Abstract
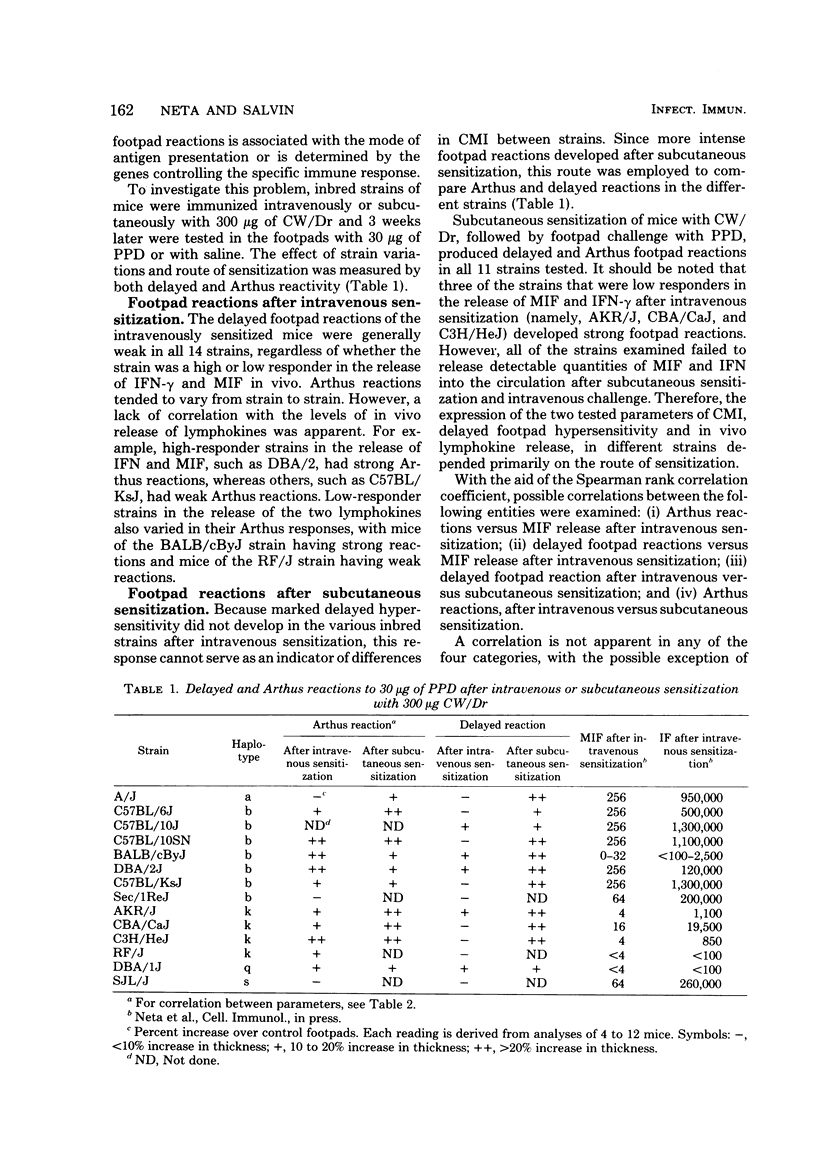
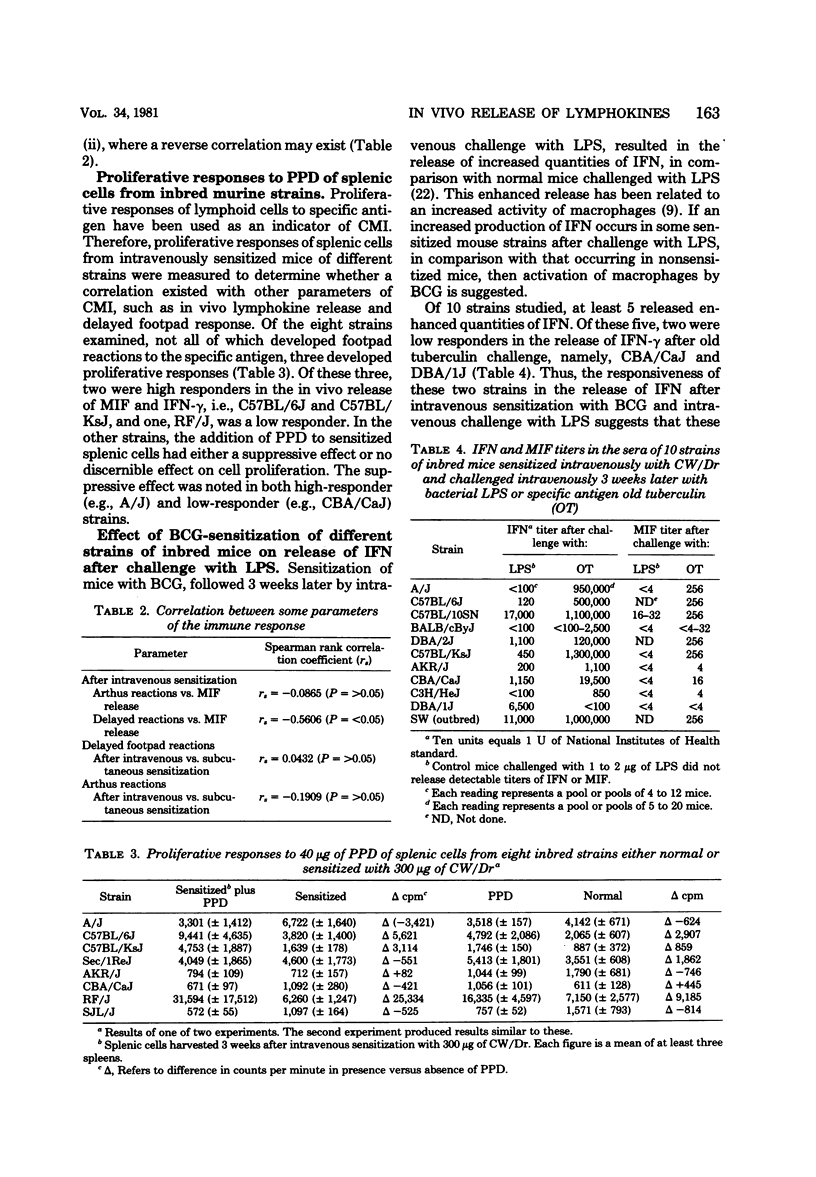
Variations exist between different strains of inbred mice in the release of lymphokines into the circulation. A number of manifestations of cell-mediated immunity in mice sensitized intravenously with Mycobacterium bovis BCG were analyzed to determine their association with the in vivo release of gamma interferon (IFN-gamma) and migration inhibitory factor. Differences occurred among the strains in the proliferative responses of splenic cells to specific antigen and in the release of IFN after the challenge of BCG-sensitized mice with lipopolysaccharide. However, the capacity of an individual strain to release migration inhibitory factor and IFN-gamma into the circulation did not parallel the extent either of the proliferative responses or of the release of IFN induced by lipopolysaccharide. Not all of the strains developed marked delayed footpad reactions to challenge with PPD regardless of the extent of their responses by other parameters. Delayed footpad reactions did develop in mice sensitized via the subcutaneous route, although this sensitization did not result in the capacity to release migration inhibitory factor and IFN-gamma into the circulation of individual inbred strains.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen E. M., Moore V. L., Stevens J. O. Strain variation in BCG-induced chronic pulmonary inflammation in mice. I. Basic model and possible genetic control by non-H-2 genes. J Immunol. 1977 Jul;119(1):343–347. [PubMed] [Google Scholar]
- Allen E. M., Moore V. L. Suppression of phytohemagglutinin and lipopolysaccharide responses in mouse spleen cells by Bacillus Calmette-Guerin. J Reticuloendothel Soc. 1979 Oct;26(4):349–356. [PubMed] [Google Scholar]
- Cheers C., McKenzie I. F., Pavlov H., Waid C., York J. Resistance and susceptibility of mice to bacterial infection: course of listeriosis in resistant or susceptible mice. Infect Immun. 1978 Mar;19(3):763–770. doi: 10.1128/iai.19.3.763-770.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civil R. H., Mahmoud A. A. Genetic differences in BCG-induced resistance to Schistosoma mansoni are not controlled by genes within the major histocompatibility complex of the mouse. J Immunol. 1978 Mar;120(3):1070–1072. [PubMed] [Google Scholar]
- Collins F. M., Watson S. R. Suppressor T-cells in BCG-infected mice. Infect Immun. 1979 Aug;25(2):491–496. doi: 10.1128/iai.25.2.491-496.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson P., Dixon M. A., Grossberg S. E. A sensitive interferon assay for many species of cells: encephalomyocarditis virus hemagglutinin yield reduction. Proc Soc Exp Biol Med. 1977 Jun;155(2):173–178. doi: 10.3181/00379727-155-39768. [DOI] [PubMed] [Google Scholar]
- Lagrange P. H., Hurtrel B. Local immune response to Mycobacterium lepraemurium in C3H and C57Bl/6 mice. Clin Exp Immunol. 1979 Dec;38(3):461–474. [PMC free article] [PubMed] [Google Scholar]
- Lagrange P. H., Mackaness G. B. Site of action of serum factors that block delayed-type hypersensitivity in mice. J Exp Med. 1978 Jul 1;148(1):235–245. doi: 10.1084/jem.148.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt H. O., Benacerraf B. Genetic control of specific immune responses. Adv Immunol. 1969;11:31–74. doi: 10.1016/s0065-2776(08)60477-0. [DOI] [PubMed] [Google Scholar]
- Nakamura R. M., Tokunaga T. Strain difference of delayed-type hypersensitivity to BCG and its genetic control in mice. Infect Immun. 1978 Dec;22(3):657–664. doi: 10.1128/iai.22.3.657-664.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neta R. Mechanisms in the in vivo release of lymphokines. II. Regulation of in vivo release of type II interferon IFN gamma. Cell Immunol. 1981 May 1;60(1):100–108. doi: 10.1016/0008-8749(81)90251-3. [DOI] [PubMed] [Google Scholar]
- Neta R., Salvin S. B. Adjuvants in the induction of suppressor cells. Infect Immun. 1979 Feb;23(2):360–365. doi: 10.1128/iai.23.2.360-365.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neta R., Salvin S. B. In vivo release of lymphokines in different strains of mice. Cell Immunol. 1980 Apr;51(1):173–178. doi: 10.1016/0008-8749(80)90247-6. [DOI] [PubMed] [Google Scholar]
- Plant J., Glynn A. A. Genetics of resistance to infection with Salmonella typhimurium in mice. J Infect Dis. 1976 Jan;133(1):72–78. doi: 10.1093/infdis/133.1.72. [DOI] [PubMed] [Google Scholar]
- Salvin S. B., Nishio J. Communications. "In vitro" cell reactions in delayed hypersensitivity. J Immunol. 1969 Jul;103(1):138–141. [PubMed] [Google Scholar]
- Salvin S. B., Ribi E., Granger D. L., Youngner J. S. Migration inhibitory factor and type II interferon in the circulation of mice sensitized with mycobacterial components. J Immunol. 1975 Jan;114(1 Pt 2):354–359. [PubMed] [Google Scholar]
- Turcotte R., Lafleur L., Labrèche M. Opposite effects of BCG on spleen and lymph node cells: lymphocyte proliferation and immunoglobulin synthesis. Infect Immun. 1978 Sep;21(3):696–704. doi: 10.1128/iai.21.3.696-704.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngner J. S., Stinebring W. R. Interferon appearance stimulated by endotoxin, bacteria, or viruses in mice pre-treated with Escherichia coli endotoxin or infected with Mycobacterium tuberculosis. Nature. 1965 Oct 30;208(5009):456–458. doi: 10.1038/208456a0. [DOI] [PubMed] [Google Scholar]


