 seconds or until a response was registered. Participants responded with their right index finger if the target was an animal and their right middle finger if the target was a tool. They were instructed that they may see some flickering to the left and right of fixation, but to focus on correctly identifying the target as fast and as accurately as possible.
Figure 1.
Schematic of trial structure in Experiments 1 and 2. Panel A. In Experiments 1 and 2, participants were instructed to categorize the target image as ‘tool’ or ‘animal’ with the index or middle fingers of the right hand. Each trial began with a jittered fixation (450, 550, 650, or 750 ms). In Experiment 1, the prime was then presented to the left or right of fixation for 33 ms; in Experiment 2 (Panel B) the prime was presented in one of eight locations arranged around central fixation. A scrambled image was presented at the same time as the prime in the other possible prime location(s). Visual masks were then presented (duration = 117 ms) in the same location as the prime as well as the locations of the scrambled stimuli. The offset of the mask was immediately followed by the target, which was on the screen for 3 seconds or until a response was given. In the follow-up prime discrimination task, the targets were replaced with question marks, and participants were told to discriminate the prime image when the question mark was presented. The trial structure for the prime discrimination task was exactly the same as in the main experiment.
After the completion of the main experiment, participants were debriefed that on some trials, primes had been briefly presented to the left or right of fixation before the target image. They then took part in a prime discrimination task. The trial structure and procedure was the same except that target images were replaced with a question mark, and there was no scrambled prime condition; participants completed 120 prime discrimination trials. Participants responded when the question mark appeared with their right index finger if the prime image was an animal, and their right middle finger if the prime image was a tool, indicating their ‘best guess’ as to what had just been presented.
Results
Only correct response times were analyzed (3.4% of all trials were errors). Anticipations (response times faster than 200 ms) and outliers (greater than 2 standard deviations above and below the mean for each participant, calculated across all conditions) were removed (3.9% of correct trials were excluded according to those criteria). Response times, priming effects, standard deviations, and error rates for each cell of the design are shown in Table 1.
Table 1.
Mean response times (ms), priming effect (ms), standard deviations (SD, ms) and error rates by target category, prime location, and prime condition in Experiment 1.
| Prime on the Left | Prime on the Right | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Target Category | Target Category | |||||||||||
| Tool | Animal | Tool | Animal | |||||||||
| RT | SD | % | RT | SD | % | RT | SD | % | RT | SD | % | |
| Identity | 487 | 64 | .04 | 481 | 52 | .03 | 480 | 61 | .03 | 480 | 54 | .04 |
| Scrambled | 493 | 60 | .04 | 490 | 50 | .03 | 493 | 60 | .04 | 490 | 50 | .03 |
| Priming Effect | 6 | 9 | 13 | 10 | ||||||||
A three-way ANOVA contrasted the factors Prime Location, Target Category, and Prime Condition. There was a main effect of Prime Condition (F(1, 16) = 21.51, MSE = 142.11, p < .001), indicating faster response times for identity primes than scrambled primes. There was no main effect of Target Category (F < 1), and a trend toward a main effect of Prime Location (F(1, 16) = 3.07, MSE = 39.93, p < .10). The three-way ANOVA with the factors Target Category, Prime Location, and Prime Condition was marginally significant (F(1, 16) = 4.16, MSE = 35.27, p = .058).
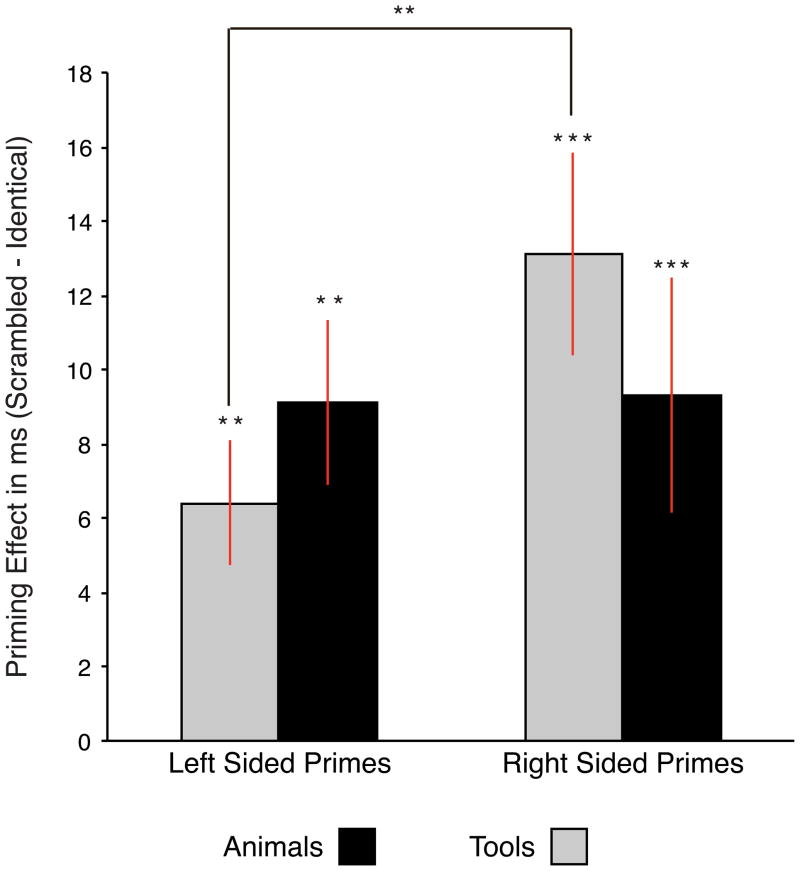
Two separate two-way ANOVAs with the factors Prime Identity and Prime Location were carried out for tool and animal targets. For tools, there was a main effect of Prime Location (F (1, 16) = 5.24, MSE = 31.18, p < .05; left-sided primes, 486 ms, SD, 64 ms; right-sided primes, 480 ms, SD, 61 ms), and Prime Identity (F (1, 16 = 23.25, MSE = 69.6, p < .001; identity prime trials, mean, 483 ms, SD, 62 ms; scrambled prime trials, mean, 493 ms, SD, 60 ms). In addition, the interaction between Prime Location and Prime Identity was significant (F (1, 16) = 5.75, MSE = 39.03, p < .05). Planned contrasts (t-tests, two-tailed) collapsed the left- and right-sided scrambled condition to form a single baseline condition for the derivation of priming effects. There were significant priming effects (identity RT < scrambled RT) for primes presented on the left (t(16) = 3.74, p < .01; mean priming effect, 6.38 ms, SD, 6.94 ms) and on the right (t(16) = 4.21, p < .01; mean priming effect, 13.12 ms, SD, 11.33 ms); consistent with the significant two way interaction described above, right-sided tool primes led to greater priming effects than left-sided tool primes (t(16) = 3.21, p < .01; mean difference in priming effects, 6.73 ms, SD = 8.66 ms).
For animal targets, there was a main effect of Prime Condition (F (1, 16) = 13.14, MSE = 109.73, p < .01; identity prime trials, mean, 480 ms, SD, 53 ms; scrambled prime trials, mean, 490 ms, SD, 50 ms), but no main effect of Prime Location (left-sided prime trials, mean, 481 ms, SD, 52 ms; right-sided prime trials, mean, 480 ms, SD, 54 ms), nor an interaction between the two factors (F < 1). Thus, while identity animal primes elicited priming effects in general for animal targets, the magnitude of the priming effect was not modulated according to whether the prime was presented on the left or the right (see Figure 2).
Figure 2.
Behavioral priming effects (Scrambled - Identity) for Experiment 1 displayed as a function of prime location and category. Error bars reflect standard errors of the mean across participants. * p < .05; ** p < .01; *** p < .001.
Prime Discrimination Data
Overall, the primes were partially visible to participants, at least as their visibility was relevant to making a categorization decision over the prime pictures (average prime discrimination accuracy = 68%; chance = 50%; cut-off for significantly different than chance at p = .05 was 59%; see Supplemental Table 1 for details). A 2×2 ANOVA contrasting Prime Location and Prime Category showed that neither the main effect of Prime Category (F < 1), nor of Prime Location (F (1, 16) = 1.78, MSE = .03, p > .20), nor the interaction (F < 1) between the two factors, was significant.
Discussion
Experiment 1 used lateralized primes together with a categorization task over tool and animal target pictures to test whether the known overrepresentation of tools in the left hemisphere affects visual object recognition. We observed that tool categorization was facilitated more when tool primes were presented on the right than when tool primes were presented on the left. In contrast, there was no modulation of categorization latencies for animal targets according to whether the primes were presented on the left or the right. The lack of modulation of animal categorization latencies by the location of the primes rules out a general RVF advantage in priming as being responsible for the pattern observed for tool targets.
In Experiment 2 we sought to replicate the principal finding of a RVF advantage for tool priming, and to also more fully characterize visual field biases in tool processing by testing eight spatial positions around central fixation. There are some indications that the upper visual field may be overrepresented by processing in the ventral stream (Previc, 1990). Given that categorization of the target images is known to be subserved by ventral stream processes, it can be predicted that the RVF advantage for tool primes will be more pronounced for the upper visual field compared to the lower visual field.
Experiment 2
Methods
Participants
Thirty-six University of Rochester undergraduate students (8 male) participated in the experiment in exchange for payment (18 to 24 years old, M = 19.8, SD = 1.7 years). Thirty-five participants were strongly right-handed (average handedness coefficient for right-handed participants = .93; average handedness coefficient for the left-handed participant = −.81). All participants had normal or corrected-to-normal vision, and gave written informed consent in accordance with the University of Rochester participant review board. One participant from Experiment 1 took part in Experiment 2, and one participant from Experiment 2 took part in control Experiment 2 (see below); all other participants did not overlap with any of the other experiments. The initial sample size for this experiment was 24 participants, at which point a interim analysis was conducted; however, in the context of addressing comments about those interim analyses, an additional twelve participants were added to Experiment 2 to increase statistical power, bringing the sample size to the current n of 36. This procedure, of analyzing data and then running additional participants, can increase false positive rate (see Simmons, Nelson, and Simonsohn, 2011). We note that the addition of 12 participants did not qualitatively change the pattern of results, and that the principal effects (described below) that are significant with 36 participants were significant with 24 participants.
Materials
The same materials used in Experiment 1 were used in Experiment 2.
Design
The experiment was a 2 (category of target; animal, tool) by 8 (prime location) by 2 (prime condition; identity, scrambled) within-subjects design. The full experimental design required 540 trials, and was distributed over 4 blocks with 135 trials per block. Each block contained 120 identity prime trials, and 15 scrambled prime trials (baseline for priming effects). Of the 120 identity prime trials, each animal stimulus and each tool stimulus was presented twice, each time in a different spatial location. The assignment of tool/animal stimuli to prime locations was chosen randomly for each block, with the constraint that all locations were equally represented across the 4 blocks. The 15 scrambled prime trials switched between 8 tool and 7 animal scrambled trials (and vice versa) across the 4 blocks. On each scrambled baseline trial, 8 different within-category scrambled primes were presented, one in each of the 8 spatial positions. The 36 participants each replicated the 4-block design 3 times (58,320 trials across all participants).
Procedure
The central target, and positions to the left and to the right of fixation subtended the same visual angle as in Experiment 1. The inside edge of prime and mask stimuli directly above and below the target was separated from the target by ~ .15 ° (outside edge of the target to inside edge of the prime). The inside corners of primes and masks presented in upper and lower left and right diagonals were ~ 2° from the nearest corner of the central target. All other aspects of the procedure and trial structure were the same as in Experiment 1 (see Figure 1B for details and a schematic of the trial structure).
After completion of the main experiment, participants were debriefed that on some trials, primes had been briefly presented across the visual field before the presentation of the target image. They then took part in a prime discrimination task. The trial structure and procedure was the same as in Experiment 2 except that target images were replaced with a question mark, and there was no scrambled prime condition; participants completed 480 prime discrimination trials (i.e., all stimuli were presented in each of the 8 prime locations). The design of the 480 trials was identical to that in the preceding categorization experiment (for a total of four blocks of 120 trials). When the question mark appeared, participants responded with their right index finger if the prime image was an animal, and their right middle finger if the prime image was a tool, indicating their ‘best guess’ as to what had just been presented.
Results
Trials on which the targets were mis-categorized (7.7% of all trials were removed) and outliers (following the same criteria as in Experiment 1, 4.5% of correct trials) were excluded. Degrees of freedom were Greenhouse-Geisser corrected when Mauchly’s Test for violation of the assumption of sphericity was significant. Response times, priming effects, standard deviations, and percent correct trials are shown in Table 2.
Table 2.
Mean response times (ms), priming effect (ms), standard deviations (SD in ms) and error rates (%) by location and stimulus category in Experiment 2.
| Response Time | Standard Deviation | Error Rate | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prime Location | Identity | Scrambled | Priming Effect | Identity | Scrambled | Identity | Scrambled | |||||||
| Tool | Animal | Tool | Animal | Tool | Animal | Tool | Animal | Tool | Animal | Tool | Animal | Tool | Animal | |
| 1 | 486 | 484 | 503 | 503 | 17 | 19 | 72 | 71 | 67 | 70 | .03 | .03 | .04 | .03 |
| 2 | 488 | 499 | 503 | 503 | 15 | 4 | 68 | 77 | 67 | 70 | .04 | .04 | .04 | .03 |
| 3 | 483 | 493 | 503 | 503 | 20 | 10 | 75 | 80 | 67 | 70 | .04 | .04 | .04 | .03 |
| 4 | 494 | 501 | 503 | 503 | 9 | 2 | 76 | 77 | 67 | 70 | .03 | .03 | .04 | .03 |
| 5 | 490 | 491 | 503 | 503 | 13 | 12 | 71 | 76 | 67 | 70 | .04 | .03 | .04 | .03 |
| 6 | 497 | 496 | 503 | 503 | 6 | 7 | 71 | 80 | 67 | 70 | .04 | .03 | .04 | .03 |
| 7 | 488 | 483 | 503 | 503 | 15 | 21 | 76 | 72 | 67 | 70 | .03 | .03 | .04 | .03 |
| 8 | 495 | 493 | 503 | 503 | 8 | 10 | 73 | 76 | 67 | 70 | .04 | .03 | .04 | .03 |
A three-way ANOVA contrasted the factors Prime Condition, Prime Location, and Target Category. There was a main effect of Prime Condition (F (1, 35) = 29.13, MSE = 1425.87, p < .001), indicating faster response times for identity primes than scrambled primes. There was also a main effect of Prime Location (F (4.68, 163.95) = 9.94, MSE = 129.56, p < .001), but no main effect of Target Category (F < 1), Critically, the three-way interaction (target category, location of the prime, and prime condition) was significant (F (7, 245) = 4.08, MSE = 77.17, p < .001).
The two-way interaction (from the three-factor ANOVA described above) between target category and prime condition was not significant (F <1), indicating that the magnitude of the priming effect did not vary for tools and animals. Thus, we calculated priming effects (Scrambled Prime RTs – Identity Prime RTs) separately for tools and animals and for each location, and performed two separate one-way ANOVAs. Each ANOVA had eight levels (the eight locations). Critically, tool priming was modulated across the visual field (F(7, 245) = 5.54, MSE = 148.76, p < .001). In addition, and going beyond Experiment 1, animal priming was also modulated across the visual field (F(4.58, 160.26) = 8.54, MSE = 273.62, p < .001).
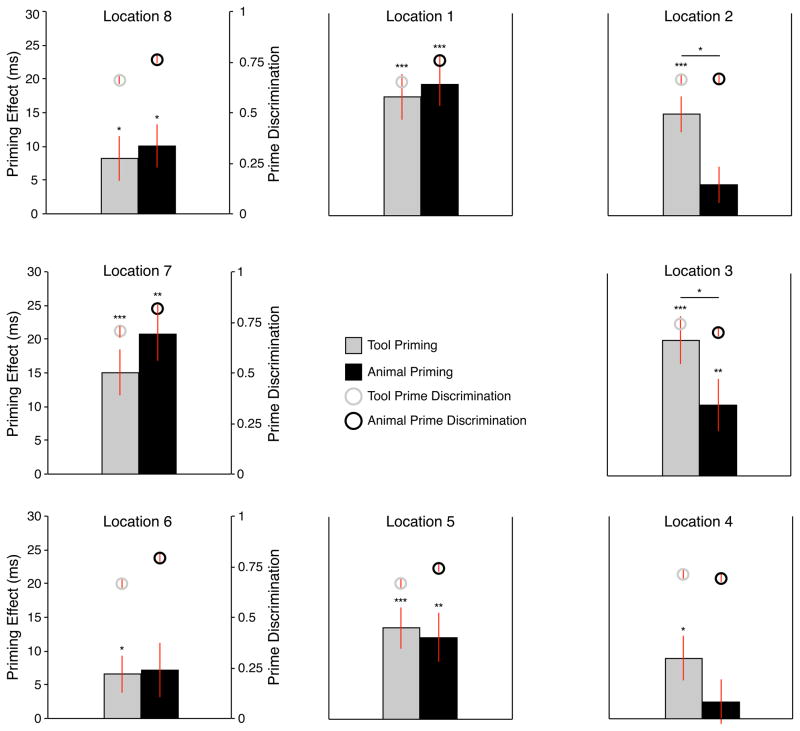
Planned contrasts (t-tests, two-tailed) were then carried out over priming effects (scrambled – identity primes). While there were significant priming effects (identity RT < scrambled RT) for tool primes presented on the left (collapsing across locations 6,7,8; t(35) = 3.71, p < .001) and on the right (collapsing across locations 2,3,4; t(35) = 5.27, p < .001), right-sided tool primes led to greater priming effects than left-sided tool primes (t(35) = 2.91, p < .01). In contrast, while animal priming effects were significant on both the left (t(35) = 3.83, p < .01) and right (t(35) = 2.03, p = .05), priming for left-sided animal primes was greater than priming for right-sided animal primes (t(35) = 4.49, p < .001).
Four of the prime locations (directly above, below, to the left and to the right) were located physically closer to the location of the subsequently presented targets, compared to the other four prime locations (which occupied the diagonals). Because it is known that physical proximity of primes to subsequently presented targets affects the magnitude of priming effects (Marzouki, Meeter, and Grainger, 2008), we tested for an effect of mere proximity of primes to targets. An ANOVA included the factor Target-Prime Distance (close, far) and Prime Condition (identity, scrambled; collapsing across categories). There was a main effect of the factor Target-Prime Distance (F (1, 35) = 40.74, MSE = 15.03, p < .001), as well as a main effect of the factor Prime Condition (F (1, 35) = 29.13, MSE = 178.23, p < .001). Importantly, analysis of the simple main effects showed priming effects for ‘close’ (F (1, 35) = 43.58, MSE = 107.50, p < .001) and ‘far’ (F (1, 23) = 13.05, MSE = 85.76, p < .01) locations.
Prime Discrimination Data
As in Experiment 1, participants were better than chance at prime discrimination (average prime discrimination accuracy = 72%; chance = 50%; cut-off for significantly different than chance at p = .05 was 55%; see Supplemental Table 2 for details). A 2 × 8 ANOVA contrasting Target Category and Prime Location showed a main effect of Target Category (F (1, 35) = 11.51, MSE = 0.05, p < .01), Prime Location (F (7, 245) = 6.40, MSE = 0.01, p < .001), and a significant interaction (F (5.21, 182.31) = 8.51, MSE = 0.13, p < .001). These results indicate that including more prime locations in Experiment 2 resulted in prime visibility not being uniform across the visual field. We return to these data below.
Discussion
Experiment 2 replicated the principal pattern observed in Experiment 1, in which there is a RVF bias toward larger identity priming effects for tool targets. The RVF bias observed for tool targets was not observed for animal targets, which rules out a general RVF bias toward greater priming effects in general. Rather, if anything, animals showed the opposite bias as tools. While further work is necessary to pin down the properties of the possible asymmetry observed for animal stimuli in Experiment 2 (a bias toward stronger priming when primes are presented in the LVF), one might point toward the observation that differential neural responses to animals tend to be stronger in right hemisphere regions (e.g., Anzellotti, Mahon, Schwarzbach, and Caramazza, 2010; Chao, Haxby, and Martin, 1999; Martin and Weisberg, 2003; Mormann, Dubois, Kornblith, Milosavljevic, Cerf, Ison, et al., 2011; Nopponey et al., 2006).
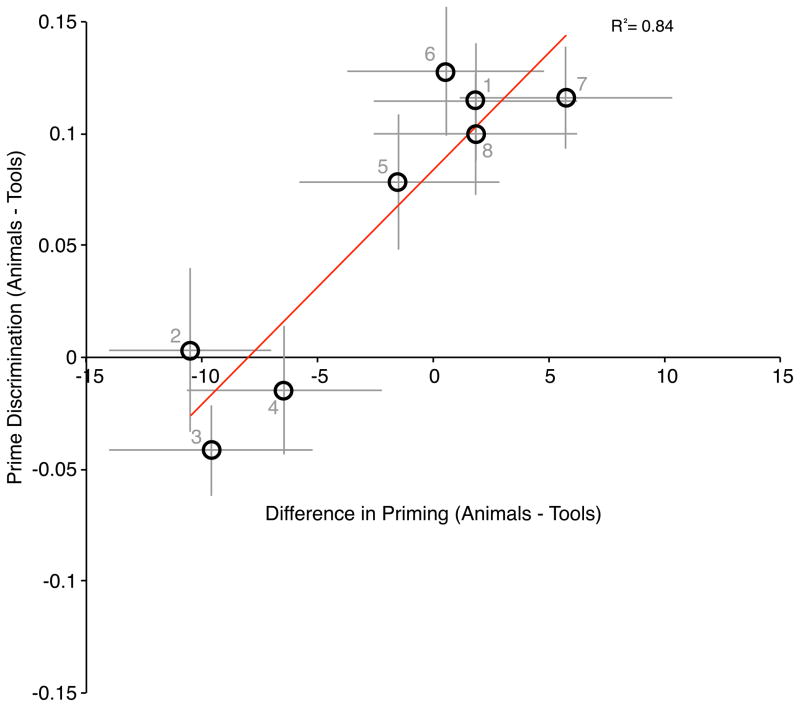
Finally, and extending the results of Experiment 1, there were differences in prime visibility by location in the prime discrimination task. Inspection of the patterns present in Figure 3 indicates that larger tool priming effects are associated with increased discrimination of the primes, across the eight possible prime locations. This positive relationship can be quantified by correlating category-biases in prime discrimination accuracy (animal prime discrimination proportion correct at each prime location minus tool prime proportion correct at each prime location) with the category-bias in identity priming (animal priming effects at each prime location minus tool priming effects at each prime location) across the 8 locations (linear R2 = .84, p < .01; see Figure 4). The fact that there is a relationship between prime discrimination accuracy and identity priming, in a category-specific manner across the visual field, reinforces and extends the principal finding: visual object recognition for tools is differentially sensitive in RVF locations, both when using accuracy (prime discrimination) and when using RT (identity priming).
Figure 3.
Priming Effects and prime discrimination in Experiment 2. Behavioral priming effects (Scrambled - Identity) and proportion correct prime discrimination displayed by experimental condition. Error bars reflect standard errors of the mean across participants. Planned contrasts (one-sample t-tests) depict significant priming effects for both tool and animal targets in locations 1 (directly above fixation), 3 (directly to the right of fixation), 5 (directly below fixation) and 7 (directly to the left of fixation) (all ps < .01). Tool priming effects in the diagonals were all significant; prime location 2: upper right (t(35) = 5.59, p < .001); prime location 4: lower right (t(35) = 2.69, p < .05); prime location 8: upper left (t(35) = 2.44, p < .02); prime location 6: lower left (t(35) = 2.39, p < .05). Analysis of animal priming effects in the diagonals found significant priming effects only for the upper left position (t(35) = 3.15, p < .01). All one-way t-tests reported as significant survived FDR correction (q < .05). Legend for alpha levels in figure: * p < .05; ** p < .01; *** p < .001.
Figure 4.
Relationship between the difference in animal and tool prime discrimination and the difference in animal and tool identity priming (Animal – Tool). Those difference values are plotted by location of the primes in Experiment 2. Numbers below the data points refer to the location of the prime as schematized in Figure 3. When the same analysis was carried for each subject individually, and the resulting r-values Fisher-transformed, the distribution of Fisher-transformed r-values across subjects was significantly different than zero (t(35) = 2.12, p < .05).
An objection that may be raised against the conclusion that identity priming for tool targets shows a RVF advantage is that participants may have adopted a strategy of initiating their categorization response upon the presentation of the prime and not the target. This alternative account is prima facie reasonable in light of the fact that prime discrimination thresholds were above chance (albeit, slightly). Thus, it may be argued that the RVF advantage is not an effect due to priming per se, but rather of categorizing a tool as a tool when presented on the right (with tool categorization decisions being initiated upon presentation of the prime). In order to address this objection, a control experiment was run in which tools were visibly presented either to the right or the left of central fixation with no primes, and participants (n = 16) were simply instructed to indicate if a tool had been presented (Go/No-Go task). This type of paradigm has previously been used (Thorpe, Fize, and Marlot, 1996) to demonstrate faster detection responses to faces than to other classes of stimuli. Thus, in order to have an internal control, we also included faces, places, as well as animals as stimuli. Across blocks participants were instructed as to which (of the four stimulus types) was the current ‘go stimulus’—they were to withhold a response when anything but the target category was presented and push a button when the target stimulus was presented (see Supplemental Online Materials for all details). If the objection outlined above is valid, then participants should be faster to detect the presence of a tool when it is presented in the RVF, then when it is presented in the LVF. Contrary to that prediction, there were no differences for tool detection when tools were presented to the left or to the right of fixation (t < 1). However, and as first described by Thorpe and colleagues (1996) detection of faces was faster than detection of tools, animals, and place stimuli, regardless of side of presentation (t-tests, two-tailed; all ps < .05; see Supplemental Figure 1). Importantly, recognition of tools was not differentially modulated by the location of stimulus presentation (left-sided ‘Go’ tool trials, mean RT, 421 ms, SD, 43 ms; right-sided ‘Go’ tool trials, mean, 426 ms, SD, 50 ms; t < 1). These data rule out an interpretation of the RVF bias toward larger identity priming for tool targets in terms of a ‘general speed advantage’ for responding to tool images when presented in the RVF.
General Discussion
Certain classes of stimuli are preferentially processed by the right or left visual fields, according to whether they have a dominant representation in the left or right hemispheres. For instance, it is well known that the left hemisphere in most individuals is specialized for language, and that printed words are processed faster and/or more efficiently in the RVF (Bub and Lewine, 1988; Chiarello, Nuding, and Pollock, 1988; Hunter and Brysbaert, 2008; Knecht et al., 2000). On the basis of neuropsychological and functional neuroimaging results, we outlined the hypothesis that the RVF would show an advantage for tools, and that this advantage should manifest in relatively larger priming effects for tool primes presented in the RVF. Our findings support this prediction, and also show that the RVF advantage for tool targets is not a general RVF advantage for any type of stimulus (i.e., the RVF advantage was not observed for animal targets).
Given that prime discrimination thresholds were consistently above chance in Experiments 1 and 2, one issue that needs to be addressed is exactly how visible were the masked primes. In order to test whether participants are able to extract the identity of the primes from the degraded format in which they were presented, a second control experiment was run in which participants (n = 19) indicated whether a lateralized prime did or did not match a centrally presented word (for details, see Supplemental Online Materials). The same materials and procedures from Experiment 1 were used; the only difference was that targets were capitalized words that shared the same identity as the prime, or were within-category foils. Participants were at chance to decide if the prime images and target words matched, regardless of whether the primes were presented in the RVF or the LVF (χ2; across participants minimum p-value, 0.15, maximum p-value, 0.66). These data indicate that while participants were able to categorize the prime images at better than chance levels, in fact, little diagnostic information relevant to object identification was extractable from the degraded primes.
We reviewed findings in the introduction indicating that important aspects of conceptual and motor processes relevant to tool use are over-represented in the left hemisphere (Boronat et al., 2005; Canessa et al., 2008; Chao and Martin, 2000; Handy et al., 2003; Johnson-Frey, Newman-Norlund, and Grafton, 2005; Kellenbach et al., 2003; Kroliczak and Frey, 2009; Kroliczak, et al., 2011; Mahon et al., 2007; Nopponey et al., 2006). We also discussed the embodied hypothesis of tool recognition, according to which simulation of the motor-relevant information about tool use in the dorsal stream is a necessary and intermediary step in the visual recognition of tools (e.g., Barsalou, 2008; Gallese and Lakoff, 2005; for general discussion see also Prinz, 1987). For instance, in a series of experiments, Helbig and colleagues (Helbig, Graf, and Kiefer, 2006; Helbig, Steinwender, Graf, and Kiefer, 2010) found that presentation of manipulable objects with congruent action-related properties improved accuracy to subsequently named objects. Participants were required to name a prime image and then a target image that immediately followed the prime image. Naming accuracy for trials with congruent target-prime relationships (e.g., nutcracker-pliers) was significantly better than naming accuracy for trials with incongruent target-prime relationships (e.g., nutcracker-spoon). Helbig and colleagues (2010) extended this effect when it was found that 2000 ms movies depicting congruent action properties improved accuracy for a subsequently presented picture-word stimulus (participants had to indicate if the word matched the picture). On the basis of those findings it was argued that motor-relevant information must be retrieved in order to recognize objects. However, we know on the basis of patient research that retrieval of motor information is not a necessary step in visual object recognition (e.g., Negri et al., 2007a; Ochipa, Rothi, and Heilman, 1989; Rothi, Ochipa, and Heilman, 1991).
The combination of findings that we have reported further refine an explanation of why the motor system is activated, and motor information automatically retrieved, when performing visual recognition tasks over manipulable objects. On the assumption that categorization is subserved by ventral stream processes, then the pattern of findings we have reported could be explained by assuming privileged connectivity between ventral stream regions and left hemisphere parietal structures that represent object-associated manipulations. This conclusion would also suggest that priming effects such as those reported by Helbig, Keifer and colleagues derive from connectivity between object recognition processes and motor-relevant information.
This interpretation resonates with the findings of Almeida and colleagues (2008; 2010) who used a technique to block direct analysis of primes by ventral temporal structures but which allowed direct access of the primes to dorsal structures (Continuous Flash Suppression; Fang and He, 2005). Almeida and colleagues (2008) found that when primes were selectively presented to the dorsal stream, there was a priming effect for tool targets and not for animal targets. What is common to the studies of Almeida and colleagues and our present study is the observation that signatures of dorsal stream processing can be observed on response times for visual processing of manipulable objects.
It is important to consider an alternative explanation of our findings that does not appeal either to the embodied cognition hypothesis of tool recognition, or the idea that ventral-dorsal connectivity is the source of the RVF bias toward greater tool priming. Konen and Kastner (2008) found that parietal responses (using fMRI) to tool stimuli were invariant to image transformations. Invariance to image transformations is a property that one would expect of a system that is involved in visual recognition; or rather, and minimally, those data indicate that there are representations of objects in parietal cortex that are abstracted away from the visual input. Thus, it might be argued that object recognition occurs, at least in part, in parietal regions; as tool representations are known independently to be lateralized in the dorsal stream, that proposal could potentially explain the RVF advantage we have observed. While this interpretation of what might be occurring in parietal cortex was not proposed Konen and Kastner on the basis of their findings, it is important to consider whether such an account could explain our data. There are two arguments against this type of interpretation. First, lesions to parietal regions do not typically result in impairments for object recognition, while ventral stream lesions can impair object recognition. Second, in Experiment 2, the magnitude of the RVF advantage for tool priming was, if anything, larger in the upper visual field than the lower visual field. On the assumption that upper visual field locations are more indicative of ventral stream processing, our data suggest that the more likely explanation of the observed variance in categorization latencies in this task is yoked to processes occurring in the ventral stream (for potentially relevant fMRI findings, see Koutstaal, Wagner, Rotte, Maril, Buckner, and Schacter et al., 2001).
In summary, the observation of a RVF advantage for visual processing of tools in a categorization task suggests that while motor information may not be strictly necessary for visual object recognition, there may be important interactions between the motor and visual systems online during object recognition (Chatterjee, 2010; Kemmerer and Gonzalez Castillo, 2010; Mahon and Caramazza, 2008). This type of a model, in which there is dynamic information exchange between motor and visual representations would provide an alternative framework for interpreting the oft reported finding that motor information is activated when viewing manipulable objects. Importantly, it may be possible to directly evaluate this interpretation of the behavioral evidence we have reported by relating subject-by-subject variation in BOLD effects elicited by viewing tool stimuli in different cortical regions to subject-by-subject variation in the magnitude of the RVF advantage for tool primes.
Supplementary Material
Table 3.
Mean response times (ms), standard deviations (SD in ms) and error rates (%) by location and stimulus category in Experiment 3.
| Image on the Left | Image on the Right | |||||
|---|---|---|---|---|---|---|
| RT | SD | % | RT | SD | % | |
| Tools | 421 | 43 | .05 | 426 | 50 | .06 |
| Animals | 435 | 53 | .08 | 434 | 60 | .07 |
| Faces | 398 | 46 | .04 | 399 | 44 | .02 |
| Places | 470 | 60 | .13 | 472 | 51 | .12 |
Acknowledgments
We are grateful to Alena Stasenko for assistance in data collection. FEG was supported by the Psi Chi Undergraduate Research Grant. BZM was supported by NIH training grant 5 T32 19942-13 and R21NS076176-01A1. JA was supported by Fundação para a Ciência e a Tecnologia fellowship SFRH/BPD/70970/2010. This research was supported in part by Norman and Arlene Leenhouts.
References
- Almeida J, Mahon BZ, Nakayama K, Caramazza A. Unconscious processing dissociates along categorical lines. Proceedings of the National Academy of Sciences. 2008;105:5214–15218. doi: 10.1073/pnas.0805867105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida J, Mahon BZ, Caramazza A. The role of the dorsal visual processing stream in tool identification. Psychological Science. 2010;21:772–778. doi: 10.1177/0956797610371343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allport DA. Distributed memory, modular subsystems and dysphasia. In: Newman SK, Epstein R, editors. Current Perspectives in Dysphasia. New York: Churchill Livingstone; 1985. pp. 207–244. [Google Scholar]
- Anzellotti S, Mahon BZ, Schwarzbach J, Caramazza A. Journal of Cognitive Neuroscience. 2011;23:2059–2067. doi: 10.1162/jocn.2010.21567. [DOI] [PubMed] [Google Scholar]
- Barsalou LW. Grounded cognition. Annual Review of Psychology. 2008;59:617– 645. doi: 10.1146/annurev.psych.59.103006.093639. [DOI] [PubMed] [Google Scholar]
- Boronat CB, Buxbaum LJ, Coslett HB, Tang K, Saffran EM, Kimberg DY, et al. Distinctions between manipulation and function knowledge of objects: evidence from functional magnetic resonance imaging. Cognitive Brain Research. 2005;23:361–373. doi: 10.1016/j.cogbrainres.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Bub DN, Lewine J. Different modes of word recognition in the left and right visual fields. Brain and Language. 1988;33:161–188. doi: 10.1016/0093-934x(88)90060-0. [DOI] [PubMed] [Google Scholar]
- Buccino G, Binkofski F, Fink GR, Fadiga L, Fogassi L, Gallese V, et al. Action observation activates premotor and parietal areas in a somatotopic manner: an fMRI study. European Journal of Neuroscience. 2001;13:400–404. [PubMed] [Google Scholar]
- Canessa N, Borgo F, Cappa SF, Perani D, Falini A, Buccino G, et al. The different neural correlates of action and functional knowledge in semantic memory: an fMRI study. Cerebral Cortex. 2008;18:740–751. doi: 10.1093/cercor/bhm110. [DOI] [PubMed] [Google Scholar]
- Chao LL, Haxby JV, Martin A. Attribute-based neural substrates in temporal cortex for perceiving and knowing about object. Nature Neuroscience. 1999;2:913–919. doi: 10.1038/13217. [DOI] [PubMed] [Google Scholar]
- Chao LL, Martin A. Representation of manipulable man-made objects in the dorsal stream. Neuroimage. 2000;12:478–484. doi: 10.1006/nimg.2000.0635. [DOI] [PubMed] [Google Scholar]
- Chatterjee A. Disembodying cognition. Language and Cognition. 2010;2:79–116. doi: 10.1515/LANGCOG.2010.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarello C, Nuding S, Pollock A. Lexical decision and naming asymmetries: influence of response selection and response bias. Brain and Language. 1988;34:302–314. doi: 10.1016/0093-934x(88)90141-1. [DOI] [PubMed] [Google Scholar]
- Di Lollo V, Enns JT, Rensink RA. Competition for consciousness among visual events: the psychophysics of reentrant visual pathways. Journal of Experimental Psychology: General. 2000;129:481–507. doi: 10.1037//0096-3445.129.4.481. [DOI] [PubMed] [Google Scholar]
- Engel SA, Glover GH, Wandell BA. Retinotopic organization in human visual cortex and spatial precision of functional MRI. Cerebral Cortex. 1997;7:181–192. doi: 10.1093/cercor/7.2.181. [DOI] [PubMed] [Google Scholar]
- Fang F, He S. Cortical responses to invisible objects in the human dorsal and ventral pathways. Nature Neuroscience. 2005;8:1380–1385. doi: 10.1038/nn1537. [DOI] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. Distributed hierarchical processing in primate visual cortex. Cerebral Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- Finkbeiner M, Almeida J, Caramazza A. Letter identification processes in reading: Distractor interference reveals a left-lateralized, domain-specific mechanism. Cognitive Neuropsychology. 2006;23:1083–1103. doi: 10.1080/02643290600665778. [DOI] [PubMed] [Google Scholar]
- Gallese V, Lakoff G. The brain’s concepts: The role of the sensory-motor system in conceptual knowledge. Cognitive Neuropsychology. 2005;22:455–479. doi: 10.1080/02643290442000310. [DOI] [PubMed] [Google Scholar]
- Gazzaniga MS, Smylie CS. Dissociation of language and cognition: a psychological profile of two disconnected right hemispheres. Brain. 1984;107:145–153. doi: 10.1093/brain/107.1.145. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends in Neurosciences. 1992;15:20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Kroliczak G, Westwood DA. Dual routes to action: contributions of the dorsal and ventral streams to adaptive behavior. Progress in Brain Research. 2005;149:269–283. doi: 10.1016/S0079-6123(05)49019-6. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Milner AD, Jakobson LS, Carey DP. A neurological dissociation between perceiving objects and grasping them. Nature. 1991;349:154–156. doi: 10.1038/349154a0. [DOI] [PubMed] [Google Scholar]
- Handy TD, Grafton ST, Shroff NM, Ketay S, Gazzaniga MS. Graspable objects grab attention when the potential for action is recognized. Nature Neuroscience. 2003;6:421–427. doi: 10.1038/nn1031. [DOI] [PubMed] [Google Scholar]
- Hauk O, Johnsrude I, Pulvermüller F. Somatotopic representation of action words in human motor and premotor cortex. Neuron. 2004;41:301–307. doi: 10.1016/s0896-6273(03)00838-9. [DOI] [PubMed] [Google Scholar]
- Helbig HB, Graf M, Kiefer M. The role of action representations in visual object recognition. Experimental Brain Research. 2006;174:221–228. doi: 10.1007/s00221-006-0443-5. [DOI] [PubMed] [Google Scholar]
- Helbig HB, Steinwender J, Graf M, Kiefer M. Action observation can prime visual object recognition. Experimental Brain Research. 2010;200:251–258. doi: 10.1007/s00221-009-1953-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges JR, Spatt J, Patterson K. “What” and “how”: Evidence for the dissociation of object knowledge and mechanical problem-solving skills in the human brain. Proceedings of the National Academy of Sciences. 1999;96:9444–9448. doi: 10.1073/pnas.96.16.9444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter ZR, Brysbaert M. Visual half-field experiments are a good measure of cerebral language dominance if used properly: evidence from fMRI. Neuropsychologia. 2008;46:316–325. doi: 10.1016/j.neuropsychologia.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Johnson-Frey SH, Newman-Norlund R, Grafton ST. A distributed left hemisphere network active during planning of everyday tool use skills. Cerebral Cortex. 2005;15:681–695. doi: 10.1093/cercor/bhh169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Frey SH. The neural bases of complex tool use in humans. Trends in Cognitive Sciences. 2004;8:71–78. doi: 10.1016/j.tics.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Kellenbach ML, Brett M, Patterson K. Actions speak louder than functions: the importance of manipulability and action in tool representation. Journal of Cognitive Neuroscience. 2003;15:20–46. doi: 10.1162/089892903321107800. [DOI] [PubMed] [Google Scholar]
- Kemmerer D, Gonzalez Castillo J. The two-level theory of verb meaning: An approach to integrating the semantics of action with the mirror neuron system. Brain and Language. 2010;112:54–76. doi: 10.1016/j.bandl.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer M, Pulvermüller F. Conceptual representations in mind and brain: Theoretical developments, current evidence and future direction. Cortex. doi: 10.1016/j.cortex.2011.04.006. in press. [DOI] [PubMed] [Google Scholar]
- Knecht S, Dräger B, Deppe M, Bobe L, Lohmann H, Flöel A, et al. Handedness and hemispheric language dominance in healthy humans. Brain. 2000;123:2512–2518. doi: 10.1093/brain/123.12.2512. [DOI] [PubMed] [Google Scholar]
- Konen CS, Kastner S. Two hierarchically organized neural systems for object information in human visual cortex. Nature Neuroscience. 2008;11:224–231. doi: 10.1038/nn2036. [DOI] [PubMed] [Google Scholar]
- Koutstaal W, Wagner AD, Rotte M, Maril A, Buckner RL, Schacter DL. Perceptual specificity in visual object priming: functional magnetic resonance imaging evidence for a laterality difference in fusiform cortex. Neuropsychologia. 2001;39:184–199. doi: 10.1016/s0028-3932(00)00087-7. [DOI] [PubMed] [Google Scholar]
- Kroliczak G, Frey SH. A common network in the left cerebral hemisphere represents planning of tool use pantomimes and familiar intransitive gestures at the hand-independent level. Cerebral Cortex. 2009;19:2396–2410. doi: 10.1093/cercor/bhn261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroliczak G, Piper BA, Frey SH. Atypical lateralization of language predicts cerebral asymmetries in parietal gesture representations. Neuropsychologia. 2011;49:1698–1702. doi: 10.1016/j.neuropsychologia.2011.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JW. Cortical networks related to human use of tools. Neuroscientist. 2006;12:211–231. doi: 10.1177/1073858406288327. [DOI] [PubMed] [Google Scholar]
- Mahon BZ, Caramazza A. The orchestration of the sensory-motor system: Clues from neuropsychology. Cognitive Neuropsychology. 2005;22:480–494. doi: 10.1080/02643290442000446. [DOI] [PubMed] [Google Scholar]
- Mahon BZ, Milleville S, Negri GAL, Rumiati RI, Caramazza A, Martin A. Action-related properties of objects shape object representations in the ventral stream. Neuron. 2007;55:507–520. doi: 10.1016/j.neuron.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon BZ, Caramazza A. A critical look at the embodied cognition hypothesis and a new proposal for grounding conceptual content. Journal of Physiology – Paris. 2008;102:59–70. doi: 10.1016/j.jphysparis.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Martin A. The representation of object concepts in the brain. Annual Review of Psychology. 2007;58:25–45. doi: 10.1146/annurev.psych.57.102904.190143. [DOI] [PubMed] [Google Scholar]
- Martin A, Weisberg J. Neural foundations for understanding social and mechanical concepts. Cognitive Neuropsychology. 2003;20:575–587. doi: 10.1080/02643290342000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzouki Y, Meeter M, Grainger J. Effects of prime-target spatial separation and attentional deployment on masked repetition priming. Perception and Psychophysics. 2008;70:1393–1400. doi: 10.3758/PP.70.7.1393. [DOI] [PubMed] [Google Scholar]
- Mormann F, Dubois J, Kornblith S, Milosavljevic M, Cerf M, Ison M, et al. A category-specific response to animals in the right human amygdala. Nature Neuroscience. 2011;14:1247–1249. doi: 10.1038/nn.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negri GAL, Rumiati RI, Zadini A, Ukmar M, Mahon BZ, Caramazza A. What is the role of motor simulation in action and object recognition? Evidence from apraxia. Cognitive Neuropsychology. 2007a;24:795–816. doi: 10.1080/02643290701707412. [DOI] [PubMed] [Google Scholar]
- Negri GA, Lunardelli A, Reverberi C, Gigli GL, Rumiati RI. Degraded semantic knowledge and accurate object use. Cortex. 2007b;43:376–388. doi: 10.1016/s0010-9452(08)70463-5. [DOI] [PubMed] [Google Scholar]
- Noppeney U, Price CJ, Penny WD, Friston KJ. Two distinct neural mechanisms for category-selective responses. Cerebral Cortex. 2006;16:437–445. doi: 10.1093/cercor/bhi123. [DOI] [PubMed] [Google Scholar]
- Ochipa C, Rothi LJG, Heilman KM. Ideational apraxia: A deficit in tool selection and use. Annals of Neurology. 1989;25:190–193. doi: 10.1002/ana.410250214. [DOI] [PubMed] [Google Scholar]
- Oliveri M, Finocchiaro C, Shapiro K, Gangitano M, Caramazza A, Pascuale- Leone A. All talk and no action: A transcranial magnetic stimulation study of motor cortex activation during action word production. Journal of Cognitive Neuroscience. 2004;16:374–381. doi: 10.1162/089892904322926719. [DOI] [PubMed] [Google Scholar]
- Pazzaglia M, Pizzamiglio L, Pes E, Aglioti SM. The sound of actions in apraxia. Current Biology. 2008;18:1766–1772. doi: 10.1016/j.cub.2008.09.061. [DOI] [PubMed] [Google Scholar]
- Previc C. Functional specialization in the lower and upper visual fields in humans: Its ecological origins and neurophysiological implications. Behavioral and Brain Sciences. 1990;13:519–575. [Google Scholar]
- Prinz W. Ideo-motor action. In: Heuer H, Sanders AF, editors. Perspectives on perception and action. Hillsdale, NJ: Erlbaum; 1987. pp. 47–76. [Google Scholar]
- Pulvermüller F. Brain mechanisms linking language and action. Nature Reviews Neuroscience. 2005;6:576–582. doi: 10.1038/nrn1706. [DOI] [PubMed] [Google Scholar]
- Pulvermüller F, Hauk O, Nikulin VV, Ilmoniemi RJ. Functional links between motor and language systems. European Journal of Neuroscience. 2005;21:793–797. doi: 10.1111/j.1460-9568.2005.03900.x. [DOI] [PubMed] [Google Scholar]
- Roby-Brami A, Hermsdörfer J, Roy A, Jacobs S. A neuropsychological perspective on the link between language and praxis in modern humans. Philosophical Transactions of The Royal Society of London. 2012;367:144–160. doi: 10.1098/rstb.2011.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothi LJG, Ochipa C, Heilman KM. A cognitive neuropsychological model of limb praxis. Cognitive Neuropsychology. 1991;8:443–458. [Google Scholar]
- Simmons WK, Barsalou LW. The similarity-in-topography principle: Reconciling theories of conceptual knowledge. Cognitive Neuropsychology. 2003;20:451–486. doi: 10.1080/02643290342000032. [DOI] [PubMed] [Google Scholar]
- Simmons JP, Nelson LD, Simonsohn U. False-positive psychology: Undisclosed flexibility in data collection and analysis allows presenting anything as significant. Psychological Science. 2011;22:1359–1366. doi: 10.1177/0956797611417632. [DOI] [PubMed] [Google Scholar]
- Sirigu A, Duhamel J, Poncet M. The role of sensorimotor experience in object recognition. Brain. 1991;144:2555–2573. doi: 10.1093/brain/114.6.2555. [DOI] [PubMed] [Google Scholar]
- Thorpe S, Fize D, Marlot C. Speed of processing in the human visual system. Nature. 1996;381:520–522. doi: 10.1038/381520a0. [DOI] [PubMed] [Google Scholar]
- Tranel D, Damasio H, Damasio AR. A neural basis for the retrieval of conceptual knowledge. Neuropsychologia. 1997;35:1319–1327. doi: 10.1016/s0028-3932(97)00085-7. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Mishkin M. Two cortical visual systems. In: Ingle DJ, Goodale MA, Mansfield RJW, editors. Analysis of Visual Behavior. Cambridge, MA: MIT Press; 1982. pp. 549–86. [Google Scholar]
- Ungerleider LG. Functional brain imaging studies of cortical mechanisms for memory. Science. 1995;270:769–775. doi: 10.1126/science.270.5237.769. [DOI] [PubMed] [Google Scholar]
- VanRullen R, Koch C. Visual selective behavioral can be triggered by a feed-forward process. Journal of Cognitive Neuroscience. 2003;15:209–217. doi: 10.1162/089892903321208141. [DOI] [PubMed] [Google Scholar]
- Verma A, Brysbaert M. A right visual field advantage for tool-recognition in the visual half-field paradigm. Neuropsychologia. 2011;49:2342–2348. doi: 10.1016/j.neuropsychologia.2011.04.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.