Abstract
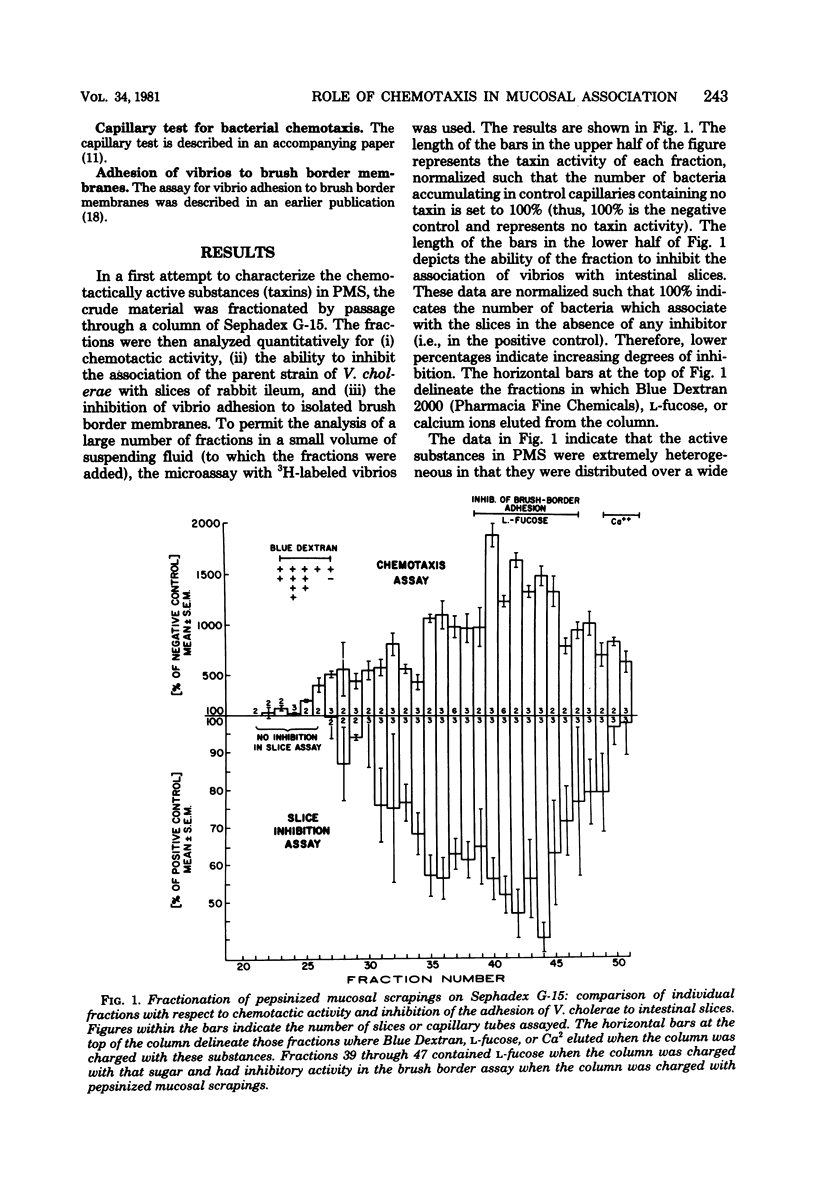
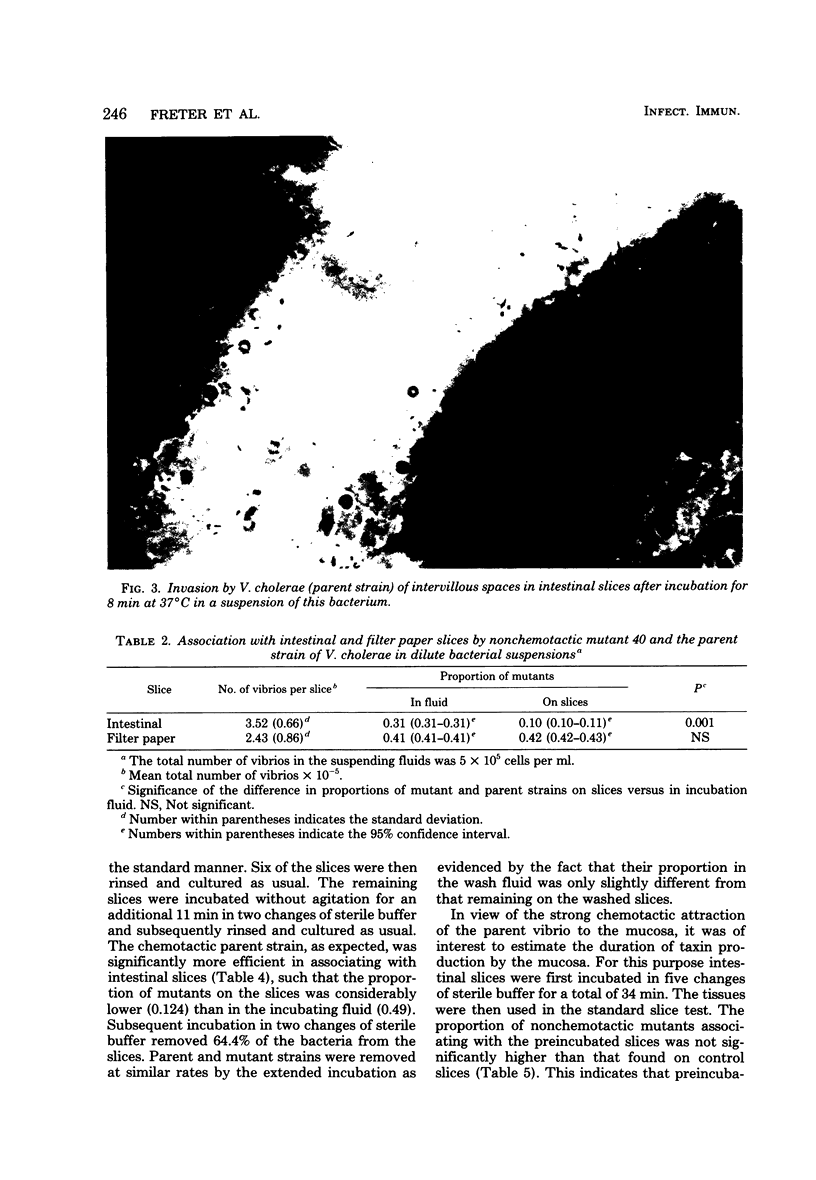
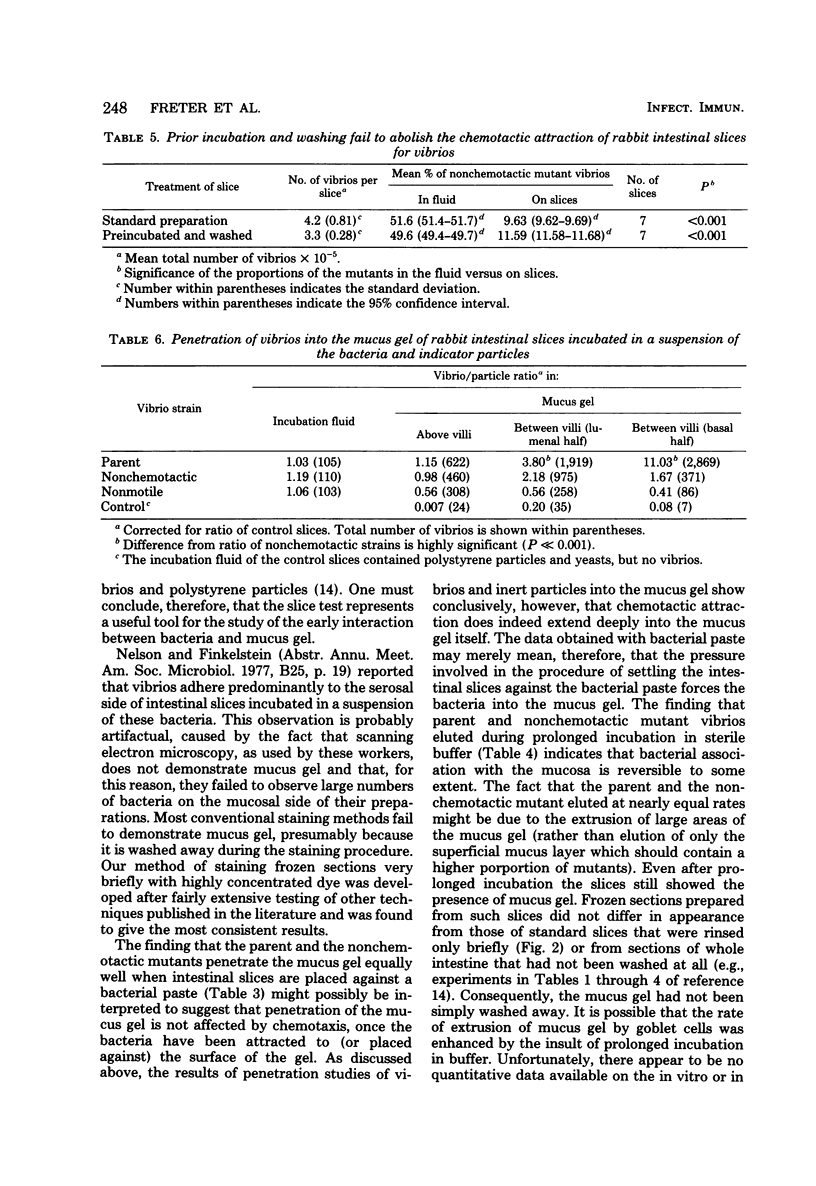
Various Sephadex G-15 fractions of pepsin-digested mucosal extract inhibited the in vitro association of cholera vibrios with mucosal slices. Inhibitory activity paralleled the taxin activity of the fractions for these bacteria. This supports the theory that inhibition of mucosal association by pepsin-digested mucosal scrapings was due to the blocking of taxin receptors on the bacterial surface. Nonchemotactic mutants were significantly less efficient than the chemotactic parent or revertant strains in associating with mucosal slices in vitro. Control experiments in which filter paper disks replaced the mucosal slices showed a comparable extent of association of chemotactic and nonchemotactic vibrios with this material. Histological studies indicated that vibrios associated predominantly with the mucus gel of the intestinal slices rather than with the mucosal epithelium or the serosal surface. Intestinal slices attracted chemotactic vibrios even after prolonged washing, suggesting continuous production of the taxin by the tissue. Inert polystyrene particles 1.1 micrometers in diameter penetrated the mucus gel of intestinal slices very poorly, but nevertheless could be detected in low numbers in the deep intervillous spaces within 15 min. In contrast, chemotactic vibrios reached the deep intervillous spaces in significantly higher numbers, whereas motile, non-chemotactic vibrios reacted like the inert particles. It is concluded that mucus gel represents a partial barrier to the penetration of particles of bacterial size and that this barrier can be invaded efficiently by motile bacteria, but only when their locomotion is guided by chemotactic stimuli.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J. Chemotaxis in bacteria. Annu Rev Biochem. 1975;44:341–356. doi: 10.1146/annurev.bi.44.070175.002013. [DOI] [PubMed] [Google Scholar]
- Allweiss B., Dostal J., Carey K. E., Edwards T. F., Freter R. The role of chemotaxis in the ecology of bacterial pathogens of mucosal surfaces. Nature. 1977 Mar 31;266(5601):448–450. doi: 10.1038/266448a0. [DOI] [PubMed] [Google Scholar]
- Bellamy J. E., Knop J., Steele E. J., Chaicumpa W., Rowley D. Antibody cross-linking as a factor in immunity to cholera in infant mice. J Infect Dis. 1975 Aug;132(2):181–188. doi: 10.1093/infdis/132.2.181. [DOI] [PubMed] [Google Scholar]
- Chet I., Mitchell R. Ecological aspects of microbial chemotactic behavior. Annu Rev Microbiol. 1976;30:221–239. doi: 10.1146/annurev.mi.30.100176.001253. [DOI] [PubMed] [Google Scholar]
- Edwards P. A. Is mucus a selective barrier to macromolecules? Br Med Bull. 1978 Jan;34(1):55–56. doi: 10.1093/oxfordjournals.bmb.a071459. [DOI] [PubMed] [Google Scholar]
- Freter R., Jones G. W. Adhesive properties of Vibrio cholerae: nature of the interaction with intact mucosal surfaces. Infect Immun. 1976 Jul;14(1):246–256. doi: 10.1128/iai.14.1.246-256.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter R., O'Brien P. C., Halstead S. A. Adhesion and chemotaxis as determinants of bacterial association with mucosal surfaces. Adv Exp Med Biol. 1978;107:429–437. doi: 10.1007/978-1-4684-3369-2_48. [DOI] [PubMed] [Google Scholar]
- Freter R., O'Brien P. C., Macsai M. S. Effect of chemotaxis on the interaction of cholera vibrios with intestinal mucosa. Am J Clin Nutr. 1979 Jan;32(1):128–132. doi: 10.1093/ajcn/32.1.128. [DOI] [PubMed] [Google Scholar]
- Freter R., O'Brien P. C., Macsai M. S. Role of chemotaxis in the association of motile bacteria with intestinal mucosa: in vivo studies. Infect Immun. 1981 Oct;34(1):234–240. doi: 10.1128/iai.34.1.234-240.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter R., O'Brien P. C. Role of chemotaxis in the association of motile bacteria with intestinal mucosa: chemotactic responses of Vibrio cholerae and description of motile nonchemotactic mutants. Infect Immun. 1981 Oct;34(1):215–221. doi: 10.1128/iai.34.1.215-221.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guentzel M. N., Berry L. J. Motility as a virulence factor for Vibrio cholerae. Infect Immun. 1975 May;11(5):890–897. doi: 10.1128/iai.11.5.890-897.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guentzel M. N., Field L. H., Eubanks E. R., Berry L. J. Use of fluorescent antibody in studies of immunity to cholera in infant mice. Infect Immun. 1977 Feb;15(2):539–548. doi: 10.1128/iai.15.2.539-548.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Freter R. Adhesive properties of Vibrio cholerae: nature of the interaction with isolated rabbit brush border membranes and human erythrocytes. Infect Immun. 1976 Jul;14(1):240–245. doi: 10.1128/iai.14.1.240-245.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake A. M., Bloch K. J., Neutra M. R., Walker W. A. Intestinal goblet cell mucus release. II. In vivo stimulation by antigen in the immunized rat. J Immunol. 1979 Mar;122(3):834–837. [PubMed] [Google Scholar]
- Schrank G. D., Verwey W. F. Distribution of cholera organisms in experimental Vibrio cholerae infections: proposed mechanisms of pathogenesis and antibacterial immunity. Infect Immun. 1976 Jan;13(1):195–203. doi: 10.1128/iai.13.1.195-203.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]