Abstract
Objective
To describe how retinal venular diameter changes over time for an individual and to examine differences in these changes among people with different risk profiles.
Design
Population-based cohort study.
Participants
4600 persons aged 43–86 years from the Beaver Dam Eye Study who participated in at least 1 examination and had venular diameter measured in the right eye.
Methods
Data from 4 examinations over 15 years were analyzed. Retinal venular diameter was measured from photographs at each examination by computer-assisted methods and summarized as the central retinal venular equivalent (CRVE). Associations of risk factors to concurrent CRVE measurements and changes in CRVE over time were determined using multivariate analyses.
Main Outcome Measure
CRVE.
Results
CRVE tended to narrow with age. Mean CRVE was about 5 µm smaller (225 vs. 230 µm) for the average 70-year-old compared to the average 50-year-old, and was about 13 µm smaller (217 vs. 230 µm) for the average 85-year-old compared to the average 50-year-old. Male sex (beta estimate [β]=5.34; 95% confidence interval [CI] 3.58, 6.90), current smoking (β=9.38; 95% CI 8.26, 10.49), and higher white blood cell count (per 1000/µL: β=0.95; 95% CI 0.74, 1.16) were independently associated with larger concurrent CRVE while higher mean arterial blood pressure (per 5 mmHg: β=−0.36; 95% CI −0.50, −0.23) and higher high-density lipoprotein cholesterol (per 10 mg/dL: β=−0.89; 95% CI −1.15, −0.63) were independently associated with smaller concurrent CRVE. History of cardiovascular disease (β=−0.16; 95% CI −0.26, −0.06) and presence of chronic kidney disease (β=−0.20; 95% CI −0.34, −0.05) were associated with a greater decrease in CRVE over time.
Conclusions
These data show that retinal venular diameter tended to narrow with age, and that concurrent venular diameter is independently associated with sex, blood pressure, high-density lipoprotein cholesterol, white blood cell count, and current smoking, and change in CRVE is independently associated with a history of cardiovascular disease and presence of chronic kidney disease. The different independent effects of these interrelated factors on CRVE highlight the complex relationship between systemic diseases and conditions and CRVE, and the difficulty in determining specific causes of change in CRVE over time.
The retinal microvasculature has been used as a “window into the body” to study the relationship of ocular and systemic diseases and conditions on the microvasculature. The central retinal venular equivalent (CRVE), a measure of retinal venular diameter, has been shown to be related to various systemic and environmental factors including diabetes, hyperglycemia and the metabolic syndrome, serum total cholesterol and inflammatory markers, body mass index (BMI), and smoking,1–13 and when considered independently of these risk factors is related to coronary heart disease14 and stroke.15–17
However, many of these relationships are cross-sectional, and it is unclear if changes in a risk factor (e.g., glucose levels) cause changes in CRVE, or vice versa, or if a common factor causes changes in both. Furthermore, it has been observed that retinal vessel diameter tends to be smaller in older individuals2,8,18; however, to our knowledge, no longitudinal studies have yet described how CRVE tends to change for an individual over the course of his or her life and what factors might be related to those changes. The purpose of this report is to describe how CRVE changes with age over 15 years and to examine factors related to those changes. This report makes use of data from 4 examinations, each spaced 5 years apart, in the population-based Beaver Dam Eye Study (BDES).
METHODS
Population
A private census of Beaver Dam, Wisconsin, was performed in 1987–1988 to identify all residents 43–84 years of age.19 Four thousand nine hundred and twenty-six of 5924 persons identified participated in the baseline examination (exam 1) in 1988–1990. Ninety-nine percent of the population was white and 56% was female. The cohort was re-examined at 5- (n=3722), 10- (n=2962), and 15-year (n=2375) follow-up examinations (exam 2, exam 3, and exam 4, respectively). There was greater than 80% participation among survivors at each examination.19–22 Differences between participants and nonparticipants have been presented elsewhere.19–22 In general, participants at each examination were younger, had lower blood pressure, and had fewer co-morbid conditions at baseline than nonparticipants. Informed consent was obtained from each participant before every examination. All data were collected with Institutional Review Board approval from the University of Wisconsin-Madison in conformity with all federal and state laws, the work was compliant with the Health Insurance Portability and Accountability Act (HIPAA), and the study adhered to the tenets of the Declaration of Helsinki.
Procedures
Participants underwent a standardized interview and examination using the same protocols for measurements each time.23 A questionnaire was administered that included questions on the history of physician-diagnosed diabetes, cigarette smoking, hypertension, and use of medications (e.g., blood pressure lowering, statins, nonsteroidal anti-inflammatory drugs [NSAIDs]). Height, weight, and blood pressures were measured. Intraocular pressure was measured using applanation tonometry. The number of years smoked and length of time since quitting smoking were determined from self-report.
Serum total and high density lipoprotein (HDL) cholesterol, glycosylated hemoglobin, and a complete blood count were determined.24,25 At all examinations, additional blood samples were stored at −80°C until the time of laboratory analysis. Frozen samples from baseline were analyzed for serum creatinine, soluble vascular cell adhesion molecule-1, serum 8-isoprostane and high sensitivity C-reactive protein (CRP).26,27 The Modification of Diet in Renal Disease study equation was used to determine estimated glomerular filtration rate (eGFR).26
Participants’ pupils were pharmacologically dilated. A slit-lamp camera (Topcon America Corporation, Paramus, NJ) was used to photograph the lens of each eye, and the photographs were graded for the presence and severity of nuclear sclerosis.28 Stereoscopic 30° color fundus photographs centered on the disc (Diabetic Retinopathy Study standard field 1) were obtained for each eye.29,30 Age-related macular degeneration (AMD) was graded according to the Wisconsin Age-Related Maculopathy Grading System.31,32
Retinal vessels were measured from the digitized images of standard field 1 using a semiautomated computer program designed for this project (IVAN, University of Wisconsin- Madison, Ferrier NJ) and summarized as central retinal arteriolar equivalent (CRAE) and CRVE.33,34 In brief, the grading protocol required vessel diameters from the six largest arterioles and six largest venules located in a zone 0.5 to 1.0 disc diameters from the disc margin.34,35 All fundus photographs were taken on a single camera and graded by one of four vessel measurement graders at the University of Wisconsin Ocular Epidemiology Reading Center. Every 6 months, the graders measured a sample of 50 eyes to determine inter- and intra-grader variability; intraclass correlation coefficients were extremely high (>0.90) for both inter- and intra-grader comparisons for both arteriolar and venular measurements. When grading the same eye, the average standard deviation of the measurement of CRVE among the graders was 2.44 µm (95% CI 2.06, 2.82 µm).
Definitions
Image quality was evaluated as good, fair, or poor. Nuclear sclerosis was graded by comparing images to standard photographs using a 5-step scale and categorized as ≤2, 3, >3, or cataract surgery. Refraction was categorized as moderately to highly myopic (<−3 diopters), mildly myopic (−3 to −1 diopters), emmetropic (−1 to 1 diopters), mildly hyperopic (1 to 3 diopters), and hyperopic (>3 diopters). Current smokers were identified as persons having smoked ≥100 cigarettes in their lifetime and were smoking at the time of the examination. Past smokers had smoked ≥100 cigarettes in their lifetime but had stopped by the time of the examination. Pack-years was defined as the average number of cigarettes smoked per day divided by 20, then multiplied by the number of years smoked. BMI was defined as weight in kilograms divided by the square of height in meters. Current heavy drinking was defined as consuming ≥4 servings of alcoholic beverages daily.
Total glycosylated hemoglobin was determined using affinity chromatography (Isolab Inc., Akron, OH, USA)36 at exams 1 and 2 and by ion capture assay (Abbott Laboratories, Abbott Park, IL, USA) at exam 3. Diabetes was defined as self-report of diabetes with treatment or elevated glycosylated hemoglobin level. White blood cell (WBC) count and hematocrit level were determined by the Coulter counter method. Mean arterial blood pressure (MABP) was defined as systolic blood pressure + (2 × diastolic blood pressure) ÷ 3. Hypertension was defined as systolic blood pressure >140 mmHg and/or diastolic blood pressure >90 mmHg and/or antihypertensive medication use. History of cardiovascular disease (CVD) was defined as a history of a myocardial infarction, angina, stroke, or brain hemorrhage. Chronic kidney disease (CKD) was defined as eGFR of <45 mL/min/1.73 m2.
Statistical Methods
Results are presented for the right eye only. Eyes in which one of the 6 largest venules was ungradable were excluded. Retinal photographs taken with non-Zeiss cameras, or eyes with late AMD, photocoagulation, photodynamic or any intravitreal treatment for AMD, retinal vessel occlusions, retinopathy, macular edema, or other pathology were excluded.
All analyses were performed with SAS version 9.2 (Cary, NC). Figure 1 was created by plotting the mean CRVE for all individuals at a given age, ignoring visit, and a local estimation line was fit using SAS PROC SGRENDER. In Figure 2 (available at http://aaojournal.org), change in CRVE was calculated by subtracting CRVE at the beginning of a 5-year interval from CRVE at the end of that interval (e.g., CRVE at exam 2 minus CRVE at baseline, CRVE at exam 3 minus CRVE at exam 2) and categorized by age at the beginning of the interval.
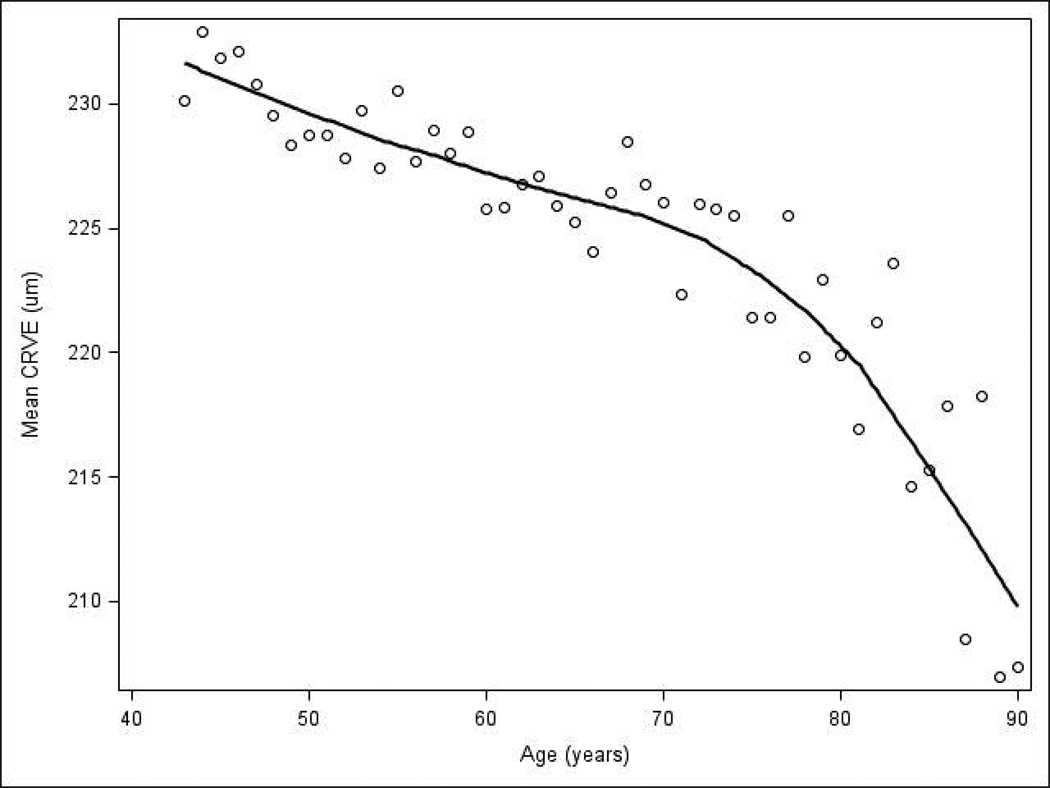
Figure 1.
Mean central retinal venular equivalent (CRVE) for all participants at a given age.
The relationship of each risk factor to CRVE was modeled using a multilevel model for change37 implemented with SAS PROC MIXED. This model was used because it allowed for summarization of the way CRVE changes over time for an individual and examination of differences in these changes between people with different risk profiles. Each model contained a term that measured the association between each risk factor and concurrent CRVE (both measured at the same examination) and a separate term that measured the association between each risk factor and change in CRVE over time. The data were structured in a person-period dataset where each participant had multiple records, one for each examination. An unstructured error covariance matrix accounted for correlation between measurements from the same individual. Age centered at 65 years (the overall mean age in our population across all exams) was used as the time scale.
Each risk factor was first modeled separately; those model results were then used to test hypotheses about the relationship of each risk factor to CRVE and to create plots that showed each relationship graphically. All models additionally adjusted for image focus, cataract status (presence of nuclear cataract in categories described above, or cataract surgery), refraction, and height centered at 65 inches. To test whether there was a quadratic effect of age on CRVE, all of the individual risk factor models were re-run using an age-squared transformation, and the Akaike information criterion (AIC) was compared to the same model where age was entered as a linear term. Using the transformed values of age did not improve the fit of any model.
We plotted the estimated trajectory of CRVE for an individual from ages 43 to 90 years, derived from the individual risk factor models described above, assuming good image focus, no cataract, emmetropic refraction, and height of 65 inches. For dichotomous risk factors, we plotted the expected trajectory for an individual with and without a risk factor (e.g., a current smoker vs. a non-smoker). For continuous risk factors, we plotted the expected trajectory for individuals with high and low values of the risk factor. The lines on the figures assume that the measurement of the risk factor is constant over time, but they can be used to estimate effects of change in the risk factor at a certain age. For example, if an individual stopped smoking at age 65, the trajectory of his/her CRVE would be expected to follow that of a current smoker from ages 43–65 and then change to that of a non-smoker from ages 65–90.
Because of collinearity among several predictors (MABP and hypertension; creatinine, eGFR, and presence of CKD; CRP and WBC count), we created several multivariate models with all risk factors that were significant individually (also adjusting for image focus, cataract status, refraction, and height) and used backward selection until a set of final models where all the terms were significant was determined. We then compared the resulting models and selected the one with the lowest AIC as our final multivariate model.
In order to utilize data from all 4 exams in our multivariate models, in cases where a variable was not measured at every exam, the missing values were imputed to be the same as at the time the variable was last measured. For example, because serum total cholesterol was not measured at exam 4, we imputed its value to be the same as it was at exam 3 for each individual.
RESULTS
Of the 4926 individuals who participated in the BDES exam 1, 4739 had gradable CRVE in the right eye. Of these, 12 were excluded because their photos were taken using a non-Zeiss camera, 63 because late AMD or treatment for AMD was present, 18 because retinal vessel occlusion was present, 81 because moderate to severe nonproliferative or proliferative retinopathy (ETDRS severity ≥ level 31) or macular edema was present, and 47 because other ocular pathology was present, leaving 4518 individuals who contributed data for exam 1. Similarly, 3242 of 3494 participants with gradable CRVE at exam 2, 2349 of 2569 participants with gradable CRVE at exam 3, and 1820 of 2191 participants with gradable CRVE at exam 4 contributed data to the analysis of change in CRVE. In total, 4600 unique participants were included in the analyses.
Characteristics of these individuals are displayed in Table 1 (available at http://aaojournal.org). Controlling for age, participants at later exams had narrower CRVE, lower MABP, lower serum total and HDL cholesterol, and a lower frequency of current smoking and nuclear sclerosis. Participants at later exams had greater BMI, higher glycosylated hemoglobin levels, and higher frequencies of cataract surgery, history of taking aspirin and NSAIDs, diabetes, CVD, and CKD. Percentages of men and women were similar among participants at earlier and later exams.
Figure 1 shows the relationship between mean CRVE and age aggregated over all 4 examinations. Mean CRVE was about 5 µm smaller (about 225 µm vs. 230 µm) for the average 70-year-old compared to the average 50-year-old, and was about 13 µm smaller (about 217 µm vs. 230 µm) for the average 85-year-old compared to the average 50-year-old. CRVE tended to decrease over a 5-year interval and was not significantly different in the 3 youngest age groups; however, the mean 5-year decrease in CRVE was greater (more negative) for individuals over 80 years old compared to individuals less than 60 years old (P<0.01, Figure 2 [available at http://aaojournal.org]). Change in CRVE was small compared to the standard deviation for change. For example, change in CRVE for the youngest age group was −3.4 µm and the standard deviation was 13.5 µm.
Table 2 presents relationships of risk factors with concurrent CRVE and the change in CRVE over time. Figure 3 shows the expected CRVE for ages 43 to 90, stratified by risk level for selected characteristics in Table 2. Figure 4 (available at http://aaojournal.org) shows the relationships of all risk factors that were statistically significantly related to concurrent CRVE or change in CRVE.
Table 2.
Relationships of Risk Factors with Concurrent Central Retinal Venular Equivalent and the Rate of Change in Central Retinal Venular Equivalent Over Time
| Concurrent CRVE | Rate of Change in CRVE | |||||
|---|---|---|---|---|---|---|
| Risk Factor* | β Estimate | 95% CI | P value | β Estimate | 95% CI | P value |
| Sex (men vs. women) | 7.13 | 5.45, 8.81 | <.001 | 0.04 | −0.04, 0.11 | 0.33 |
| Mean arterial blood pressure (per 5 mmHg) | −0.33 | −0.46, −0.20 | <.001 | −0.01 | −0.02, 0.004 | 0.23 |
| Hypertension present | −0.50 | −1.19, 0.20 | 0.16 | −0.07 | −0.13, −0.01 | 0.02 |
| Intraocular pressure (per 2 mmHg) | 0.01 | −0.19, 0.22 | 0.89 | 0.001 | −0.02, 0.02 | 0.87 |
| Body mass index (per 3 kg/m2) | 0.33 | 0.08, 0.58 | 0.01 | 0.03 | 0.01, 0.05 | <.001 |
| History of cardiovascular disease | 0.96 | −0.15, 2.06 | 0.09 | −0.11 | −0.20, −0.02 | 0.01 |
| Diabetes present | −0.09 | −0.73, 0.56 | 0.79 | −0.03 | −0.09, 0.02 | 0.25 |
| Glycosylated hemoglobin (per 1%) | 0.39 | 0.12, 0.66 | 0.01 | 0.01 | −0.02, 0.03 | 0.60 |
| Total cholesterol (per 20 mg/dL) | 0.04 | −0.12, 0.19 | 0.66 | 0.02 | 0.01, 0.03 | <.001 |
| HDL cholesterol (per 10 mg/dL) | −1.08 | −1.33, −0.83 | <.001 | 0.005 | −0.01, 0.02 | 0.63 |
| Current nonsteroidal anti-inflammatory drug use | −1.50 | −2.02, −0.98 | <.001 | −0.01 | −0.06, 0.04 | 0.80 |
| Current aspirin use | 0.55 | −0.08, 1.18 | 0.09 | −0.06 | −0.12, −0.001 | 0.04 |
| Current heavy drinking | 1.43 | −0.85, 3.72 | 0.22 | 0.04 | −0.18, 0.26 | 0.75 |
| Current smoking | 9.55 | 8.40, 10.70 | <.001 | 0.04 | −0.05, 0.13 | 0.39 |
| Pack-years (per 5) | 0.40 | 0.30, 0.50 | <.001 | −0.01 | −0.02, −0.003 | 0.01 |
| Years since stopping smoking (per 5) | −0.98 | −1.25, −0.71 | <.001 | −0.02 | −0.04, 0.001 | 0.07 |
| Years smoked (per 5) | 1.23 | 1.02, 1.44 | <.001 | −0.04 | −0.05, −0.02 | <.001 |
| Chronic kidney disease present | −0.42 | −2.38, 1.54 | 0.67 | −0.21 | −0.35, −0.07 | <.001 |
| Serum creatinine (per 0.1 mg/dL) | −0.09 | −0.24, 0.05 | 0.20 | −0.02 | −0.03, −0.004 | 0.01 |
| Estimated GFR (per 1 mL/min/1.73 m2) | 0.02 | 0.0006, 0.04 | 0.04 | 0.001 | −0.0005, 0.003 | 0.18 |
| White blood cell count (per 1000/µL) | 1.19 | 0.99, 1.40 | <.001 | −0.02 | −0.03, 0.00 | 0.02 |
| Serum 8-isoprostane (per pg/mL) | 0.002 | −0.01, 0.01 | 0.57 | 0.0001 | −0.001, 0.001 | 0.79 |
| Serum sVCAM-1 (per ng/mL) | −0.001 | −0.004, 0.002 | 0.47 | −0.00003 | −0.0002, 0.0002 | 0.81 |
| Serum C-reactive protein (per mg/L) | 0.16 | 0.10, 0.21 | <.001 | −0.007 | −0.01, −0.001 | <.001 |
CI, confidence interval; CRVE, central retinal venular equivalent; GFR, glomerular filtration rate; HDL, high density lipoprotein; sVCAM-1, soluble vascular cell adhesion molecule-1.
All are adjusted for image focus, cataract status, refraction, and height and use age as the time scale.
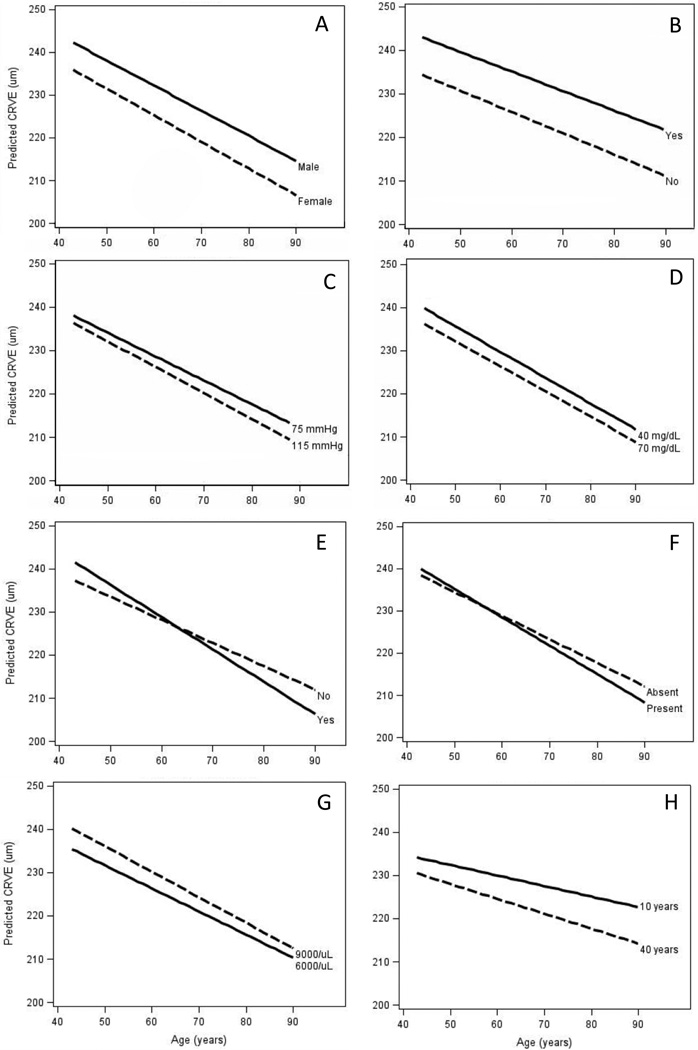
Figure 3.
Estimated central retinal venular equivalent (CRVE) for persons aged 43 to 90 years, stratified by risk level for selected characteristics: A. Sex; B. Current smoking; C. Mean arterial blood pressure; D. Serum high-density lipoprotein cholesterol; E. Chronic kidney disease; F. History of cardiovascular disease; G. White blood cell count; H. Years since stopping smoking.
Being male (Figure 3A; beta estimate [β]=7.12, 95% confidence interval [CI] 5.45, 8.81), having higher glycosylated hemoglobin (per 1%: β=0.39; 95% CI 0.12, 0.66), being a current smoker (Figure 3B; β=9.55, 95% CI 8.40, 10.70), and having a higher eGFR (per 1 mL/min/1.73 m2: β=0.02, 95% CI 0.001, 0.04) were associated with larger concurrent CRVE but not change in CRVE.
Higher MABP (Figure 3C; per 5 mmHg: β=−0.33, 95% CI −0.46, −0.20), higher HDL cholesterol (Figure 3D; per 10 mg/dL: β=−1.08, 95% CI −1.33, −0.83), and NSAID use (β=−1.50, 95% CI −2.02, −0.98) were associated with smaller concurrent CRVE but not change in CRVE.
Presence of hypertension (β=−0.07, 95% CI −0.13, −0.01) and presence of CKD (Figure 3E; β=−0.21; 95% CI −0.35, −0.07) or higher serum creatinine (per 1 mg/dL: β=−0.17; 95% CI −0.29, −0.04) were associated with a greater decrease in CRVE over time but not concurrent CRVE. Higher serum total cholesterol (per 20 mg/dL: β=0.02; 95% CI 0.01, 0.03) was associated with a smaller decrease in CRVE over time but not with concurrent CRVE.
Greater BMI was associated with larger concurrent CRVE (per 3 kg/m2: β=0.33; 95% CI 0.08, 0.58) and with a less negative change in CRVE over time (per 3 kg/m2: β=0.03; 95% CI 0.01, 0.05). Regular aspirin use had a borderline significant relationship with larger concurrent CRVE (β=0.55; 95% CI −0.08, 1.18) and was associated with a greater decrease in CRVE over time (β=−0.06; 95% CI −0.12, −0.001). Similarly, history of CVD (Figure 3F; β=0.96; 95% CI −0.15, 2.06), higher WBC count (Figure 3G; per 1000/µL: β=1.19; 95% CI 0.98, 1.40), and higher CRP (per mg/L: β=0.16; 95% CI 0.10, 0.21) were associated with larger concurrent CRVE and with a greater decrease in CRVE (CVD present: β=−0.11; 95% CI −0.20, −0.02; WBC per 1000/µL: β=−0.01; 95% CI −0.03, −0.001; CRP per mg/L: β=−0.007; 95% CI −0.01, −0.001).
In a sub-analysis of past and current smokers, more pack-years smoked (per 5 pack-years: β=0.40; 95% CI 0.30, 0.50) and more years smoked (per 5 years: β=1.23; 95% CI 1.02, 1.44) were both associated with larger concurrent CRVE and a greater decrease in CRVE over time (per 5 pack-years: β=−0.01; 95% CI −0.02, −0.003; per 5 years: β=−0.04; 95% CI −0.05, −0.02). In a further sub-analysis of past smokers, the number of years since stopping smoking was related to smaller concurrent CRVE (Figure 3H; per 5 years: β=−0.98; 95% CI −1.25, −0.71) and a greater decrease in CRVE over time (per 5 years: β=−0.02; 95% CI −0.04, 0.002).
Intraocular pressure, presence of diabetes, history of heavy drinking, serum 8-isoprostane, and soluble vascular cell adhesion molecule −1 were not related to concurrent CRVE or change in CRVE. Model results for individual risk factors were similar when imputed data was not used.
We combined all significant terms into a set of multivariate models as described earlier and arrived at one final multivariate model. Models including MABP had a lower (better) AIC compared to models with hypertension, models including WBC count had lower AIC compared to models with CRP, and models including presence of CKD had lower AIC than models with eGFR or serum creatinine. Male sex (β=5.34; 95% CI 3.58, 6.90), current smoking (β=9.38; 95% CI 8.26, 10.49), and higher WBC count (per 1000/µL: β=0.95; 95% CI 0.74, 1.16) were independently associated with larger concurrent CRVE while higher MABP (per 5 mmHg: β=−0.36; 95% CI −0.50, −0.23) and higher HDL cholesterol (per 10 mg/dL: β=−0.89; 95% CI −1.15, −0.63) were independently associated with smaller concurrent CRVE (Table 3). History of CVD (β=−0.16; 95% CI −0.26, −0.06) and presence of CKD (β=−0.20; 95% CI −0.34, −0.05) were independently associated with a greater decrease in CRVE over time but not concurrent CRVE.
Table 3.
Multivariate Relationships of Risk Factors with Concurrent Central Retinal Venular Equivalent and the Rate of Change in Central Retinal Venular Equivalent Over Time
| Concurrent CRVE | Change in CRVE Over Time | |||||
|---|---|---|---|---|---|---|
| Risk Factor | β Estimate | 95% CI | P value | β Estimate | 95% CI | P value |
| Sex (men vs. women) | 5.24 | 3.58, 6.90 | <.001 | |||
| MABP (per 5 mmHg) | −0.36 | −0.50, −0.23 | <.001 | |||
| History of cardiovascular disease | 0.77 | −0.38, 1.92 | 0.19 | −0.16 | −0.26, −0.06 | 0.001 |
| HDL cholesterol (per 10 mg/dL) | −0.94 | −0.89, −0.63 | <.001 | |||
| Current smoking | 9.38 | 8.26, 10.49 | <.001 | |||
| History of chronic kidney disease | −0.67 | −2.69, 1.35 | 0.52 | −0.20 | −0.34, −0.05 | 0.008 |
| WBC count (per 1000/µL) | 0.95 | 0.74, 1.16 | <.001 | |||
CI, confidence interval; CRVE, central retinal venular equivalent; HDL, high density lipoprotein; MABP, mean arterial blood pressure; WBC, white blood cell.
Additional adjustment for CRAE in the multivariate model did not alter the relationship between any risk factor and CRVE, except that greater MABP became significantly associated with larger rather than smaller CRVE (per 5 mmHg: β=0.46; 95% CI 0.34, 0.58).
Model results were similar when data was limited to values that were not imputed (data not shown).
DISCUSSION
The BDES offered an opportunity to examine associations of various factors (e.g., smoking status, serum total and HDL cholesterol, glycosylated hemoglobin level, inflammatory markers) previously shown to be associated with CRVE to changes in CRVE over time. Our study shows that CRVE tended to decrease with age, and many factors were related to concurrent CRVE and change in CRVE. However, in multivariate analysis, few of these relationships persisted, especially relationships with change in CRVE. Adjusting for image focus, cataract status, refraction, and height, and using age as the time scale, we found that sex, MABP, serum HDL cholesterol, history of current smoking, and WBC count were independently associated with concurrent retinal venular diameter, and history of CVD and presence of CKD were associated with change in retinal venular diameter over time.
We found a relationship between 2 measures of generalized inflammation, higher WBC count and higher serum CRP, and wider concurrent CRVE. This result is consistent with previous findings from the Rotterdam Study, the Multi-Ethnic Study of Atherosclerosis, the Atherosclerosis Risk in Communities study, and in a multi-ethnic Asian population.1–4,8,9 While the mechanism behind these associations remains unclear, they may be related to disruption of the endothelial surface layer by oxidized low density lipoproteins or activated leukocytes.1–3,9,38 Our study found that WBC count is a stronger predictor of CRVE than CRP. In multivariate analysis, CRP drops out of the multivariate model when both CRP and WBC are included, and, comparing models with the same control variables, the model with WBC count alone had a lower AIC than the model with CRP alone. The reason for this is uncertain, since CRP has been extensively used as a nonspecific biomarker of inflammation and is associated with CVD and other vascular diseases.
In our study, smoking was also associated with a wider concurrent venular diameter, an effect consistent with previously reported associations of current smoking with wider CRVE.1,39–41 Smoking-induced increase in nitrous oxide production, potassium channel activation,39 and possible elastic tissue degeneration might also explain this association.40–43 The effect of smoking may also be mediated by an inflammatory effect. Ikram et al. have hypothesized that inflammation secondary to smoking may disrupt and thin the endothelial surface layer, thereby increasing the apparent intraluminal caliber of retinal blood vessels.1 Our sub-analysis in past smokers suggests that the longer the period of time since stopping smoking, the more the widening effect on the retinal venules is reversed.
Several previous studies have observed the relationship of increased BMI to wider concurrent CRVE.4,8,44 BMI may be a marker of systemic inflammation, as the relationship of BMI and CRVE and change in CRVE was attenuated after adjusting for other potential factors associated with inflammation such as measures of CKD (serum cystatin C and creatinine levels) and smoking status. The underlying reasons for the inverse association of change in BMI with CRVE are unclear. This may possibly represent regression to the mean or be a result of unmeasured confounding (e.g., change in estrogen levels) associated with changing BMI.
We hypothesized that NSAID use would be associated with narrowing of the CRVE due to its anti-inflammatory effect. We did not find an independent association of any antiinflammatory agents with CRVE. Few studies have examined this. In a cross-sectional analysis, NSAID use in general was shown to be related to wider CRVE in the BDES,44 although the Blue Mountains Eye Study did not find an association of aspirin use with CRVE.45 When we excluded WBC count, a measure of systemic inflammation for which NSAIDs may have been taken, from the multivariate model, NSAID use became independently associated with smaller concurrent CRVE but not change in CRVE, and in a separate multivariate model excluding WBC count, aspirin use specifically was associated with a greater decrease in CRVE over time but not concurrent CRVE (data not shown).
We found a significant association between increased glycosylated hemoglobin and wider concurrent CRVE, which did not persist in multivariate analysis. This is consistent with crosssectional findings from different studies that glycemia is associated with wider CRVE,8,10–12 and that wider CRVE may be associated with an increased incidence of diabetes.46 However, glycosylated hemoglobin but not diabetes status was associated with wider concurrent CRVE over time, an observation consistent with some studies in Asian populations.10,11 In the Multi-Ethnic Study of Atherosclerosis, the association of CRVE with diabetes was only present in Hispanics and Chinese.12 Including diabetic persons with moderate to severe nonproliferative retinopathy or proliferative retinopathy in the analyses did not change these relationships (data not shown).
Higher HDL cholesterol was independently associated with smaller concurrent CRVE, which is consistent with findings from the Rotterdam Study, the Multi-Ethnic Study of Atherosclerosis, the Atherosclerosis Risk in Communities study, and earlier cross-sectional results from the BDES.1–4,8 We also found an association between history of CVD and more narrowing of CRVE over time but not concurrent CRVE. While other studies have found relationships between markers of CVD risk and wider CRVE,1–4,8 to our knowledge no other studies have examined CRVE in individuals with and without a history of CVD. The relationship of CVD with narrowing CRVE may be a result of selective survival, i.e., persons with CVD who have wider CRVE may be more likely to die sooner than those with CVD who have narrower CRVE. In our study we found that while adjusting for age and sex, individuals with CVD and larger CRVE at one exam were more likely not to participate at the next exam (P<0.01).
We found a relationship between higher MABP and smaller concurrent CRVE, which is likely to be a result of the effect of hypertension on ocular blood flow similar to observations of the central retinal arteriole equivalent (CRAE). However, when CRAE was added to the final multivariate model, the relationship between MABP and CRVE was reversed, i.e., higher MABP became significantly associated with wider CRVE. A larger CRVE for a given value of CRAE implies a smaller arteriole-to-venule ratio, which several studies have shown to be associated with higher blood pressure and hypertension.47–49
We also found a relationship between measures of kidney function and CRVE. Higher eGFR was associated with larger concurrent CRVE, which is consistent with results from another study where individuals with the lowest quartiles of CRVE had (non-significantly) higher rates of CKD.50 Higher creatinine and presence of CKD were both associated with a larger decrease in CRVE over time, and when comparing models with the same control variables, the model with CKD had a lower AIC than the model with creatinine. The relationship between CKD and change in CRVE may be due to a common cause, selective survival, or regression to the mean. To our knowledge, the only earlier study on the relationship of CKD to change in retinal venular diameter found no relationship between eGFR or presence of CKD to incident venular widening.51
Although CRVE tended to be narrower in older individuals, change in CRVE for an individual over a 5-year period was small. Reasons for this relative lack of overall change of CRVE in these large cohorts remain speculative at best. Compensatory ocular control of retinal blood flow, e.g., autonomic system may maintain an individual’s retinal blood vessel diameters over time. Furthermore, genetic factors may control retinal vessel caliber, independent of blood pressure and other factors.52 A 2011 study reported that 6-year-old daughters but not sons of hypertensive parents were more likely to have narrower retinal arterioles than daughters of parents who did not have hypertension.53 It is also possible that changes in some factors (e.g., a history of CVD) associated with wider CRVE may be balanced by factors associated with narrower CRVE (e.g., stopping smoking). The relationship of smaller CRVE with increasing age may partially be due to selective survival. In a meta-analysis of participants in the BDES and Blue Mountains Eye Study, wider CRVE was associated with an increased risk of coronary heart disease and stroke mortality.54
We found male sex to be associated with larger concurrent CRVE, even after controlling for height. A similar cross-sectional relationship was observed in a cohort of African-American and white individuals in the Atherosclerosis Risk in Communities study where mean CRVE was 2 µm larger in men than in women.8 It is unclear why men have larger CRVE than women, independent of height. Hormonal differences between men and women may partially explain this difference. In a cross-sectional study in the BDES, current users of estrogen replacement therapy had narrower retinal venular diameters than past users or those who never used it. However, we found no relationship between measures of natural hormonal status in women (e.g., pregnancy, age at menarche) and retinal vessel diameter.55
Strengths of this study include its long-term follow-up, use of standardized grading protocols, and its large population-based structure. However, caution must be used when interpreting the findings. One limitation is that the CRVE may be a summary of different retinal vessels at different time points. When determining change in CRVE, the individual vessels selected for measurement were not necessarily the same each time, and even when the same vessels were measured, different lengths may have been measured at different gradings. However, the effect of this is thought to be small; we graded the same vessels side by side and compared the results to the standard grading approach used in the study, and found that the differences were not statistically significant.56
Another limitation is the natural interrelatedness of different risk factors measured. Risk factors change throughout a person’s life (e.g., an individual develops high blood pressure, starts taking anti-hypertensive medication, and his/her blood pressure returns to normal), and they interact with each other (e.g., hypertension, diabetes, and kidney disease) in ways that are not easily measured. We speculate that this is why most of the factors that were related to change in CRVE in individual models dropped out when combined in multivariate analysis. Also, using the multivariate model results to interpret how changes in one risk factor should affect CRVE requires assuming that the values of all the other risk factors are unchanged, an assumption that may be incorrect.
In summary, these population-based data describe an overall narrowing of retinal venular caliber in the general adult population with age. We confirmed earlier cross-sectional associations of inflammation as measured by WBC count and conditions associated with inflammation (e.g., smoking and obesity) and higher glycosylated hemoglobin with wider CRVE. We show that a history of CVD and CKD are associated with change in retinal venular diameter over time. However, there were few consistent relationships between concurrent and prospective effects of these factors over time. The different independent effects of these interrelated factors and longitudinal changes in them highlight the complex relationship between systemic diseases and associated factors and CRVE over time.
Supplementary Material
Acknowledgments
Financial Support: This study was supported by National Institutes of Health grant EY06594 (BEK Klein and R Klein) and by Research to Prevent Blindness (BEK Klein and R Klein, Senior Scientific Investigator Awards), New York, NY. The National Eye Institute provided funding for entire study including data collection and analysis; RPB provided additional support for data analysis. Neither funding organization had a role in the design or conduct of this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Eye Institute or the National Institutes of Health.
Conflict of Interest: No conflicting relationship exists for any author.
REFERENCES
- 1.Ikram MK, de Jong FJ, Vingerling JR, et al. Are retinal arteriolar or venular diameters associated with markers for cardiovascular disorders? The Rotterdam Study. Invest Ophthalmol Vis Sci. 2004;45:2129–2134. doi: 10.1167/iovs.03-1390. [DOI] [PubMed] [Google Scholar]
- 2.Wong TY, Islam FM, Klein R, et al. Retinal vascular caliber, cardiovascular risk factors, and inflammation: the Multi-Ethnic Study of Atherosclerosis (MESA) Invest Ophthalmol Vis Sci. 2006;47:2341–2350. doi: 10.1167/iovs.05-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klein R, Klein BE, Knudtson MD, et al. Are inflammatory factors related to retinal vessel caliber? The Beaver Dam Eye Study. Arch Ophthalmol. 2006;124:87–94. doi: 10.1001/archopht.124.1.87. [DOI] [PubMed] [Google Scholar]
- 4.Wong TY, Duncan BB, Golden SH, et al. Associations between the metabolic syndrome and retinal microvascular signs: the Atherosclerosis Risk In Communities study. Invest Ophthalmol Vis Sci. 2004;45:2949–2954. doi: 10.1167/iovs.04-0069. [DOI] [PubMed] [Google Scholar]
- 5.Ikram MK, Janssen JA, Roos AM, et al. Retinal vessel diameters and risk of impaired fasting glucose or diabetes: the Rotterdam study. Diabetes. 2006;55:506–510. doi: 10.2337/diabetes.55.02.06.db05-0546. [DOI] [PubMed] [Google Scholar]
- 6.Klein R, Klein BE, Moss SE, et al. Retinal vascular caliber in persons with type 2 diabetes: the Wisconsin Epidemiological Study of Diabetic Retinopathy: XX. Ophthalmology. 2006;113:1488–1498. doi: 10.1016/j.ophtha.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 7.Klein BE, Klein R, Myers CE, Lee KE. Complete blood cell count and retinal vessel diameters. Arch Ophthalmol. 2011;129:490–497. doi: 10.1001/archophthalmol.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liew G, Sharrett AR, Wang JJ, et al. Relative importance of systemic determinants of retinal arteriolar and venular caliber: the Atherosclerosis Risk in Communities study. Arch Ophthalmol. 2008;126:1404–1410. doi: 10.1001/archopht.126.10.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheung CY, Wong TY, Lamoureux EL, et al. C-reactive protein and retinal microvascular caliber in a multiethnic Asian population. Am J Epidemiol. 2010;171:206–213. doi: 10.1093/aje/kwp357. [DOI] [PubMed] [Google Scholar]
- 10.Jeganathan VS, Sabanayagam C, Tai ES, et al. Retinal vascular caliber and diabetes in a multiethnic Asian population. Microcirculation. 2009;16:534–543. doi: 10.1080/10739680902975222. [DOI] [PubMed] [Google Scholar]
- 11.Islam FM, Nguyen TT, Wang JJ, et al. Quantitative retinal vascular calibre changes in diabetes and retinopathy: the Singapore Malay Eye Study. Eye (Lond) 2009;23:1719–1724. doi: 10.1038/eye.2008.362. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen TT, Wang JJ, Sharrett AR, et al. Relationship of retinal vascular caliber with diabetes and retinopathy: the Multi-Ethnic Study of Atherosclerosis (MESA) Diabetes Care. 2008;31:544–549. doi: 10.2337/dc07-1528. [DOI] [PubMed] [Google Scholar]
- 13.Tikellis G, Wang JJ, Tapp R, et al. The relationship of retinal vascular calibre to diabetes and retinopathy: the Australian Diabetes, Obesity and Lifestyle (AusDiab) study. Diabetologia. 2007;50:2263–2271. doi: 10.1007/s00125-007-0822-x. [DOI] [PubMed] [Google Scholar]
- 14.McGeechan K, Liew G, Macaskill P, et al. Meta-analysis: retinal vessel caliber and risk for coronary heart disease. Ann Intern Med. 2009;151:404–413. doi: 10.7326/0003-4819-151-6-200909150-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ikram MK, de Jong FJ, Bos MJ, et al. Retinal vessel diameters and risk of stroke: the Rotterdam Study. Neurology. 2006;66:1339–1343. doi: 10.1212/01.wnl.0000210533.24338.ea. [DOI] [PubMed] [Google Scholar]
- 16.McGeechan K, Liew G, Macaskill P, et al. Prediction of incident stroke events based on retinal vessel caliber: a systematic review and individual-participant meta-analysis. Am J Epidemiol. 2009;170:1323–1332. doi: 10.1093/aje/kwp306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong TY, Kamineni A, Klein R, et al. Quantitative retinal venular caliber and risk of cardiovascular disease in older persons: the Cardiovascular Health Study. Arch Intern Med. 2006;166:2388–2394. doi: 10.1001/archinte.166.21.2388. [DOI] [PubMed] [Google Scholar]
- 18.Kifley A, Liew G, Wang JJ, et al. Long-term effects of smoking on retinal microvascular caliber. Am J Epidemiol. 2007;166:1288–1297. doi: 10.1093/aje/kwm255. [DOI] [PubMed] [Google Scholar]
- 19.Klein R, Klein BE, Linton KL, De Mets DL. The Beaver Dam Eye Study: visual acuity. Ophthalmology. 1991;98:1310–1315. doi: 10.1016/s0161-6420(91)32137-7. [DOI] [PubMed] [Google Scholar]
- 20.Klein R, Klein BE, Lee KE. Changes in visual acuity in a population. The Beaver Dam Eye Study. Ophthalmology. 1996;103:1169–1178. doi: 10.1016/s0161-6420(96)30526-5. [DOI] [PubMed] [Google Scholar]
- 21.Klein R, Klein BE, Lee KE, et al. Changes in visual acuity in a population over a 10-year period: The Beaver Dam Eye Study. Ophthalmology. 2001;108:1757–1766. doi: 10.1016/s0161-6420(01)00769-2. [DOI] [PubMed] [Google Scholar]
- 22.Klein R, Klein BE, Lee KE, et al. Changes in visual acuity in a population over a 15-year period: the Beaver Dam Eye Study. Am J Ophthalmol. 2006;142:539–549. doi: 10.1016/j.ajo.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 23.Klein R, Klein BE. Manual of Operations (Revised). Report for 16 Jun 87 - 31 May 92. Springfield, VA: US Dept of Commerce; 1991. Beaver Dam Eye Study; pp. 182–227. NTIS Publication PB91-149823. [Google Scholar]
- 24.Allain CC, Poon LS, Chan CS, et al. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–475. [PubMed] [Google Scholar]
- 25.Lopes-Virella MF, Stone P, Ellis S, Colwell JA. Cholesterol determination in high-density lipoproteins separated by three different methods. Clin Chem. 1977;23:882–884. [PubMed] [Google Scholar]
- 26.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 27.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 28.Klein BE, Klein R, Linton KL, et al. Assessment of cataracts from photographs in the Beaver Dam Eye Study. Ophthalmology. 1990;97:1428–1433. doi: 10.1016/s0161-6420(90)32391-6. [DOI] [PubMed] [Google Scholar]
- 29.Diabetic Retinopathy Study Research Group. Report Number 6. Design, methods, and baseline results. Invest Ophthalmol Vis Sci. 1981;21:1–209. [PubMed] [Google Scholar]
- 30.Diabetic Retinopathy Study Research Group. Report Number 7. A modification of the Airlie House classification of diabetic retinopathy. Invest Ophthalmol Vis Sci. 1981;21:210–226. [PubMed] [Google Scholar]
- 31.Klein R, Davis MD, Magli YL, et al. The Wisconsin age-related maculopathy grading system. Ophthalmology. 1991;98:1128–1134. doi: 10.1016/s0161-6420(91)32186-9. [DOI] [PubMed] [Google Scholar]
- 32.Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology. 1992;99:933–943. doi: 10.1016/s0161-6420(92)31871-8. [DOI] [PubMed] [Google Scholar]
- 33.Sun C, Liew G, Wang JJ, et al. Retinal vascular caliber, blood pressure, and cardiovascular risk factors in an Asian population: the Singapore Malay Eye Study. Invest Ophthalmol Vis Sci. 2008;49:1784–1790. doi: 10.1167/iovs.07-1450. [DOI] [PubMed] [Google Scholar]
- 34.Knudtson MD, Lee KE, Hubbard LD, et al. Revised formulas for summarizing retinal vessel diameters. Curr Eye Res. 2003;27:143–149. doi: 10.1076/ceyr.27.3.143.16049. [DOI] [PubMed] [Google Scholar]
- 35.Knudtson MD, Klein BE, Klein R, et al. Variation associated with measurement of retinal vessel diameters at different points in the pulse cycle. Br J Ophthalmol. 2004;88:57–61. doi: 10.1136/bjo.88.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klenk DC, Hermanson GT, Krohn RI, et al. Determination of glycosylated hemoglobin by affinity chromatography: comparison with colorimetric and ion-exchange methods, and effects of common interferences. Clin Chem. 1982;28:2088–2094. [PubMed] [Google Scholar]
- 37.Singer JD, Willett JB. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. New York: Oxford University Press; 2003. [Google Scholar]
- 38.Vink H, Constantinescu AA, Spaan JA. Oxidized lipoproteins degrade the endothelial surface layer : implications for platelet-endothelial cell adhesion. Circulation. 2000;101:1500–1502. doi: 10.1161/01.cir.101.13.1500. [DOI] [PubMed] [Google Scholar]
- 39.Iida M, Iida H, Dohi S, et al. Mechanisms underlying cerebrovascular effects of cigarette smoking in rats in vivo. Stroke. 1998;29:1656–1665. doi: 10.1161/01.str.29.8.1656. [DOI] [PubMed] [Google Scholar]
- 40.Frances C, Boisnic S, Hartmann DJ, et al. Changes in the elastic tissue of the non-sun-exposed skin of cigarette smokers. Br J Dermatol. 1991;125:43–47. doi: 10.1111/j.1365-2133.1991.tb06037.x. [DOI] [PubMed] [Google Scholar]
- 41.Rogot E, Murray JL. Smoking and causes of death among U.S. veterans: 16 years of observation. Public Health Rep. 1980;95:213–222. [PMC free article] [PubMed] [Google Scholar]
- 42.Sharrett AR, Sorlie PD, Chambless LE, et al. Relative importance of various risk factors for asymptomatic carotid atherosclerosis versus coronary heart disease incidence: the Atherosclerosis Risk in Communities Study. Am J Epidemiol. 1999;149:843–852. doi: 10.1093/oxfordjournals.aje.a009900. [DOI] [PubMed] [Google Scholar]
- 43.Sharrett AR, Ding J, Criqui MH, et al. Smoking, diabetes, and blood cholesterol differ in their associations with subclinical atherosclerosis: the Multiethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2006;186:441–447. doi: 10.1016/j.atherosclerosis.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 44.Wong TY, Knudtson MD, Klein BE, et al. Medication use and retinal vessel diameters. Am J Ophthalmol. 2005;139:373–375. doi: 10.1016/j.ajo.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 45.Liew G, Mitchell P, Leeder SR, et al. Regular aspirin use and retinal microvascular signs: the Blue Mountains Eye Study. J Hypertens. 2006;24:1329–1335. doi: 10.1097/01.hjh.0000234113.33025.33. [DOI] [PubMed] [Google Scholar]
- 46.Kifley A, Wang JJ, Cugati S, et al. Retinal vascular caliber and the long-term risk of diabetes and impaired fasting glucose: the Blue Mountains Eye Study. Microcirculation. 2008;15:373–377. doi: 10.1080/10739680701812220. [DOI] [PubMed] [Google Scholar]
- 47.Hubbard LD, Brothers RJ, King WN, et al. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities Study. Ophthalmology. 1999;106:2269–2280. doi: 10.1016/s0161-6420(99)90525-0. [DOI] [PubMed] [Google Scholar]
- 48.Sharrett AR, Hubbard LD, Cooper LS, et al. Retinal arteriolar diameters and elevated blood pressure: the Atherosclerosis Risk in Communities Study. Am J Epidemiol. 1999;150:263–270. doi: 10.1093/oxfordjournals.aje.a009997. [DOI] [PubMed] [Google Scholar]
- 49.Kawasaki R, Cheung N, Wang JJ, et al. Retinal vessel diameters and risk of hypertension: the Multiethnic Study of Atherosclerosis. J Hypertens. 2009;27:2386–2393. doi: 10.1097/HJH.0b013e3283310f7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sabanayagam C, Shankar A, Koh D, et al. Retinal microvascular caliber and chronic kidney disease in an Asian population. Am J Epidemiol. 2009;169:625–632. doi: 10.1093/aje/kwn367. [DOI] [PubMed] [Google Scholar]
- 51.Sabanayagam C, Shankar A, Klein BE, et al. Bidirectional association of retinal vessel diameters and estimated GFR decline: the Beaver Dam CKD Study. Am J Kidney Dis. 2011;57:682–691. doi: 10.1053/j.ajkd.2010.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xing C, Klein BE, Klein R, et al. Genome-wide linkage study of retinal vessel diameters in the Beaver Dam Eye Study. Hypertension. 2006;47:797–802. doi: 10.1161/01.HYP.0000208330.68355.72. [DOI] [PubMed] [Google Scholar]
- 53.Gopinath B, Baur LA, Hardy LL, et al. Parental history of hypertension is associated with narrower retinal arteriolar caliber in young girls. Hypertension. 2011;58:425–430. doi: 10.1161/HYPERTENSIONAHA.111.177022. [DOI] [PubMed] [Google Scholar]
- 54.Wang JJ, Liew G, Klein R, et al. Retinal vessel diameter and cardiovascular mortality: pooled data analysis from two older populations. Eur Heart J. 2007;28:1984–1992. doi: 10.1093/eurheartj/ehm221. [DOI] [PubMed] [Google Scholar]
- 55.Wong TY, Knudtson MD, Klein BE, et al. Estrogen replacement therapy and retinal vascular caliber. Ophthalmology. 2005;112:553–558. doi: 10.1016/j.ophtha.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 56.Klein R, Myers CE, Knudtson MD, et al. The relationship of blood pressure and other factors to serial retinal arteriolar diameter measurements over time: the Beaver Dam Eye Study. Arch Ophthalmol. 2012 doi: 10.1001/archophthalmol.2012.560. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.