Abstract
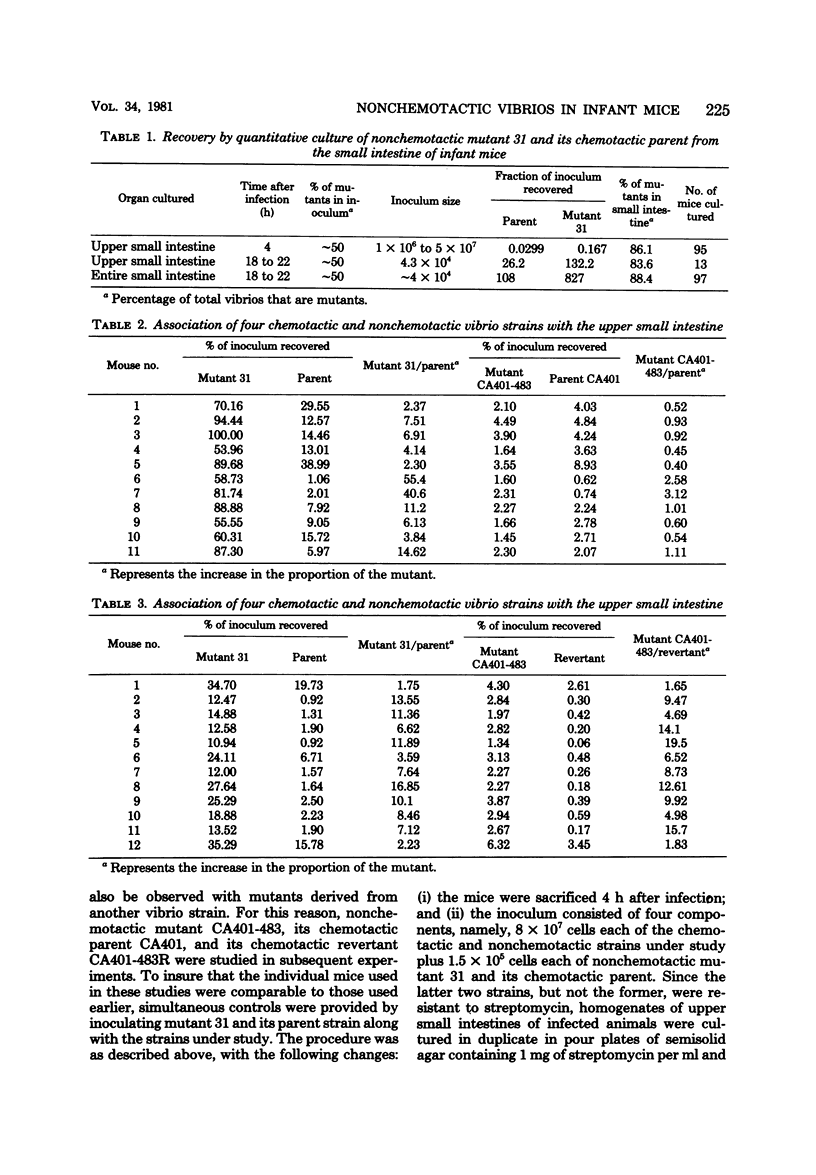
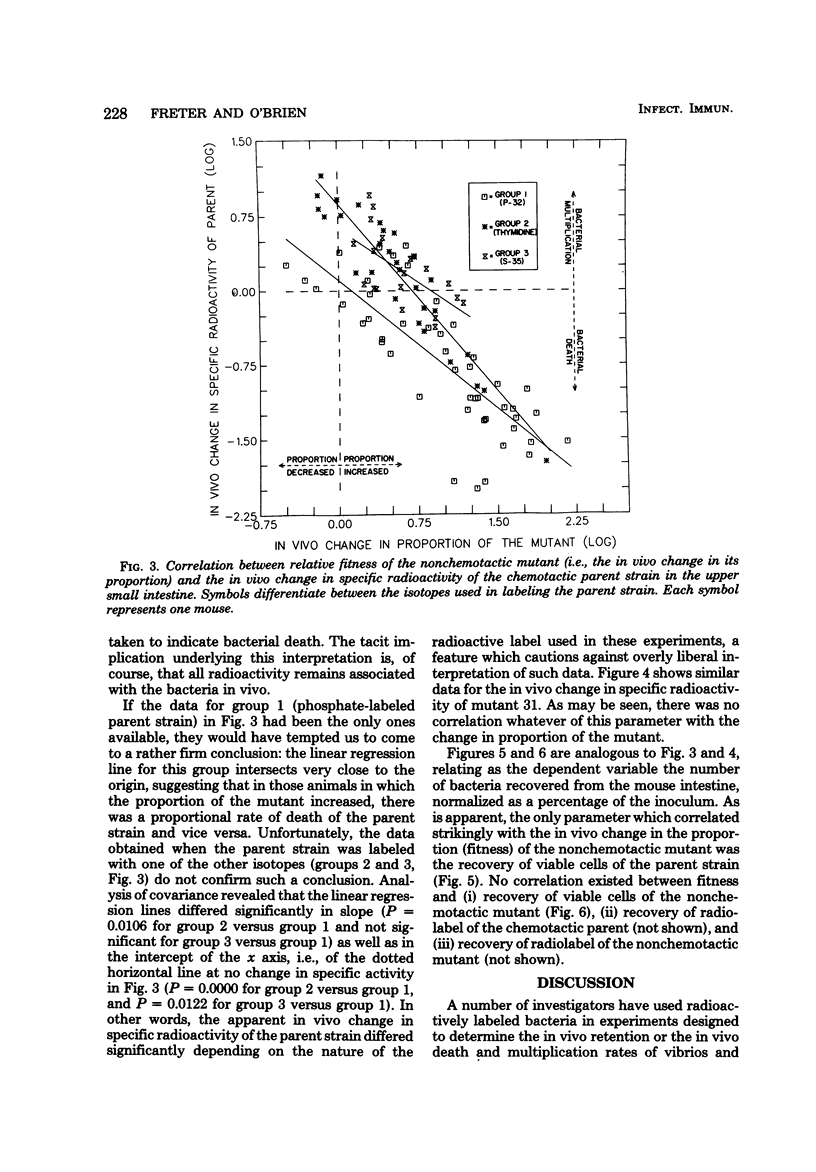
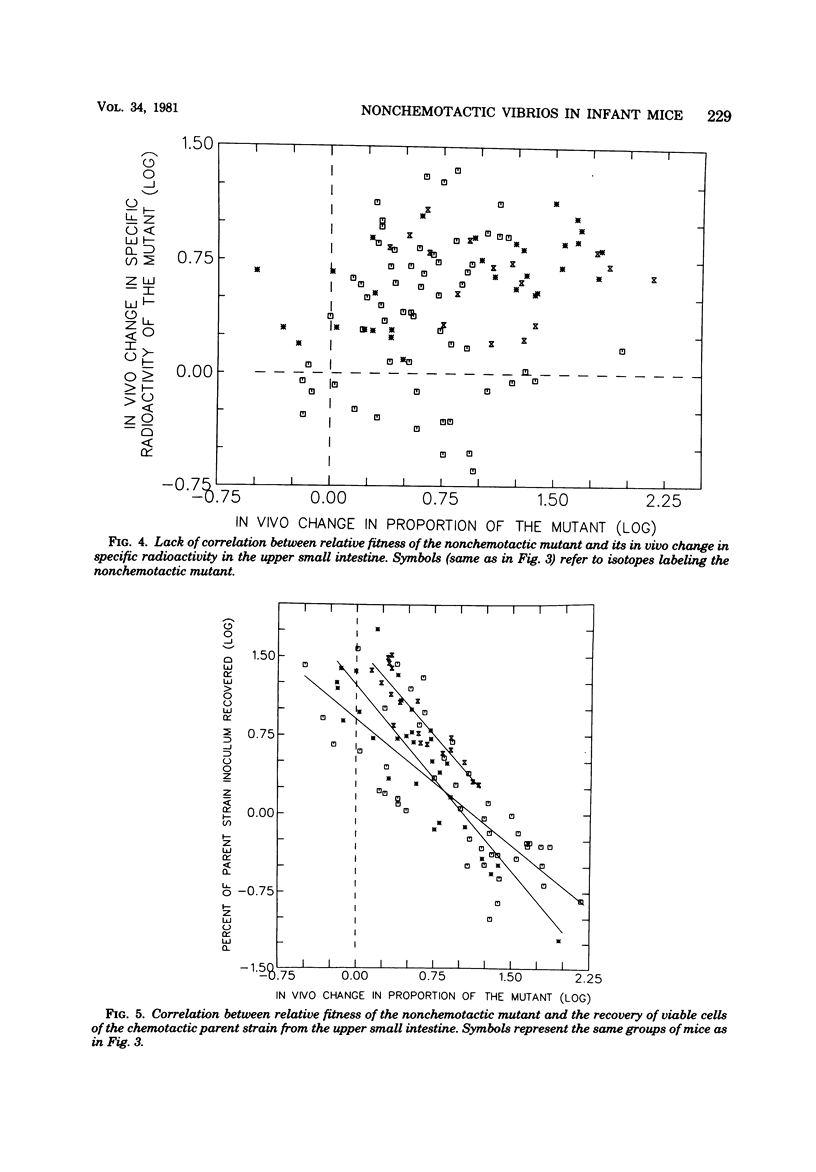
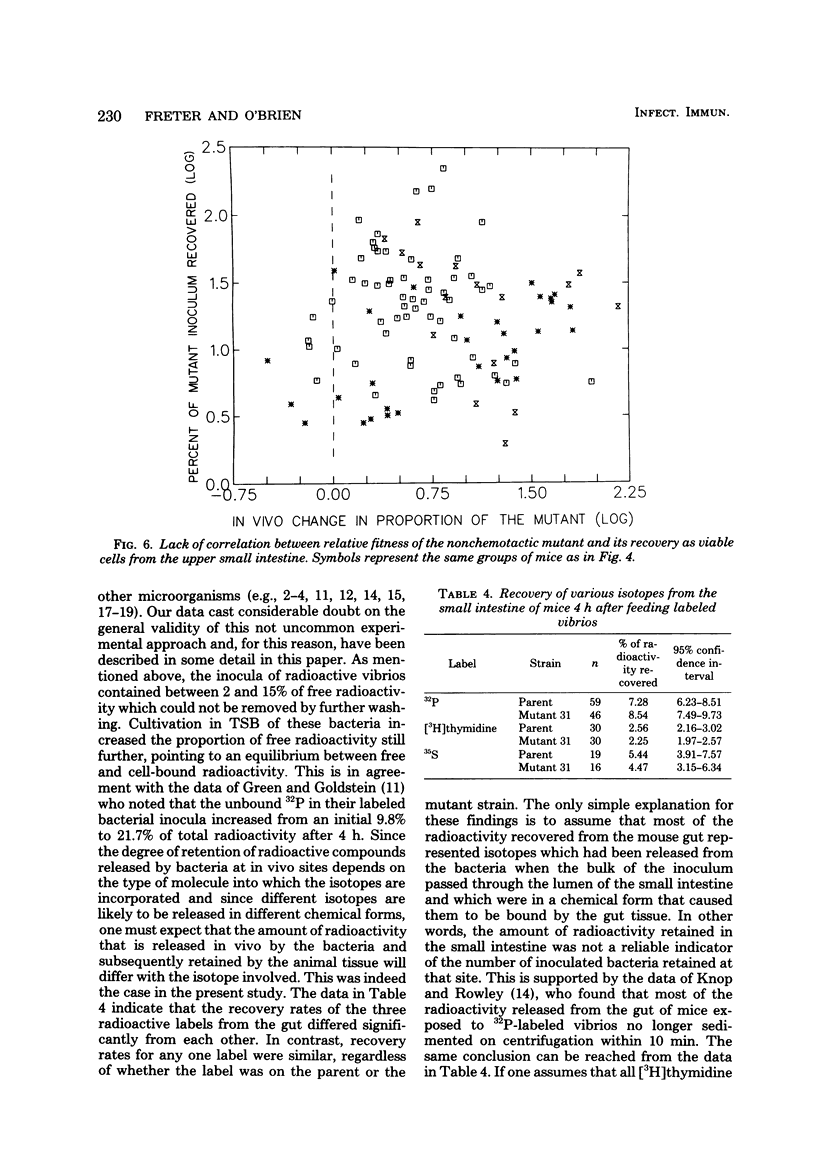
Contrary to earlier findings with all other in vivo and in vitro models of cholera studied, nonchemotactic vibrio mutants showed a relatively greater fitness in 5-day-old infant mice as compared with chemotactic parent or chemotactic revertant strains. This trend was manifest in the relatively greater number of nonchemotactic mutants recovered from the upper small intestine at 4 and 18 h after intragastric infection. The same trend was also revealed in the significantly greater virulence (in terms of time to death) of nonchemotactic mutants as compared with the chemotactic parent or revertant strains. Histological studies in infant mice of the penetration of chemotactic and nonchemotactic vibrios into the mucus gel of the small intestine yielded the same findings as in all other models studied, i.e., significantly greater penetration by chemotactic vibrios. There was no correlation between the relative fitness of nonchemotactic vibrios in the small intestine of infant mice and the rate of recovery of viable nonchemotactic vibrios from that site. In contrast, excellent correlation was found between the relative fitness of nonchemotactic vibrios and a decrease in the recovery of viable cells of the chemotactic strain from the small intestine. This indicates that the relatively greater fitness of the nonchemotactic vibrios in infant mice was only apparent and that the observed phenomenon was actually due to an antibacterial mechanism which prevented the accumulation of the chemotactic strains in the small intestine rather than to any stimulating effect on the nonchemotactic mutant itself. To study the in vivo fate of the inoculum in infant mice, vibrios were labeled with either 32P, 35S, or [3H]thymidine. Specific activity determinations of the 32P label were compatible with the assumption of an accelerated rate of death of the chemotactic parent strain in the small intestine. Results with the other isotopes, however, were significantly different. Indeed, the amount of radioactivity retained in the small intestine after feeding labeled bacteria correlated more closely with the isotope used than with the strain of vibrio under study. Consequently, considerable doubt must be cast on the general validity of this not uncommon technique for determining the in vivo location and the death or survival of radioactively labeled bacteria.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baselski V. S., Medina R. A., Parker C. D. In vivo and in vitro characterization of virulence-deficient mutants of Vibrio cholerae. Infect Immun. 1979 Apr;24(1):111–116. doi: 10.1128/iai.24.1.111-116.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baselski V. S., Medina R. A., Parker C. D. Survival and multiplication of Vibrio cholerae in the upper bowel of infant mice. Infect Immun. 1978 Nov;22(2):435–440. doi: 10.1128/iai.22.2.435-440.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baselski V. S., Parker C. D. Intestinal distribution of Vibrio cholerae in orally infected infant mice: kinetics of recovery of radiolabel and viable cells. Infect Immun. 1978 Aug;21(2):518–525. doi: 10.1128/iai.21.2.518-525.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baselski V., Briggs R., Parker C. Intestinal fluid accumulation induced by oral challenge with Vibrio cholerae or cholera toxin in infant mice. Infect Immun. 1977 Mar;15(3):704–712. doi: 10.1128/iai.15.3.704-712.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom L., Rowley D. Re-examination of small intestinal disposal of Vibrio cholerae in mice. Aust J Exp Biol Med Sci. 1977 Aug;55(4):385–391. doi: 10.1038/icb.1977.35. [DOI] [PubMed] [Google Scholar]
- Freter R., Allweiss B., O'Brien P. C., Halstead S. A., Macsai M. S. Role of chemotaxis in the association of motile bacteria with intestinal mucosa: in vitro studies. Infect Immun. 1981 Oct;34(1):241–249. doi: 10.1128/iai.34.1.241-249.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter R. Mechanism of Action of Intestinal Antibody in Experimental Cholera II. Antibody-Mediated Antibacterial Reaction at the Mucosal Surface. Infect Immun. 1970 Nov;2(5):556–562. doi: 10.1128/iai.2.5.556-562.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter R., O'Brien P. C., Macsai M. S. Role of chemotaxis in the association of motile bacteria with intestinal mucosa: in vivo studies. Infect Immun. 1981 Oct;34(1):234–240. doi: 10.1128/iai.34.1.234-240.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter R., O'Brien P. C. Role of chemotaxis in the association of motile bacteria with intestinal mucosa: chemotactic responses of Vibrio cholerae and description of motile nonchemotactic mutants. Infect Immun. 1981 Oct;34(1):215–221. doi: 10.1128/iai.34.1.215-221.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN G. M., KASS E. H. THE ROLE OF THE ALVEOLAR MACROPHAGE IN THE CLEARANCE OF BACTERIA FROM THE LUNG. J Exp Med. 1964 Jan 1;119:167–176. doi: 10.1084/jem.119.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green G. M., Goldstein E. A method for quantitating intrapulmonary bacterial inactivation in individual animals. J Lab Clin Med. 1966 Oct;68(4):669–677. [PubMed] [Google Scholar]
- Knop J., Rowley D. Protection against cholera. A bactericidal mechanism on the mucosal surface of the small intestine of mice. Aust J Exp Biol Med Sci. 1975 Apr;53(2):155–165. [PubMed] [Google Scholar]
- LaForce F. M. Effect of aerosol immunization with RE 595 Salmonella minnesota on lung bactericidal activity against Serratia marcescens, Enterobacter cloacae, and Pseudomonas aeruginosa. Am Rev Respir Dis. 1977 Aug;116(2):241–249. doi: 10.1164/arrd.1977.116.2.241. [DOI] [PubMed] [Google Scholar]
- MEYNELL G. G., SUBBAIAH T. V. Antibacterial mechanisms of the mouse gut. I. Kinetics of infection by Salmonella typhi-murium in normal and streptomycin-treated mice studied with abortive transductants. Br J Exp Pathol. 1963 Apr;44:197–208. [PMC free article] [PubMed] [Google Scholar]
- Norden C. W., Green G. M., Kass E. H. Antibacterial mechanisms of the urinary bladder. J Clin Invest. 1968 Dec;47(12):2689–2700. doi: 10.1172/JCI105952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perers L., Andåker L., Edebo L., Stendahl O., Tagesson C. Association of some enterobacteria with the intestinal mucosa of mouse in relation to their partition in aqueous polymer two-phase systems. Acta Pathol Microbiol Scand B. 1977 Oct;85B(5):308–316. doi: 10.1111/j.1699-0463.1977.tb01980.x. [DOI] [PubMed] [Google Scholar]
- THORBECKE G. J., BENACERRAF B. Some histological and functional aspects of lymphoid tissue in germfree animals. II. Studies on phagocytosis in vivo. Ann N Y Acad Sci. 1959 May 8;78:247–253. doi: 10.1111/j.1749-6632.1959.tb53107.x. [DOI] [PubMed] [Google Scholar]


