Abstract
We present a timesaving strategy for acquiring 3D magic angle spinning NMR spectra for chemical shift assignments in proteins and protein assemblies in the solid state. By simultaneous application of non-uniform sampling (NUS) and paramagnetic-relaxation-assisted condensed data collection (PACC), we can attain 16-fold time reduction in the 3D experiments without sacrificing the signal-to-noise ratio or the resolution. We demonstrate that with appropriate concentration of paramagnetic dopant introduced into the sample the overwhelming majority of chemical shifts are not perturbed, with the exception of a limited number of shifts corresponding to residues located at the surface of the protein, which exhibit small perturbations. This approach enables multi-dimensional MAS spectroscopy in samples of intrinsically low sensitivity and/or high spectral congestion where traditional experiments fail, and is especially beneficial for structural and dynamics studies of large proteins and protein assemblies.
Keywords: solid-state NMR; magic angle spinning; MAS; PACC, non-uniform-sampling
INTRODUCTION
Magic angle spinning (MAS) solid-state NMR spectroscopy is an emerging structural biology technique and is unique among other methods as it can provide atomic-level structure and dynamics information in biological systems that are very large, insoluble and lack long-range order, and thus not amenable to X-ray crystallography or solution NMR spectroscopy.1–9 Most of the protocols for extracting structural or dynamics information by NMR require site-specific chemical shift assignments as the initial step.10–12 Resonance assignments by MAS NMR require acquisition of a set of homonuclear and heteronuclear multidimensional (typically 3D) spectra to establish internuclear (intra-residue and sequential) connectivities in order to identify the amino acid type and its position in the polypeptide chain.13,14 These multidimensional spectra must have adequate sensitivity and resolution in order to enable reliable resonance assignments.15 Both requirements in turn often translate into long experiment times, for instance when the NMR samples are of intrinsically low sensitivity and/or high chemical shift degeneracy, such as in proteins and protein assemblies of high molecular weight and/or in isotopically dilute samples. Such long experimental times are generally impractical for a number of reasons, such as sample and/or instrument instability, and general instrument time constraints. Timesaving strategies are therefore highly desirable in multidimensional MAS NMR spectroscopy, as they would expand the range of biological systems amenable to detailed structural and dynamics analysis.
Among the methods developed with the goal of reducing the experiment time in the MAS NMR measurements, Paramagnetic-relaxation-Assisted Condensed data Collection (PACC) introduced by Ishii and coworkers permits 5–20 fold timesaving by decreasing the recycle delay.16 This approach requires i) paramagnetic doping (typically by Cu(II)-EDTA) of the protein samples resulting in the reduced 1H longitudinal relaxation time and ii) very fast MAS frequencies (40 kHz or higher) allowing for low-power proton decoupling during RF pulses and acquisition time. Similar approaches have been applied to protein systems that have natural metal centers or artificially introduced paramagnetic tags.17–21 Recently, Jaroniec and coworkers extended the PACC approach to 3D experiments for resonance assignment as well as for acquiring distance restraints.21 An apparent advantage of these paramagnetic-relaxation-assisted approaches is that no major hardware upgrades are required except for a need to have a fast MAS probe capable of rotation frequencies of at least 40 kHz.
Another unexplored advantage of PACC is that it can be applied in conjunction with other timesaving strategies such as non-uniform sampling (NUS) protocols22–26, which would result in further significant reductions in experiment time. An experiment otherwise occurring in the “sensitivity-limited regime” can be brought into the “sampling-limited regime”27 when PACC is applied. In the “sampling-limited regime”, many sparse sampling techniques have been introduced to save time in multidimensional NMR experiments by omitting acquisition of some FIDs24–26. Recently, we have demonstrated that in biological solids, NUS data collection gives rise to large sensitivity enhancements (ca. two-fold in each indirect dimension) when random exponentially biased sampling schedules are used,28 and these signal enhancements originate in the time domain as was shown previously.29 Time-domain signals acquired non-uniformly require processing algorithms other than conventional Discrete Fourier Transform (DFT). For example, radial sampling requires the time domain signal to be processed with G-matrix Fourier transform15, projection reconstruction30, automated projection spectroscopy (APSY),31 projection decomposition (PRODECOMP)32 or high resolution iterative frequency identification for NMR (HIFI-NMR). 33 Random non-uniformly sampled data can be processed with maximum entropy reconstruction,24 multidimensional decomposition (MDD),34 forward maximum entropy reconstruction (FM),35 l1-norm regularization (also called compressed sensing),36–38 Spectroscopy by Integration of Frequency and Time domain information (SIFT),39 non-uniform Fourier transformation (NU-FT),40 and Gridding-FFT (GFFT)41. Recently, we have introduced a Maximum Entropy Interpolation (MINT) processing protocol that attains full linearity between the time and the frequency domains and permits direct analysis of the frequency-domain spectra acquired by NUS with respect to those recorded by the conventional uniform sampling (US) protocol. With NUS/MINT we have demonstrated that inherent properties of solid-state signals (relatively short T2*) permit collection of datasets possessing both enhanced sensitivity and retained resolution.28
To the best of our knowledge, the above non-Cartesian sampling techniques in conjunction with nonlinear data processing protocols to date have only occasionally been applied in solid-state NMR spectroscopy,42–45 despite their excellent potential to facilitate data acquisition without compromising on the spectral quality. We expect that the introduction of new techniques for sensitivity and resolution enhancement in solid-state NMR spectroscopy (e.g. dynamic nuclear polarization (DNP),46 paramagnetic-relaxation-assisted condensed data collection (PACC),16 In-Phase-Anti-Phase spectroscopy (IPAP),47 and Spin-State-Selective Excitation (S3E)48) will make the applications of non-uniform sampling techniques more widespread.
In this report, we present a timesaving strategy dubbed NUS-PACC for acquiring 3D MAS solid-state NMR spectra, which is based on the simultaneous use of PACC16 and random exponentially biased non-uniform sampling (NUS) in the indirect dimensions. We adopted this approach in order to be able to work with sensitivity-limited samples, such as assemblies of U-13C,15N-enriched microtubule-associated proteins in complex with natural abundance microtubules8 and sparsely U-13C,15N- (amino-acid specifically) enriched assemblies of HIV-1 capsid proteins,9 where we hit severe sensitivity limitations preventing us from collecting heteronuclear 3D MAS NMR spectra for resonance assignments and/or structure determination. Using this NUS-PACC method, we demonstrate that at least 16-fold timesaving is attained in each of three 3D experiments, NCOCA, NCACO and NCACB, which are key experiments for resonance assignments of proteins and protein assemblies by MAS NMR. We discuss the sample and the experimental conditions required for accurate measurements of the chemical shifts. The approach demonstrated in our work is expected to be generally beneficial for samples of intrinsically low sensitivity and high spectral congestion.
EXPERIMENTS AND METHODS
Expression and purification of isotopically enriched LC8
Expression and purification of U-15N and U-15N,13C isotopically enriched dynein light chain LC8 was reported by us previously. 49
Preparation of solution and solid-state NMR samples of U-15N and U-15N,13C LC8 doped with Cu(II)-EDTA
For the preparation of solution NMR sample, 500 ul of 10 mg/ml LC8 solutions different Cu(II)-EDTA concentrations (0 mM, 5 mM, 10 mM, 20 mM, and 50 mM) with 10% D2O were placed into solution NMR tubes. For the preparation of solid-state NMR samples, U-15N or U-13C,15N enriched purified LC8 solutions were dialyzed against 10 mM MES buffer (10 mM MgCl2, pH 6.0) and concentrated to 30 and 25 mg/ml, respectively. The 1M Cu(II)-EDTA stock solution (dissolved in 10 mM MES buffer described above) was added to the concentrated U-15N LC8 solution and to the 32% w/v solution of PEG-3350 (also dissolved in 10 mM MES buffer) in several aliquots to reach the final concentration of Cu(II)-EDTA in the protein and PEG-3350 solutions of 5 mM, 10 mM, 20 mM, and 50 mM. The above PEG-3350 solutions containing Cu(II)-EDTA at desired concentrations were gradually added to the solutions of LC8 containing the same Cu(II)-EDTA concentrations to generate protein precipitates, following the controlled precipitation protocol.50 A control LC8 sample containing no Cu(II)-EDTA was also prepared. The solid-state samples of LC8 containing different concentrations of Cu(II)-EDTA (0 mM, 5 mM, 10 mM, 20mM and 50 mM) were packed into 3.2, 1.8, or 1.6 mm MAS rotors for subsequent NUS-PACC or conventional experiments. The final amounts of protein precipitate packed in a rotor ranged from 4.8 to 15 mg, depending on the rotor size.
Solid-state NMR spectroscopy
Magic angle spinning solid-state NMR spectra of the U-15N and U-13C,15N labeled LC8 samples containing different concentration of the paramagnetic dopant were acquired at 14.1 T (600 MHz) on a narrow bore Varian InfinityPlus instrument outfitted with three probes: i) a 3.2 mm triple resonance Varian T3 probe; ii) a 1.8 mm triple resonance fast-MAS probe developed and built in the laboratory of our collaborator, Dr. Ago Samoson (Technical University of Tallinn); and iii) a 1.6 mm triple resonance fast-MAS Varian probe. Larmor frequencies are 599.5 MHz for 1H, 150.7 MHz for 13C, and 60.7 MHz for 15N. The experiments were conducted at the MAS frequencies of 10±0.001 or 40±0.001 kHz controlled by a Varian MAS controller. The temperature was calibrated for each probe at different MAS frequencies using either a PbNO3 51 or KBr52 temperature sensor, and the actual temperature at the sample was maintained to within ±1 °C throughout the experiments using the Varian temperature controller. The NMR experiments were carried out at the following temperatures: i) for 10 kHz MAS frequency, T = −16.7 °C (apparent temperature of −20 °C); ii) for 40 kHz MAS frequency, T = −15 °C (apparent temperature of −35 °C). 1H, 13C and 15N chemical shifts were referenced with respect to DSS, adamantane and ammonium chloride used as external referencing standards.53
1H T1 and 15N T2 measurements
1H T1 and 15N T2 of Cu(II)-EDTA doped U-15N LC8 samples were measured at 10 kHz with 15N CP-based inversion recovery and spin-echo experiments, respectively.54 1H T1 and 15N T2 of U-13C, 15N Cu(II)-EDTA doped LC8 samples were measured according to the same procedures as for 15N enriched LC8 (vide supra), at MAS frequencies of 10 and 40 kHz.
NUS-PACC experiments on Cu(II)-EDTA-doped LC8
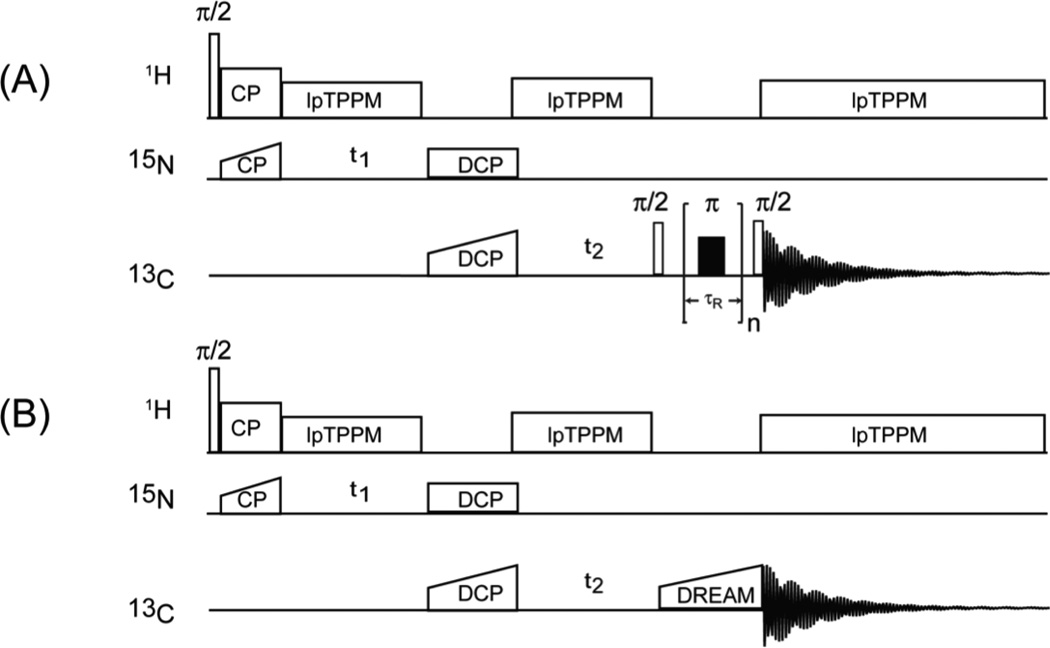
The typical 90-degree pulse lengths were 2.7 µs (1H) and 4.17 µs (13C). The 1H-15N cross polarization radio frequency fields were set to ωH + ωN(center) = ωMAS with a linear ramp on ωN (center at 10 kHz). 15N-13C DCP/SPECIFIC-CP55,56 radio frequency fields were set to ωN + ωC(center) = ωMAS with tangential ramp on ωC (center at 20 kHz).18 10 kHz low-power TPPM57,58 decoupling was used during the acquisition and evolution periods in the indirect dimensions. RFDR59,60 or DREAM61 homonuclear magnetization transfer schemes were used following the DCP step to acquire NCACX, NCOCX, NCACB, NCACO or NCOCA spectra (pulse sequences are shown in Figures 1A and 1B). Rotor-synchronized XY-16 sequences (40 kHz π pulse at the center of each rotor period) were applied for RFDR, and the mixing time was 2.4 ms. In the DREAM sequence, tangential ramp centered at 16 kHz with 2.4 ms mixing time was used for C'-Cα and Cα-C' magnetization transfers; tangential ramp centered at 20 kHz with 2.5 ms mixing time was used for Cα-Cβ magnetization transfer. No proton decoupling was applied during RFDR or DREAM period. For NCACO and NCACB experiments, the 13C carrier frequency was placed at 56.6 ppm; for NCOCA experiment- at 174.6 ppm; no frequency offset was applied in any experiment during the DREAM mixing.
Figure 1.
Pulse sequences for NUS-PACC experiments: (A) DCP-RFDR pulse sequence for NCOCX and NCACX 3D experiments; (B) DCP-DREAM pulse sequence for NCOCA, NCACO and NCACB 3D experiments.
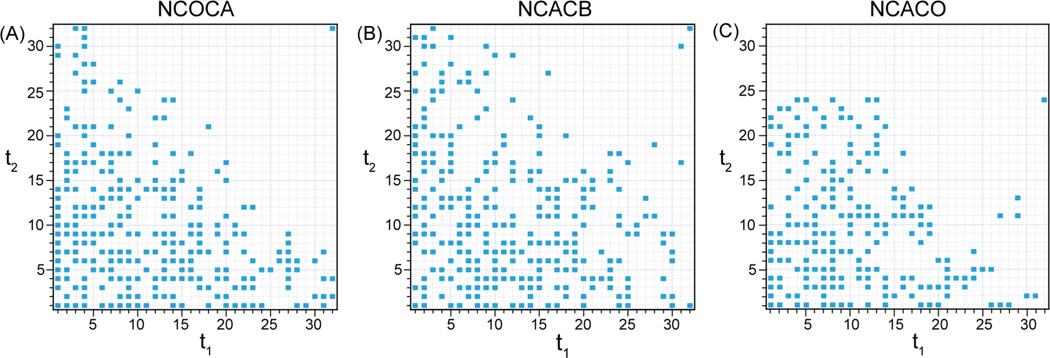
In the non-uniformly sampled 3D experiments (DCP-DREAM-based NCACB, NCACO and NCOCA, the pulse sequence is shown in Figure 1B), four FIDs were acquired in the (RR, IR, RI, II) sequence for each NUS (t1, t2) combination, corresponding to a States-States62 hypercomplex acquisition mode used for phase-sensitive detection in the indirect dimensions. Three random non-uniform sampling schedule (see Figure 2) was created by ScheduleTool (software documentation available at http://sbtools.uchc.edu/nmr/sample_scheduler/).63 The 15N T2 * value was used as a guide for constructing the non-uniform sampling schedule for 3D NCOCA, NCACO and NCACB experiments as described.28 15N T2 * was estimated from 1D 15N CPMAS and 2D NCA spectra. For all samples, the NUS schedule contained 25% hypercomplex points with respect to the corresponding US schedule. For the 5 mM Cu(II)-EDTA LC8 sample, 256 hypercomplex NUS points were acquired (the maximum increment in the NUS dataset for both indirect dimensions corresponds to point #32 in the US dataset that would be acquired with the same dwell time) for NCOCA and NCACB experiments, and 192 hypercomplex NUS points were acquired (the maximum increments in the NUS dataset for 15N and 13C indirect dimensions correspond to points #32 and #24, respectively, in the corresponding US dataset that would be acquired with the same dwell time) for NCACO. The total experiment times are 9 hours for NCOCA (64 scans), 14 hours for NCACO (128 scans) and 27 hours for NCACB (192 scans). To evaluate the effects of NUS on chemical shift deviation, two US NCACB experiments were carried out with experimental settings identical as those in the NUS-PACC NCACB experiment except that the evolution times in the indirect dimensions were sequentially incremented, and the final number of points collected in the indirect 15N and 13C dimensions were 32×32 (experiment 1) and 16×12 (experiment 2). For the 10 mM Cu(II)-EDTA LC8 sample, 384 hypercomplex points were acquired (the maximum increments in the NUS dataset for 15N and 13C indirect dimensions correspond to points #32 and #48, respectively, in the corresponding US dataset that would be acquired with the same dwell time). The total experiment time is 54 hours for NCACB (256 scans). For the 50 mM Cu(II)-EDTA LC8 sample, 324 hypercomplex points were acquired (the maximum increment for both indirect dimensions corresponds to point #36 in the US dataset that would be acquired with the same dwell time).
Figure 2.
Sampling schedule for NUS-PACC 3D (A) NCOCA, (B) NCACB and (C) NCACO experiments for the 5 mM Cu(II)-EDTA sample. Each point represents four FIDs combined from the real (R) and imaginary (I) parts in the order of (t1, t2): RR, IR, RI, II. For the sampling schedules of NCOCA and NCACB experiments, 25% points of a 32×32 uniform sampling schedules were used. For the sampling schedule of the NCACO experiment, 25% points of a 32 × 24 uniform sampling schedule was used.
Conventional US/FFT experiments on neat LC8
For the experiments acquired at 40 kHz, the typical 90-degree pulse lengths were 2.7 µs (1H) and 4.17 µs (13C). The 1H-15N cross polarization rf fields were set as ωH - ωN(center) = ωMAS with a linear ramp on ωN (center at 55 kHz). 15N-13C DCP/SPECIFIC-CP55,56 rf fields were set as ωN - ωC(center) = ωMAS with tangential ramp on ωC (center at 25 kHz).18 10 kHz low-power TPPM57,58 decoupling was used during the acquisition and indirect-dimension evolution periods. DREAM61 homonuclear magnetization transfer schemes were used following the DCP step to acquire NCACB spectra. In the DREAM sequence, tangential ramp centered at 20 kHz with 1.9 ms mixing time was used for Cα-Cβ magnetization transfer. No decoupling was applied during the DREAM period. States-States62 hypercomplex acquisition mode was used for phase-sensitive detection in the indirect dimensions. The NCACB spectrum was acquired with 144 scans, 18 complex points in t1 and 18 complex points in t2.
Solution NMR spectroscopy
1H-15N HSQC spectra of U-15N-LC8 samples containing different concentrations of paramagnetic Cu(II)-EDTA dopant (0 mM, 5 mM, 10 mM, 20mM and 50 mM, see above) were acquired at 14.1 T on a Bruker Avance spectrometer. Larmor frequencies are 600.133 MHz (1H), 60.8 MHz (15N). All experiments were conducted at 298 K. Chemical shifts were referenced to DSS.64 Additional acquisition and processing parameters are specified in the Supporting Information.
NMR data processing and analysis
The NUS 3D data were converted by in-house written python scripts in conjunction with the bin2pipe module in NMRPipe65 from the Chemagnetics format to a format in which each data point is in an IEEE float-32 format, whereas those points in the uniform grid omitted by NUS were zero filled, and the real/imaginary parts were interleaved in every dimension.
The converted data were processed with DFT in the direct dimension and with maximum entropy reconstruction in the indirect dimensions as implemented in the program Rowland NMR Toolkit (software documentation available at http://rnmrtk.uchc.edu).66–68 In the direct dimension, a 90-degree or 60-degree sine bell apodization function was applied to the converted data, followed by Lorentizan-to-Gaussian apodization, zero filling to 1024 points, Fourier transform and phase correction. In the indirect dimensions, the 1024 planes were reconstructed to 128 × 128 2D slices. MINT processing was applied as described.28
In parallel, the converted data were also processed with the MDD algorithm.34 Three software packages were used to implement the MDD data processing: mddNMR,34 MDDGUI (software courtesy of Cheryl Arrowsmith and Aleksandras Gutmanas) and NMRPipe.65 In addition, in-house written python scripts were used to aid the conversion of data from the NMRPipe format to the mdd format as our current implementation of NUS in spinsight/Chemagnetics does not generate parameter files (e.g. Bruker acqus, Varian procpar files) that could be parsed by mddNMR and MDDGUI. In the direct dimension, a 60-degree sine bell apodization function was applied to the converted data, followed by Lorentzian-to-Gaussian apodization, zero filling to 1024 points, Fourier transform and phase correction. The resulting interferograms were divided into small regions of interest (ROIs, each ROI is 1.0 ppm and two neighboring ROIs have 0.5 ppm overlap) and converted to mdd format. Each mdd file for a small ROI was subjected to multidimensional decomposition carried by mddNMR in order to reconstruct a full grid interferogram. The full grid interferograms for each ROI were combined and converted back to the NMRPipe format, and the two indirect dimensions were processed by NMRPipe with forward linear prediction of the number of points equal to that in the experimental data, 30, 60 or 90-degree sine bell followed by Lorentzian-to-Gaussian apodization, zero filling, Fourier transform and phase correction.
The conventional uniformly sampled data were processed in rnmrtk (following the MINT protocol, as described in NUS data processing) and in NMRPipe.65 For the processing by NMRPipe, we applied forward linear prediction in the indirect dimensions of the number of points equal to that in the experimental data; 90-degree or 60-degree sine bell was applied to each dimension followed by Lorentzian-to-Gaussian apodization, zero filling, Fourier transform and phase correction.
The processed spectra were analyzed in Sparky.69
RESULTS AND DISCUSSION
Effects of Paramagnetic Doping of Cu(II)-EDTA on 1H T1 and 15N T2 of LC8
Under the optimal sample conditions, in the presence of a paramagnetic dopant, the 1H longitudinal relaxation time T1 should be significantly shortened while the 15N and 13C transverse relaxation times T2 should be close to those of neat samples. The previous study by Ishii and coworkers demonstrated that the concentration of the paramagnetic dopant has the most dramatic effect on the relaxation times, and that the optimal concentration of the paramagnetic dopant is sample specific.16 To examine the effect of paramagnetic doping on the protein under study, dynein light chain 8 (LC8), we measured 1H T1 and 15N T2 for four U-15N-enriched LC8 samples: neat protein and protein doped with 5, 10, 20, and 50 mM Cu(II)-EDTA. The dopant concentration dependencies of 1H T1 and 15N T2 for LC8 measured at the MAS frequencies of 10 kHz are listed in Table 1 and shown in Figure S1 of the Supporting Information). For the LC8 sample doped with 50 mM Cu(II)-EDTA, 1H T1 dropped to 11.4 % (48 ms) of that for the neat sample (~422 ms) while the 15N T2 has remained the same as for the neat protein (29 ms for the doped and 23 ms for the neat samples, respectively, shown in Figure S1 of the Supporting Information). Similar measurements were also carried out at the MAS frequency of 40 kHz on U-13C,15N-enriched LC8 (neat sample and samples doped with 5, 10, and 50 mM Cu(II)-EDTA). The trends are consistent with our findings for the U-15N-enriched LC8). The doubly labeled sample has short 1H T1 and long 15N T2, and these relaxation times exhibit pronounced concentration dependence (listed in Table 1 and illustrated in Figure S1 and Figure S2). The slightly longer 1H T1 for the doubly labeled sample is due to the attenuation of the effects of dipolar coupling and chemical shift anisotropy by fast MAS.
Table 1.
Dopant Concentration Dependencies of1H T1 and 15N T2 for LC8.
| MAS frequency | Cu(II) –EDTA concentrations |
1H T1 (ms) | 15N T2 (ms) |
|---|---|---|---|
| 10 kHz | 0 mM | 422 ± 8 | 23 ± 1 |
| 10 mM | 82 ± 3 | Not measured | |
| 20 mM | 83 ± 3 | Not measured | |
| 50 mM | 48 ± 1 | 29 ± 2 | |
| 40 kHz | 0 mM | 632 ± 7 | 24 ± 2 |
| 5 mM | 151 ± 2 | 21 ± 2 | |
| 10 mM | 115 ± 2 | 17 ± 2 | |
| 50 mM | 73 ± 3 | 26 ± 1 | |
Experiments at 40 kHz MAS were conducted on a U-15N, 13C labeled LC8, experiments at 10 kHz MAS frequency were conducted on a U-15N labeled LC8.
On the basis of these results we conclude that at dopant concentrations between 5 and 50 mM relaxation properties are favorable for NUS-PACC experiments.
Effects of Paramagnetic Doping of Cu(II)-EDTA on Solution and Solid-State Chemical Shifts
Dopant-induced chemical shift perturbation in solution
In light of the favorable relaxation properties of Cu-doped LC8 samples making them potentially suitable for NUS-PACC experiments, we turned our attention to the chemical shift perturbation as a function of the dopant concentration. It is well known that when present in proximity to the nucleus of interest, unpaired electrons induce chemical shift perturbations, the extent of which depends on the nature of the paramagnetic site, on the nuclear-electron distance as well as on the experimental parameters.70 Therefore, we conducted a systematic analysis of paramagnetically induced 15N and 1H shift perturbations in solution to examine the possibility of using HSQC spectra as a way of rapidly screening the dopant concentrations to find the most suitable sample conditions for NUS-PACC experiments. As shown in Table S1 of the Supporting Information, at Cu(II)-EDTA concentrations of 20 and 50 mM, significant number of peaks are shifted, and some peaks are broadened beyond detection. Not surprisingly, these resonances belong to residues located at the termini, loops, and surface of the protein. At lower concentrations of Cu(II)-EDTA, the majority of the chemical shifts are intact. These solution experiments suggest that LC8 samples prepared with 5 and 10 mM Cu(II)-EDTA may be best suited for NUS-PACC experiments.
Dopant-induced chemical shift perturbation in the solid state
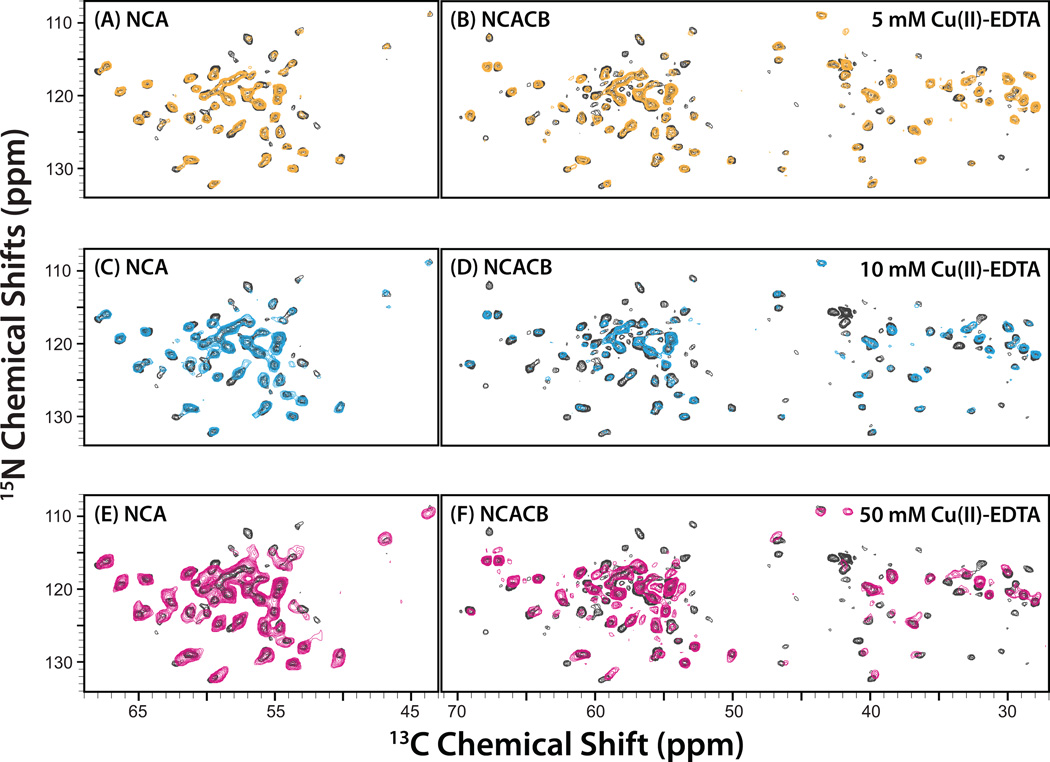
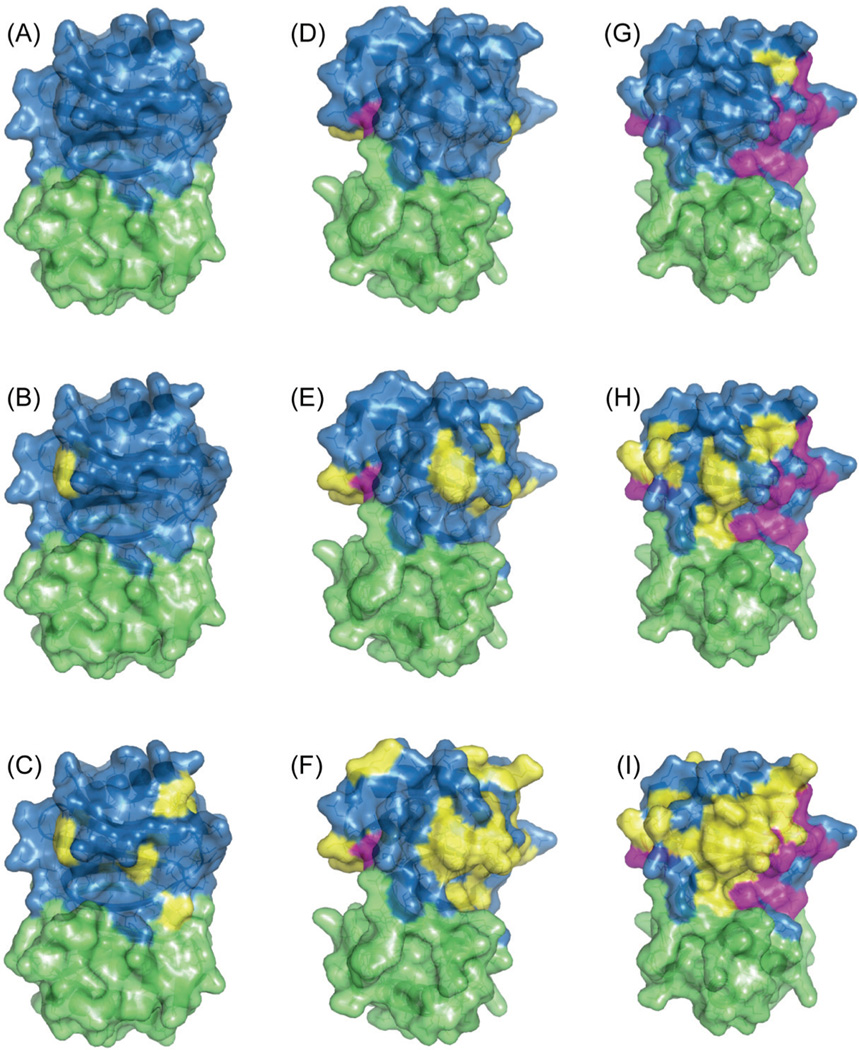
We next examined the influence of Cu(II)-EDTA on 13C and 15N solid-state chemical shifts in the 2D MAS NCA and NCACB datasets. In Figure 3, NCA and NCACB spectra are shown for LC8 doped with 5, 10, and 50 mM Cu(II)-EDTA, overlaid onto the spectra of the neat LC8 sample. It is clear that at 5 mM the effect on the spectra is modest with the majority of the peaks not affected by the paramagnetic dopant and only a few resonances exhibiting small chemical shift perturbations. For this sample, the mean chemical shift differences vs. the non-doped protein and the standard deviations in ppm are: 0.12 ± 0.11 (15N) and 0.05 ± 0.05 (13C). In contrast, higher concentrations of the Cu(II)-EDTA dopant (10 and 50 mM) induce significant changes to the spectra including chemical shift perturbations and broadening of peaks. The chemical shift perturbations for individual peaks as a function of the dopant concentration are presented in Table S2 and Figure S3 of the Supporting Information. In Figure 4, the peaks that are either missing or have perturbed chemical shifts are mapped onto the X-ray structure of LC8. Not surprisingly, the peaks affected by the paramagnetic copper are located on the surface of the protein.
Figure 3.
2D NCA (left) and NCACB (right) spectra of U-13C,15N-LC8 doped with 5 mM (top, yellow), 10 mM (middle, blue), and 50 mM (bottom, magenta) Cu(II)-EDTA, overlaid onto the corresponding spectra of the neat protein sample (black).
Figure 4.
Surface representation of the X-ray structure of LC8 (2PG175) depicting the residues either missing (in magenta) or with perturbed peaks (in yellow) in 3D NCACB spectra of samples doped with different concentration of Cu(II)-EDTA: 5 mM (left column), 10 mM (middle column), and 50 mM (right column). The two monomers of LC8 are shown in blue and green. The top, middle and bottom rows present the residues with perturbations larger than 0.5 ppm, 0.2 ppm, and 0.1 ppm for 13C, respectively.
Effects of Non-Uniform Sampling and Experimental Conditions on Chemical Shift Accuracy in the Solid State
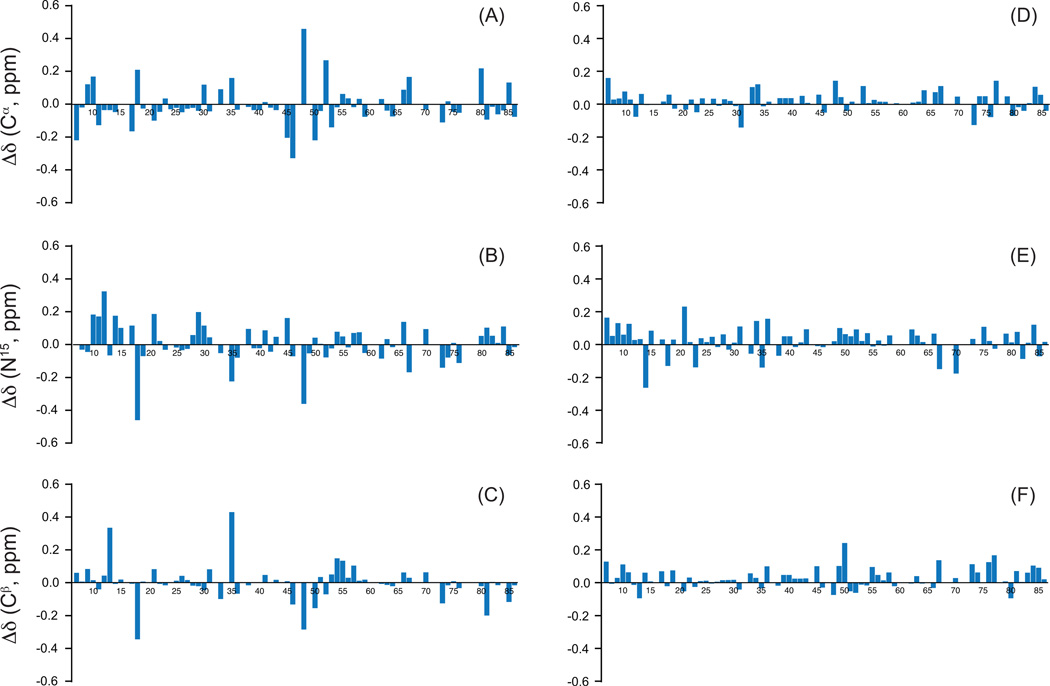
We next analyzed whether non-uniform sampling of the data points in the indirect dimension(s) may affect the accuracy of the measured frequencies and hence introduce additional artificial chemical shift variations in the 2D and 3D NUS spectra, for the LC8 sample doped with 5 mM Cu(II)-EDTA. The results summarized in Figure 5 and in Table S3 clearly demonstrate that the effects are minor, and for the spectra acquired under similar conditions, with sufficiently long evolution times in the indirect dimensions, the mean standard deviations of the chemical shifts are 0.06 ppm (15N) and 0.04 ppm (13C), respectively. It is noteworthy that the chemical shift differences between NUS and US spectra with relatively longer evolution times are significantly smaller than those between the two US spectra, one of which has short evolution times.
Figure 5.
Chemical shift differences plotted for each residue in the LC8 sample doped with 5 mM Cu(II)-EDTA, for the following atom types: 13Ca (A, D), 15N (B, E), and 13Cb (C, F). The left panel (A–C) is the difference of chemical shifts between two 3D NCACB uniformly sampled spectra. The right panel (D–F) is the difference of chemical shifts between two 3D NCACB spectra, one acquired by non-uniform sampling in both indirect dimensions and another- by uniform sampling. The US spectrum used as the reference dataset in both the left and right panel was acquired as a 32×32 point hypercomplex matrix in two indirect dimensions; the total evolution time is 12.4 ms and 6.9 ms in the indirect 15N (t1) and 13C (t2) dimensions, respectively. The second US data used for chemical shift comparison has shorter evolution time in N and CA dimensions (50% of evolution time in N dimension, and 37.5% of evolution time in CA dimension). The NUS spectrum was acquired using a 3D NUS schedule consisting of 25% points of the corresponding reference US dataset and using the same spectral width and the same evolution time in all dimensions. Note that the chemical shift differences between the NUS and US spectra are small and below 0.2 ppm for the overwhelming majority of the residues. On the other hand, the two US spectra exhibit significantly higher chemical shift deviations.
The chemical shift accuracy is critically dependent on maximum evolution times.67 As illustrated in Figure 5 with a pair of 3D US NCACB spectra of LC8 doped with 5 mM Cu(II)-EDTA, when the evolution times are reduced in the indirect dimensions, large discrepancies in chemical shifts are observed with respect to the dataset acquired with appropriate evolution times. In this case, the mean chemical shift differences and the standard deviations are 0.09 ± 0.09 (15N) and 0.07 ± 0.09 (13C), which are comparable to or even higher than the perturbations introduced by the presence of Cu(II)-EDTA. This indicates the benefits of NUS for improving chemical shift accuracy because it allows sampling data points at longer evolution times otherwise not allowed within the constraints of short experimental time.
Taken together, the above results indicate that the LC8 sample prepared with 5 mM Cu(II)-EDTA is well suited for NUS-PACC experiments aimed at resonance assignments. In this sample, minor chemical shift perturbations occur in a fraction of surface residues. These small perturbations can be readily corrected for by comparison of 2D NCACX and NCOCX spectra of the doped and the neat samples. At the same time, if needed, LC8 samples with higher Cu(II)-EDTA dopant concentration of 10–50 mM possessing shorter T2* relaxation times can be used for rapid screening of NUS-PACC experimental conditions. Generally speaking, our findings also indicate that sample conditions have to be optimized for each system of interest prior to conducting NUS-PACC experiments to assure the desired relaxation parameters are attained with the minimal possible concentration of paramagnetic dopant so that chemical shifts are intact or minimally perturbed with respect to the non-doped sample.
13C Homonuclear Recoupling in 3D Experiments With Condensed Data Collection at Fast MAS Frequencies
In the typical 3D NCACX or NCOCX experiments, a homonuclear magnetization transfer period is added after the DCP55 or SPECIFIC-CP56 step. To allow for condensed data collection, the homonuclear recoupling period must be short and must consist of low-power RF radiation yet it must have high magnetization transfer efficiency under fast MAS conditions. Therefore, first-order recoupling sequences appear to be advantageous for the homonuclear mixing in the NUS-PACC experiments (the drawback is dipolar truncation in these methods precluding the observation of lon-grange correlations). Previous studies16,19,21 employed RFDR59,60 or DREAM61 for condensed data collection under fast magic angle spinning. We carried out a series of 1D NCACX (or NCACB, NCACO) and NCOCX (or NCOCA) type experiments on U-13C,15N-enriched LC8 doped with 50 mM Cu(II)-EDTA and compared the homonuclear transfer efficiency during the RFDR and DREAM mixing. Our results demonstrate that DCP-DREAM experiments have higher signal-to-noise ratio than the corresponding DCP-RFDR experiments performed with the same number of scans (see Table 2; the pulse sequences for DCP-RFDR and DCP-DREAM are shown in Figure 1A and B). The other advantages of DREAM are its short duration (~3–4 ms) and low-power RF radiation (~16 kHz or ~20 kHz on 13C channel), with no proton decoupling required. At the MAS frequency of 40 kHz, DREAM can be used to achieve C'-Cα, Cα-C' and Cα-Cβ homonuclear recoupling by adjusting the RF field strength on the 13C channel according to nωr = (ωrf2 + Ω12)1/2 + (ωrf2 + Ω22)1/2.61 Therefore, the same DCP-DREAM sequence can be used for three experiments: NCOCA, NCACO and NCACB. In favorable cases, these three experiments will be sufficient for establishing complete site-specific resonance assignments.
Table 2.
Signal-to-Noise Ratio of 1D DCP-RFDR and DCP-DREAM Spectra Recorded in 50 mM Cu(II)-EDTA Doped U-13C, 15N-Labeled LC8 Sample.
| DCP-RFDR | DCP-DREAM | ||||
|---|---|---|---|---|---|
| S/N* | NCACX (256 scans) |
NCOCX (64 scans) |
NCACB (256 scans) |
NCACO (256 scans) |
NCOCA (64 scans) |
| C' | 11.8 | 21.3 | - | 20.2 | 22.2 |
| Ca | 6.6 | 4.6 | 9.7 | 11.9 | 8.6 |
| Cb | 3.2 | - | 8.4 | - | - |
The signal to noise ratio at different regions are measured for the tallest peak in each region.
We note after the work on this manuscript has been completed, several classes of efficient homonuclear recoupling sequences suitable for fast-MAS conditions have emerged, first-order71 and second-order methods.72,73 These sequences should be well suited for NUS-PACC experiments, and assessment of their utility to resonance assignments and acquisition of tertiary distance restraints is currently ongoing in our laboratory.
Non-Uniformly Sampled 3D Experiments With Condensed Data Collection at MAS Frequency of 40 kHz
We recorded three non-uniformly sampled 3D DCP-DREAM spectra, NCOCA, NCACO and NCACB on U-13C,15N-enriched LC8 doped with 5 mM Cu(II)-EDTA (see Experiments and Methods). Additional data sets were also collected on the LC8 sample containing 50 mM Cu(II)-EDTA, but due to a considerable number of chemical shift perturbations and several missing peaks, we restrict the discussion to the first sample. At the MAS frequency of 40 kHz, 10 kHz low-power TPPM (lpTPPM)58 1H decoupling was implemented during t1, t2 and t3 time periods. No proton decoupling was applied for the DCP and DREAM magnetization transfers (see Figure 1B). A 500 ms recycle delay was used (to exceed three times the 1H T1). These conditions result in a four-fold timesaving by the PACC approach using the conventional uniform Cartesian sampling. Random non-uniform sampling schedule (see Figure 2) was created by the ScheduleTool63 based on the desired number of points, the desired maximum increment, and the estimated 15N and 13C T2. This NUS schedule sampled 25% (256) hypercomplex points compared to the corresponding uniform sampling schedule (32 × 32 = 1024). Overall, the combination of PACC and NUS results in 16-fold timesaving. The total experiment times are 9 hours for NCOCA (64 scans), 14 hours for NCACO (128 scans) and 27 hours for NCACB (192 scans). We note that for LC8, conventional 3D NCOCA, NCACO and NCACB experiments are very time consuming: on our 14.1 T instrument their completion requires 6, 9 and 18 days, respectively.
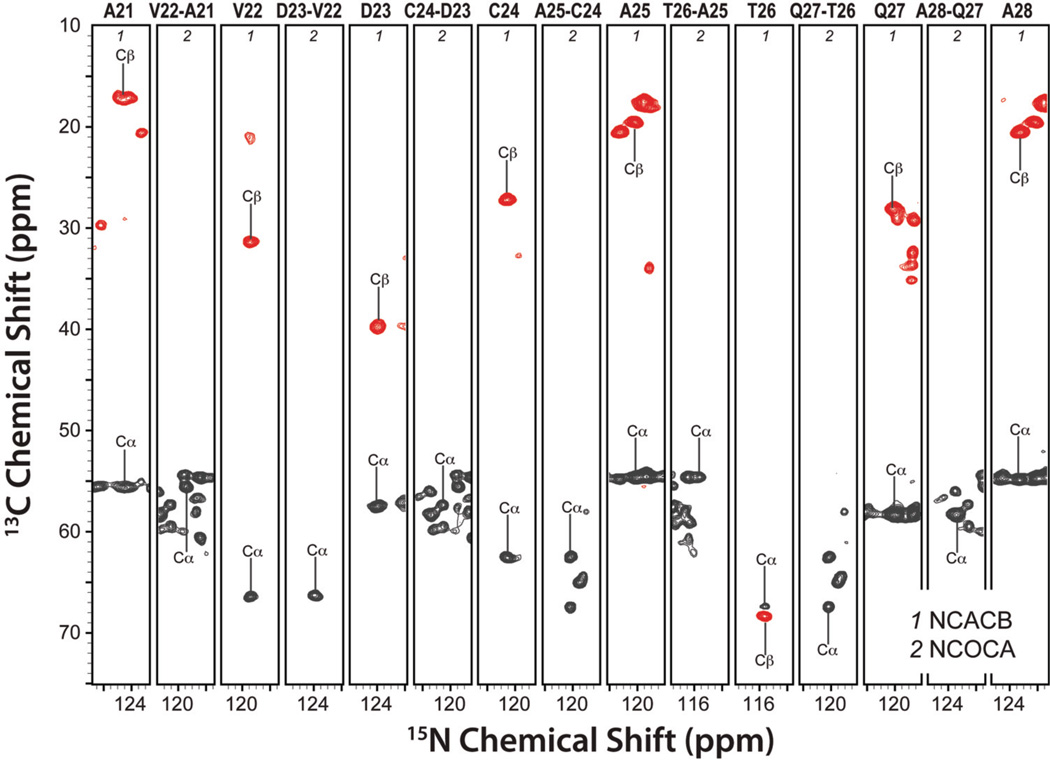
As discussed above, the chemical shifts recorded in these two 3D NUS-PACC experiments, are in excellent agreement with the shifts obtained on non-doped LC8 sample using the conventional approach (see Table S2). In Figure 6, a backbone walk for the stretch of residues 21–28 is shown, generated from the 3D NUS-PACC datasets. With the NUS-PACC experiments, we corroborated the resonance assignments of LC8 established by us previously from conventional 3D MAS experiments74. (see Table S4) From the isotropic chemical shifts and using TALOS+, we have derived the backbone torsion angles. As shown in Figure S4, the torsion angles and the secondary structure are in excellent agreement with the previous solid-state NMR,74 solution NMR,49 and X-ray75 studies.
Figure 6.
Backbone walk for A21–A28 using three-dimensional NUS-PACC experiments: DCP-DREAM based NCACB and NCOCA. Acquisition and processing parameters for each experiment are presented in the Experiments and Methods.
Peak Intensities, Dynamic Range and Data Processing Methods
MaxEnt reconstruction implemented in RNMRTK seeks optimization (in an unconstrained form) of a target function that is a combination of the entropy of the reconstructed spectrum and consistency with the experimental data.24 The relative weight of data consistency is determined by the Lagrange multiplier λ (larger λ indicates more significance is put on data consistency). We have recently demonstrated that in the limit of high λ, full linearity of the transformation between the time and the frequency domains is attained, permitting quantitative analysis of the spectral peak intensities; we dubbed this approach MINT.28
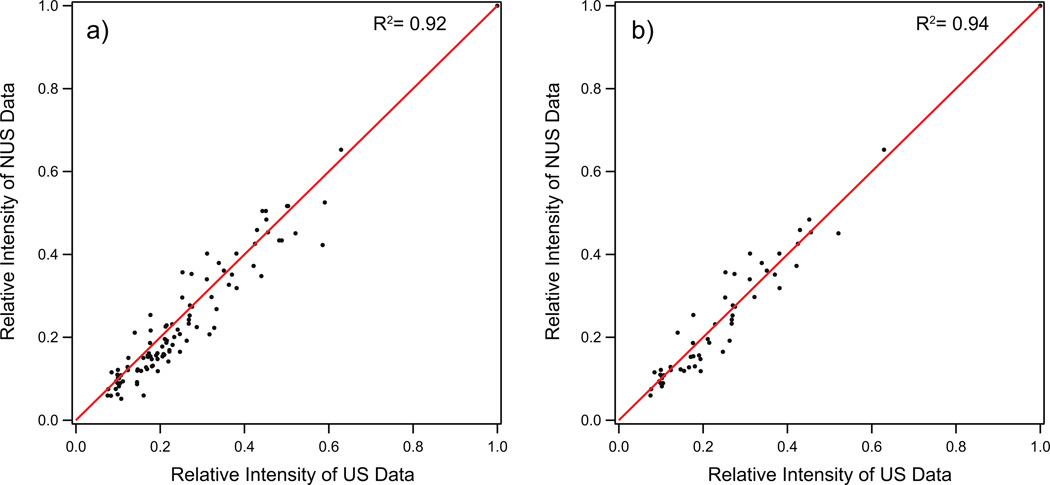
In processing the NUS-PACC data sets presented here, λ was set at 5000 for NUS-NCACB, NUS-NCACO and NUS-NCOCA experiments. This is a balanced choice of λ based on the intrinsic SNR of the NMR data, and these values generally produce high-quality spectra on data with limited dynamic range. This choice of processing parameters also enabled time-efficient reconstruction of 3D spectra discussed here, without significantly compromising the absolute peak intensities as evidenced by the highly linear correlation between peak intensities in the related 2D NUS-PACC and US datasets (see Figure 7).
Figure 7.
The correlation plot of relative peak intensity of 2D NCACB NUS spectrum vs US spectrum. (a) The correlations including diagonal-peaks (N-CA-CA) and cross-peaks (N-CA-CB) for 2D NUS vs 2D US. (b) The correlation plot of cross-peaks (N-CA-CB) only. Both spectra were processed by RNMRTK with high λ (following MINT protocol).
However, under these conditions it is possible that weak cross peaks may be overlooked in the datasets with high dynamic range.76 Many MAS NMR experiments, especially second-order dipolar recoupling methods, cover signals with broad dynamic range. For example, this occurs in homonuclear dipolar-based correlations, where diagonal peaks are often much stronger than the associated cross peaks, and cross peak intensities vary widely depending on the magnitude of the corresponding dipolar coupling. To examine the effect of dynamic range on the MaxEnt-processed NUS spectra of LC8, we also processed two NUS-PACC NCACB and NCOCA 3D data sets acquired on LC8 doped with 50 mM Cu(II)-EDTA using MDD,34 which has been formerly applied for data sets with broad dynamic range, such as solution NOESY data.77,78 Spectra generated by MaxEnt and MDD are very similar, if not identical (see Figure S5). We conclude that both numerical methods are suitable for processing DCP-DREAM-based NUS-PACC data. We note that under our experimental conditions, given generally high and uniform transfer efficiency of the DREAM mixing sequence, the dynamic range of the signal is somewhat limited. If non-uniform sampling is applied in experiments aimed at collecting distance restraints where peak intensities bear critical information and long-range correlations corresponding to generally weaker peaks are valuable, it will be interesting to explore multiple data processing algorithms for a broader range of MAS NMR data sets. For example, when distance restraints are acquired under fast-MAS conditions (e.g., R2 symmetry based RDSD,79 PARIS,80 SHANGHAI,72 PAR81,82 or PAIN-CP83), the different signals cover broad dynamic range. We expect that in such cases data processing by methods such as MINT,28 MDD77 (or its variant CO-MDD78), or FM35, might be advantageous for retaining weak signals.
Future Outlook
It is important to note that both the optimal ratio of sampled over omitted points and the overall sampling schedule affect the final spectral quality as well as the timesaving efficiency. In our approach, four-fold timesaving has resulted from the NUS protocol. However, it remains to be tested whether even more “aggressive” non-uniform sampling can be used in MAS SSNMR experiments. Recently, several new NUS sampling strategies that utilized probability and statistics theories were developed, and demonstrated that greater timesaving could be attained by designing advanced sampling schedules.84
Four-dimensional experiments were introduced by Rienstra and coworkers in the solid state several years ago.85 However, the long experimental time required for 4D experiments has so far prevented their applications to samples of low or even moderate sensitivity. Most recently, these researchers reported the application of GFT projection NMR in MAS experiments, where they demonstrated an order-of-magnitude reduction in experiment time by jointly sampling the two indirect dimensions.45 With this protocol, 4D spectroscopy can be carried out as (4,3)D GFT experiments. However, this approach is not suitable for samples with high intrinsic chemical shift degeneracy, such as microtubule-associated proteins LC874 and CAP-Gly8 or HIV-1 CA protein assemblies9,73 under investigation in our laboratory. With new numerical methods available for processing data collected with NUS in three indirect dimensions, such as FM35, msa3d of MaxEnt84, we expect that NUS-PACC can be implemented in four-dimensional MAS NMR experiments, thus enabling site-specific resonance assignments in challenging biological systems such as protein assemblies and membrane-associated proteins.
CONCLUSIONS
We have demonstrated that simultaneous use of non-uniform sampling (NUS) and paramagnetic-relaxation-assisted condensed data collection (PACC) enables dramatic timesaving in 3D MAS NMR experiments. Specifically, we have observed 16-fold timesaving in three 3D DCP-DREAM-based experiments, NCACB, NCACO and NCOCA, which constitute the basis for the resonance assignment protocol in proteins in the solid state. The net effect is a composite of the inherent sensitivity enhancements attained due to NUS (up to two-fold in each indirect dimension) and drastic reduction in spin-lattice relaxation times permitting very short recycle delays when MAS frequencies of 40 kHz and above are used. With careful screening of the paramagnetic dopant concentration appropriate conditions can be found where the chemical shift perturbations are limited to a small number of residues at the surface of the protein. These perturbations can be corrected for by comparison of shifts with those extracted from 2D spectra of a neat non-doped protein, once the resonance assignments are established. The approach introduced here can be extended to a wide variety of 3D or 4D experiments as long as they satisfy the requirement for PACC, and is expected to be highly beneficial for structural and dynamics studies of large proteins and protein assemblies by MAS NMR, especially those possessing inherently low sensitivity.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (NIH Grant R01GM085396 from NIGMS). Development of RNMRTK was supported in part by NIH P01-GM-047467. We thank Cheryl Arrowsmith and Aleksandras Gutmanas for providing the MDDGUI software.
Footnotes
SUPPORTING INFORMATION
Figures with i) 1H T1 and 15N T2* relaxation curves for neat LC8 and LC8 doped with Cu(II)-EDTA of various concentrations; ii) chemical shift deviations in 3D NCACB spectra recorded by NUS-PACC in LC8 doped with Cu(II)-EDTA at 5, 10, and 50 mM with respect to those recorded by US in neat LC8; iii) backbone torsion angles Ψ and Φ derived by TALOS+ using the chemical shifts of LC8 doped with 5 mM Cu(II)-EDTA recorded in NUS-PACC experiments; iv) 2D planes of 3D NCOCA NUS-PACC spectrum processed with MaxEnt and MDD. Tables with solution and solid-state chemical shift perturbations of LC8 for various Cu(II)-EDTA dopant concentrations, solid-state chemical shifts for LC8 doped with 5 mM Cu(II)-EDTA, chemical shift differences in a pair of 3D NCACB spectra (NUS and US) for LC8 doped with 5 mM Cu(II)-EDTA, the chemical shift assignments for the LC8 with 5 mM Cu(II)-EDTA by 3D NUS NCACB, NCACO and NCOCA spectra, standard deviations for the resolved residues in 3D NUS-PACC NCOCA spectra acquired three times, and the NUS schedules for the 3D experiments. This material is available free of charge via the Internet at http://pubs.acs.org.
Contributor Information
Shangjin Sun, Email: shangjin@udel.edu.
Si Yan, Email: syan@udel.edu.
Changmiao Guo, Email: cmguo@udel.edu.
Mingyue Li, Email: mingyue@udel.edu.
Jeffrey C. Hoch, Email: hoch@uchc.edu.
John C. Williams, Email: JWilliams@coh.org.
Tatyana Polenova, Email: tpolenov@udel.edu.
REFERENCES
- 1.McDermott A. Annu. Rev. Biophys. 2009;38:385–403. doi: 10.1146/annurev.biophys.050708.133719. [DOI] [PubMed] [Google Scholar]
- 2.Mani R, Cady SD, Tang M, Waring AJ, Lehrer RI, Hong M. Proc. Natl. Acad. Sci U.S.A. 2006;103:16242–16247. doi: 10.1073/pnas.0605079103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lange A, Giller K, Hornig S, Martin-Eauclaire MF, Pongs O, Becker S, Baldus M. Nature. 2006;440:959–962. doi: 10.1038/nature04649. [DOI] [PubMed] [Google Scholar]
- 4.Petkova AT, Yau WM, Tycko R. Biochemistry. 2006;45:498–512. doi: 10.1021/bi051952q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chimon S, Shaibat MA, Jones CR, Calero DC, Aizezi B, Ishii Y. Nat. Struct. Mol. Biol. 2007 doi: 10.1038/nsmb1345. [DOI] [PubMed] [Google Scholar]
- 6.Wasmer C, Lange A, Van Melckebeke H, Siemer AB, Riek R, Meier BH. Science. 2008;319:1523–1526. doi: 10.1126/science.1151839. [DOI] [PubMed] [Google Scholar]
- 7.Helmus JJ, Surewicz K, Nadaud PS, Surewicz WK, Jaroniec CP. Proc. Natl. Acad. Sci. U.S.A. 2008;105:6284–6289. doi: 10.1073/pnas.0711716105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun S, Siglin A, Williams JC, Polenova T. J. Am. Chem. Soc. 2009;131:10113–10126. doi: 10.1021/ja902003u. [DOI] [PubMed] [Google Scholar]
- 9.Han Y, Ahn J, Concel J, Byeon IJ, Gronenborn AM, Yang J, Polenova T. J. Am. Chem. Soc. 2010;132:1976–1987. doi: 10.1021/ja908687k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wüthrich K. NMR of proteins and nucleic acids. New York: Wiley; 1986. [Google Scholar]
- 11.Sattler M, Schleucher J, Griesinger C. Prog. Nucl. Magn. Reson. Spectrosc. 1999;34:93–158. [Google Scholar]
- 12.Baldus M. Prog. Nucl. Magn. Reson. Spectrosc. 2002;41:1–47. [Google Scholar]
- 13.Ikura M, Kay LE, Bax A. Biochemistry. 1990;29:4659–4667. doi: 10.1021/bi00471a022. [DOI] [PubMed] [Google Scholar]
- 14.Grzesiek S, Dobeli H, Gentz R, Garotta G, Labhardt AM, Bax A. Biochemistry. 1992;31:8180–8190. doi: 10.1021/bi00150a009. [DOI] [PubMed] [Google Scholar]
- 15.Kim S, Szyperski T. J. Am. Chem. Soc. 2003;125:1385–1393. doi: 10.1021/ja028197d. [DOI] [PubMed] [Google Scholar]
- 16.Wickramasinghe NP, Parthasarathy S, Jones CR, Bhardwaj C, Long F, Kotecha M, Mehboob S, Fung LW, Past J, Samoson A, Ishii Y. Nat. Methods. 2009;6:215–218. doi: 10.1038/nmeth.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Linser R, Chevelkov V, Diehl A, Reif B. J. Magn. Reson. 2007;189:209–216. doi: 10.1016/j.jmr.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Laage S, Sachleben JR, Steuernagel S, Pierattelli R, Pintacuda G, Emsley L. J. Magn. Reson. 2009;196:133–141. doi: 10.1016/j.jmr.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 19.Bertini I, Emsley L, Lelli M, Luchinat C, Mao J, Pintacuda G. J. Am. Chem. Soc. 2010;132:5558–5559. doi: 10.1021/ja100398q. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto K, Xu J, Kawulka KE, Vederas JC, Ramamoorthy A. J. Am. Chem. Soc. 2010;132:6929–6931. doi: 10.1021/ja102103n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nadaud PS, Helmus JJ, Sengupta I, Jaroniec CP. J. Am. Chem. Soc. 2010;132:9561–9563. doi: 10.1021/ja103545e. [DOI] [PubMed] [Google Scholar]
- 22.Kazimierczuk K, Stanek J, Zawadzka-Kazimierczuk A, Kozminski W. Prog. Nucl. Magn. Reson. Spectrosc. 2010;57:420–434. doi: 10.1016/j.pnmrs.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Coggins BE, Venters RA, Zhou P. Prog. Nucl. Magn. Reson. Spectrosc. 2010;57:381–419. doi: 10.1016/j.pnmrs.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoch JC, Stern AS. Meth. Enzymol. 2001;338:159–178. doi: 10.1016/s0076-6879(02)38219-3. [DOI] [PubMed] [Google Scholar]
- 25.Freeman R, Kupce E. J. Biomol. NMR. 2003;27:101–113. doi: 10.1023/a:1024960302926. [DOI] [PubMed] [Google Scholar]
- 26.Atreya HS, Szyperski T. Meth. Enzymol. 2005;394:78–108. doi: 10.1016/S0076-6879(05)94004-4. [DOI] [PubMed] [Google Scholar]
- 27.Szyperski T, Yeh DC, Sukumaran DK, Moseley HN, Montelione GT. Proc. Natl. Acad. Sci. U.S.A. 2002;99:8009–8014. doi: 10.1073/pnas.122224599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paramasivam S, Suiter CL, Hou GJ, Sun SJ, Palmer M, Hoch JC, Rovnyak D, Polenova T. J. Phys. Chem. B. 2012;116:7416–7427. doi: 10.1021/jp3032786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rovnyak D, Sarcone M, Jiang Z. Magn. Reson. Chem. 2011 doi: 10.1002/mrc.2775. [DOI] [PubMed] [Google Scholar]
- 30.Kupce E, Freeman R. J. Am. Chem. Soc. 2003;125:13958–13959. doi: 10.1021/ja038297z. [DOI] [PubMed] [Google Scholar]
- 31.Hiller S, Fiorito F, Wuthrich K, Wider G. Proc. Natl. Acad. Sci. U.S.A. 2005;102:10876–10881. doi: 10.1073/pnas.0504818102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malmodin D, Billeter M. Magn. Reson. Chem. 2006;44:S185–S195. doi: 10.1002/mrc.1824. Spec No. [DOI] [PubMed] [Google Scholar]
- 33.Eghbalnia HR, Bahrami A, Tonelli M, Hallenga K, Markley JL. J. Am. Chem. Soc. 2005;127:12528–12536. doi: 10.1021/ja052120i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jaravine V, Ibraghimov I, Orekhov VY. Nat. Methods. 2006;3:605–607. doi: 10.1038/nmeth900. [DOI] [PubMed] [Google Scholar]
- 35.Hyberts SG, Frueh DP, Arthanari H, Wagner G. J. Biomol. NMR. 2009;45:283–294. doi: 10.1007/s10858-009-9368-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holland DJ, Bostock MJ, Gladden LF, Nietlispach D. Angew. Chem. Int. Ed. 2011;50:6548–6551. doi: 10.1002/anie.201100440. [DOI] [PubMed] [Google Scholar]
- 37.Kazimierczuk K, Orekhov VY. Angew. Chem. Int. Ed. 2011;50:5556–5559. doi: 10.1002/anie.201100370. [DOI] [PubMed] [Google Scholar]
- 38.Shrot Y, Frydman L. J. Magn. Reson. 2011;209:352–358. doi: 10.1016/j.jmr.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsuki Y, Eddy MT, Herzfeld J. J. Am. Chem. Soc. 2009;131:4648–4656. doi: 10.1021/ja807893k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kazimierczuk K, Kozminski W, Zhukov I. J. Magn. Reson. 2006;179:323–328. doi: 10.1016/j.jmr.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 41.Jiang B, Jiang X, Xiao N, Zhang X, Jiang L, Mao XA, Liu M. J. Magn. Reson. 2010;204:165–168. doi: 10.1016/j.jmr.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 42.Rovnyak D, Filip C, Itin B, Stern AS, Wagner G, Griffin RG, Hoch JC. J. Magn. Reson. 2003;161:43–55. doi: 10.1016/s1090-7807(02)00189-1. [DOI] [PubMed] [Google Scholar]
- 43.Jones DH, Opella SJ. J. Magn. Reson. 2006;179:105–113. doi: 10.1016/j.jmr.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 44.Matsuki Y, Eddy MT, Griffin RG, Herzfeld J. Angew. Chem. Int. Ed. Engl. 2010 doi: 10.1002/anie.201003329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Franks WT, Atreya HS, Szyperski T, Rienstra CM. J. Biomol. NMR. 2010 doi: 10.1007/s10858-010-9451-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maly T, Debelouchina GT, Bajaj VS, Hu KN, Joo CG, Mak-Jurkauskas ML, Sirigiri JR, van der Wel PC, Herzfeld J, Temkin RJ, Griffin RG. J. Chem. Phys. 2008;128:052211. doi: 10.1063/1.2833582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duma L, Hediger S, Brutscher B, Bockmann A, Emsley L. J. Am. Chem. Soc. 2003;125:11816–11817. doi: 10.1021/ja036893n. [DOI] [PubMed] [Google Scholar]
- 48.Laage S, Lesage A, Emsley L, Bertini I, Felli IC, Pierattelli R, Pintacuda G. J. Am. Chem. Soc. 2009;131:10816–10817. doi: 10.1021/ja903542h. [DOI] [PubMed] [Google Scholar]
- 49.Lightcap CM, Sun S, Lear JD, Rodeck U, Polenova T, Williams JC. J. Biol. Chem. 2008;283:27314–27324. doi: 10.1074/jbc.M800758200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marulanda D, Tasayco ML, McDermott A, Cataldi M, Arriaran V, Polenova T. J. Am. Chem. Soc. 2004;126:16608–16620. doi: 10.1021/ja0464589. [DOI] [PubMed] [Google Scholar]
- 51.Neue G, Dybowski C. Solid State Nucl. Magn. Reson. 1997;7:333–336. doi: 10.1016/s0926-2040(96)01291-x. [DOI] [PubMed] [Google Scholar]
- 52.Thurber KR, Tycko R. J. Magn. Reson. 2009;196:84–87. doi: 10.1016/j.jmr.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morcombe CR, Zilm KW. J. Magn. Reson. 2003;162:479–486. doi: 10.1016/s1090-7807(03)00082-x. [DOI] [PubMed] [Google Scholar]
- 54.Levitt MH. Spin dynamics : basics of nuclear magnetic resonance. Chichester; New York: John Wiley & Sons; 2001. [Google Scholar]
- 55.Schaefer J, McKay RA, Stejskal EO. J. Magn. Reson. 1979;34:443–447. [Google Scholar]
- 56.Baldus M, Petkova AT, Herzfeld J, Griffin RG. Mol. Phys. 1998;95:1197–1207. [Google Scholar]
- 57.Bennett AE, Rienstra CM, Auger M, Lakshmi KV, Griffin RG. J. Chem. Phys. 1995;103:6951–6958. [Google Scholar]
- 58.Kotecha M, Wickramasinghe NP, Ishii Y. Magn. Reson. Chem. 2007;45:S221–S230. doi: 10.1002/mrc.2151. [DOI] [PubMed] [Google Scholar]
- 59.Bennett AE, Rienstra CM, Griffiths JM, Zhen W, Lansbury PT, Griffin RG. J. Chem. Phys. 1998;108:9463–9479. [Google Scholar]
- 60.Ishii Y. J. Chem. Phys. 2001;114:8473–8483. [Google Scholar]
- 61.Verel R, Baldus M, Ernst M, Meier BH. Chem. Phys. Lett. 1998;287:421–428. [Google Scholar]
- 62.States DJ, Haberkorn RA, Ruben DJ. J. Magn. Reson. 1982;48:286–292. [Google Scholar]
- 63.Maciejewski MW, Gorbatyuk V, Hoch JC. ScheduleTool. [Google Scholar]
- 64.Wishart DS, Bigam CG, Yao J, Abildgaard F, Dyson HJ, Oldfield E, Markley JL, Sykes BD. J. Biomol. NMR. 1995;6:135–140. doi: 10.1007/BF00211777. [DOI] [PubMed] [Google Scholar]
- 65.Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A. J. Biomol. NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 66.Hoch JC, Stern AS. NMR Data Processing. New York: Wiley-Liss; 1996. [Google Scholar]
- 67.Stern AS, Li KB, Hoch JC. J. Am. Chem. Soc. 2002;124:1982–1993. doi: 10.1021/ja011669o. [DOI] [PubMed] [Google Scholar]
- 68.Mobli M, Maciejewski MW, Gryk MR, Hoch JC. J. Biomol. NMR. 2007;39:133–139. doi: 10.1007/s10858-007-9180-8. [DOI] [PubMed] [Google Scholar]
- 69.Goddard TD, Kneller DG. Sparky 3. [Google Scholar]
- 70.McConnell HM, Robertson RE. J. Chem. Phys. 1958;29:1361–1365. [Google Scholar]
- 71.Shen M, Hu B, Lafon O, Trebosc J, Chen Q, Amoureux JP. J. Magn. Reson. 2012;223:107–119. doi: 10.1016/j.jmr.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 72.Hu B, Lafon O, Trebosc J, Chen Q, Amoureux J-P. J. Magn. Reson. 2011;212:320–329. doi: 10.1016/j.jmr.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 73.Byeon IJL, Hou GJ, Han Y, Suiter CL, Ahn J, Jung J, Byeon CH, Gronenborn AM, Polenova T. J. Am. Chem. Soc. 2012;134:6455–6466. doi: 10.1021/ja300937v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sun S, Butterworth AH, Paramasivam S, Yan S, Lightcap CM, Williams JC, Polenova T. Can. J. Chem. 2011;89:909–918. doi: 10.1139/v11-030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Williams JC, Roulhac PL, Roy AG, Vallee RB, Fitzgerald MC, Hendrickson WA. Proc. Natl. Acad. Sci. U.S.A. 2007;104:10028–10033. doi: 10.1073/pnas.0703614104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hyberts SG, Heffron GJ, Tarragona NG, Solanky K, Edmonds KA, Luithardt H, Fejzo J, Chorev M, Aktas H, Colson K, Falchuk KH, Halperin JA, Wagner G. J. Am. Chem. Soc. 2007;129:5108–5116. doi: 10.1021/ja068541x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tugarinov V, Kay LE, Ibraghimov I, Orekhov VY. J. Am. Chem. Soc. 2005;127:2767–2775. doi: 10.1021/ja044032o. [DOI] [PubMed] [Google Scholar]
- 78.Hiller S, Ibraghimov I, Wagner G, Orekhov VY. J. Am. Chem. Soc. 2009;131:12970–12978. doi: 10.1021/ja902012x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hou G, Yan S, Sun S, Han Y, Byeon IJ, Ahn J, Concel J, Samoson A, Gronenborn AM, Polenova T. J. Am. Chem. Soc. 2010 doi: 10.1021/ja108650x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Weingarth M, Demco DE, Bodenhausen G, Tekely P. Chem. Phys. Lett. 2009;469:342–348. [Google Scholar]
- 81.De Paepe G, Lewandowski JR, Loquet A, Bockmann A, Griffin RG. J. Chem. Phys. 2008;129:245101. doi: 10.1063/1.3036928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lewandowski JR, De Paepe G, Eddy MT, Struppe J, Maas W, Griffin RG. J. Phys. Chem. B. 2009;113:9062–9069. doi: 10.1021/jp810280t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lewandowski JR, De Paepe G, Griffin RG. J. Am. Chem. Soc. 2007;129:728–729. doi: 10.1021/ja0650394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mobli M, Stern AS, Bermel W, King GF, Hoch JC. J. Magn. Reson. 2010;204:160–164. doi: 10.1016/j.jmr.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Franks WT, Kloepper KD, Wylie BJ, Rienstra CM. J. Biomol. NMR. 2007;39:107–131. doi: 10.1007/s10858-007-9179-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.