Abstract
Objective
We recently reported expression of collagen triple helix repeat containing-1 (Cthrc1) in injured arteries and proteolytic cleavage of Cthrc1 in smooth muscle cells in vitro. The present study characterizes Cthrc1 processing and determines its biological significance.
Methods and Results
Domain-specific antibodies localized full-length Cthrc1 in the cytoplasm of vascular, gastrointestinal, and uterine smooth muscle as well as in some neurons. Unlike smooth muscle α-actin, Cthrc1 was not expressed in the embryonic myocardium. Intracellular localization of full-length Cthrc1 was sharply reduced in dedifferentiated smooth muscle of the developing neointima despite the previously shown increase in mRNA levels with accompanying extracellular Cthrc1 immunoreactivity. Immunoblotting suggested an apparent covalent association of monomeric full-length Cthrc1 with a cytoplasmic protein present in differentiated smooth muscle. Plasmin was identified as a protease that cleaved a putative propeptide generating an N-terminally truncated form of Cthrc1 with increased inhibitory activity of procollagen synthesis.
Conclusions
Our data show that the differentiated smooth muscle cell phenotype is associated with the intracellular localization of noncleaved Cthrc1 despite the presence of a signal peptide. On arterial injury, increased Cthrc1 expression with apparent extracellular localization of N-terminally truncated Cthrc1 occurs. Removal of the propeptide correlated with increased activity of the molecule.
Keywords: differentiation, plasmin, smooth muscle
Cthrc1 is a novel gene induced in adventitial fibroblasts and smooth muscle cells (SMCs) of the developing neointima after arterial injury, and it functions as an inhibitor of transforming growth factor (TGF)-β signaling.1 TGF-β signaling can be decreased by overexpressing Cthrc1, which results in a reduction of phosphorylated Smad 2/3 (pS-mad2/3) levels and decreased intimal lesion formation.1 We therefore hypothesized that increased levels of Cthrc1 in the vasculature function to inhibit dedifferentiation of SMCs.1
TGF-β has been implicated in transdifferentiation of fibroblasts to myofibroblasts3,4 by promoting expression of the SMC marker gene smooth muscle α-actin (SMA).2 Despite the involvement of TGF-β in positive regulation of smooth muscle marker gene expression, we found that in vivo TGF-β stimulates SMC proliferation,3 which is linked to dedifferentiation. Furthermore, we recently reported that the TGF-β signaling pathway is highly active in SMCs during neointima formation as demonstrated by the presence of pSmad2/3 in intimal SMCs, whereas pSmad2/3 was not detectable in uninjured medial SMCs.1 This is in contrast to adventitial fibroblasts and endothelial cells of normal vessels, which displayed constitutive TGF-β signaling as detected by the presence of prominent nuclear pSmad2/3.
In our previous studies of normal arteries, we found no detectable levels of Cthrc1 mRNA by Northern blotting or in situ hybridization and with our previously available antibodies we were not able to detect Cthrc1 protein in uninjured arteries.1 Immunoblotting of SMCs in vitro, however, revealed various lower molecular weight immunoreactive Cthrc1 species.1 As many proteins, including TGF-β family members, require proteolytic processing for the generation of active molecules, questions about the identity and function of the lower molecular weight Cthrc1 fragments were raised. This prompted us to generate antisera specific for the N terminus of unprocessed Cthrc1. These antibodies detected unprocessed full-length Cthrc1 in the cytoplasm of quiescent and differentiated smooth muscle in an apparent covalent association with a cytoplasmic protein. This identifies the proform of Cthrc1 as a novel marker for differentiated smooth muscle. In addition, cytoplasmic retention of a molecule without apparent ER-Golgi or vesicle association despite the presence of a signal peptide is a phenomenon that has not previously been reported.
Materials and Methods
Antibody Generation and Immunohistochemistry
Rabbit antisera were raised against an N-terminal peptide sequence derived from human Cthrc1 (KQKAQLRQREVVDLYNGMC, amino acids 39 to 57). This sequence is highly conserved among all vertebrates, and 2 high titer antisera were found to cross-react with mouse, rat, pig, and human specimens in an identical manner and are referred to herein as “antipro”. Mouse monoclonal antibodies were generated with 8× His-tagged purified full-length recombinant rat Cthrc1 expressed in E coli as antigen. Conditioned media from hybridoma lines were screened for anti-Cthrc1 antibodies using conditioned medium from CHO cells transduced with an adenovirus expressing Cthrc1. IgG monoclonal antibodies recognizing Cthrc1 with high affinity and specificity were obtained (clones 16D3 and 16D4, Maine Biotechnology Services Inc). These antibodies detected Cthrc1 in the conditioned medium of Cthrc1-transduced CHO cells by immunoblotting at concentrations of 50 ng/mL and below.
For immunohistochemistry with antipro serum (1:500 dilution) on paraffin-embedded paraformaldehyde-fixed tissues, antigens were retrieved in 10 mmol/L citrate buffer for 15 minutes before staining using standard procedures.4 Controls with preimmune serum (1:500 dilution) from the same rabbit were included for all tissue sections, and at this dilution nonspecific staining was negligible.
Plasmin Cleavage
His-tagged Cthrc1 was purified from conditioned media harvested from CHO cells virally transduced with full-length Cthrc1. Purified protein was incubated with 0.05 U purified human plasmin (Hematologic Technologies Inc) for 5 to 30 minutes at 37°C. As controls for the proteolysis assays, identical samples were incubated in an identical fashion in the absence of plasmin. At the end of the incubation the samples were boiled immediately. The fragments were resolved by SDS-PAGE and transferred to polyvinylidene fluoride (PVDF) membranes before N-terminal peptide sequencing by a commercial service.
For inhibition of endogenous plasmin activity in vitro, PAC1 cells were transduced with full-length Cthrc1 adenovirus in the presence or absence of 0.5 mmol/L ε-aminocaproic acid (EACA) before harvesting of conditioned media and cell lysate for immunoblotting with antipro 48 hours later.
Cell Culture and Western Blotting
PAC1 smooth cells and CHO-K1 cells were grown as described.1,2 Lysates were collected at confluence for immunoblot analysis. Cells were transduced with 6000 viral particles per cell for 4 hours. One to 4 days later, cell lysates were collected for analysis of Cthrc1 production. A segment of porcine abdominal aorta was perfused generously with phosphate-buffered saline before processing for immunoblotting with anti-Cthrc1 antiserum (1:3000),1,2 antipro antiserum (1:3000), and monoclonal antibody 16D4 (200 ng/mL) under reducing and nonreducing conditions (80 μg protein per lane). Samples from plasmin cleavage experiments were analyzed by Western blotting under similar conditions. For assessing Smad2/3 phosphorylation levels in the presence of Cthrc1Δ48, PAC1 cells were transduced with adenovirus expressing Cthrc1Δ48 or β-galactosidase adenovirus as described above. Three days later, cells were stimulated with TGF-β (5 ng/mL) and cell lysates harvested 30 minutes later for immunoblotting with antipSmad2/3 antibody as described.1
Animals
All protocols using animals were approved by the Institutional Animal Care and Use Committee and were in compliance with all federal and local guidelines. Uninjured 2-week and 4-week ligated carotid arteries along with miscellaneous other organs were harvested from wild-type and Cthrc1 transgenic mice as described.1,5 Porcine aorta samples were obtained from discarded surgical training tissues.
Results
Endogenous Cthrc1 in Smooth Muscle Cells Undergoes Proteolytic Processing
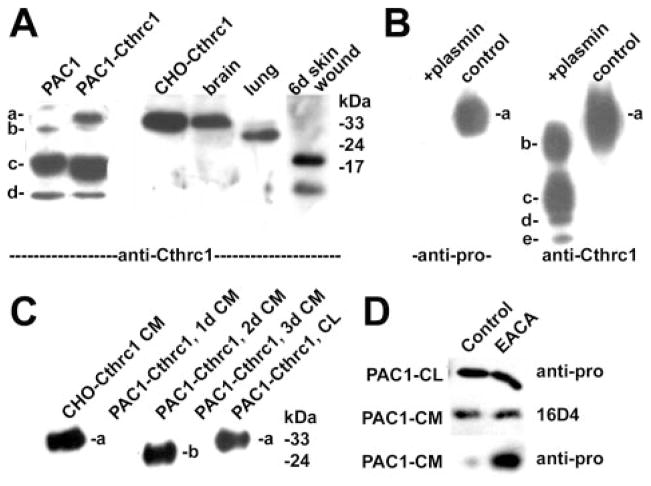
Previously we successfully raised rabbit antisera against full-length recombinant Cthrc1 expressed in E coli (referred to as “anti-Cthrc1”). These antisera recognize multiple epitopes along the molecule. When lysates of PAC1 SMCs expressing endogenous Cthrc1 were immunoblotted with these antibodies, several immunoreactive bands with apparent molecular weights below 30 kDa were observed (Figure 1A, bands labeled “b,” “c,” “d”). The majority of endogenous immunoreactive Cthrc1 in PAC1 SMCs was present in fragment c (approximately 20 kDa). In PAC1 SMCs stably transfected with a full-length Cthrc1 expression vector, an immunoreactive band consistent with unprocessed Cthrc1 (Figure 1A, band labeled “a” in lane PAC1-Cthrc1) was detected.1,2 These generated fragments can also be identified in vivo in tissue lysates from various organs. Cthrc1 found in the brain primarily corresponds to fragment a, whereas fragment b is prevalent in the lung. During wound healing in response to a skin incision Cthrc1 is processed and fragments c and d can be identified (Figure 1A).
Figure 1.
Immunoblotting for Cthrc1 (a) and cleaved fragments (b– e) in vitro and in vivo. A, Control PAC1 cell lysate reveals fragments and uncleaved a form in overexpressing cells (PAC1-Cthrc1, CHO-Cthrc1). B, Plasmin treatment rapidly generates fragments b, c, d, and e from uncleaved a form. C, Immunoblotting with anti-Cthrc1 of cell lysate (CL) and conditioned media (CM) harvested at the indicated times after Cthrc1 adenovirus trunsduction. D, Inhibition of propeptide cleavage by ε-amonicaproic acid (EACA).
Transduction of CHO-K1 cells with an adenovirus expressing full-length Cthrc1 led to the secretion of large amounts of Cthrc1 into the serum-free medium (Figure 1B, lane labeled “control”). The majority had the same apparent molecular weight as the a-form detected in PAC1 cells overexpressing Cthrc1 (Figure 1A).
For the purpose of identifying the lower molecular weight forms of Cthrc1, we raised antisera against specific Cthrc1 epitopes. Highly specific and sensitive antisera against the N-terminal peptide sequence (KQKALLRQREVVDLYN-GMC) following the signal peptide were obtained and referred to as “antipro”. The antisera previously raised against full-length recombinant Cthrc1 expressed in E coli did not cross-react with this epitope (data not shown). The antipro antibody detected only the a form in the untreated medium harvested from CHO cells overexpressing Cthrc1, and none of the other bands immunoreactive with the anti-Cthrc1 antibody (Figure 1B). This indicated that the N-terminal epitope was not present in bands b through e.
Plasmin Cleaves Secreted Cthrc1
Pilot experiments with protease inhibitors aprotinin, leupeptin, and PMSF (phenylmethylsulfonyl fluoride) indicated that a serine protease was responsible for cleavage of Cthrc1 derived from transduced CHO cells (not shown). Because plasmin is a serine protease that can activate cytokines such as TGF-β,6 we tested its ability to cleave Cthrc1. Within minutes of incubation with plasmin, purified Cthrc1 (a form) was converted into fragments b, c, d, and e (Figure 1B). These fragments had the same apparent molecular weight as those detected in PAC1 cells (Figure 1A). Only the a form was reactive with the antipro antibody, indicating that all smaller fragments lacked the putative propeptide (Figure 1A). N-terminal peptide sequencing on fragments b through e identified the N terminus of b as V49, and the N terminus of fragments c, d, and e was E98. Thus, the plasmin cleavage sites are flanking the G-X-Y domain, and fragments d and e are derived from c through further C-terminal truncation. On adenoviral overexpression of Cthrc1 in PAC1 cells, only the N-terminally truncated b form was transiently detectable in the conditioned medium 48 hours after transduction (Figure 1C, lane labeled “PAC1-Cthrc1, 2d CM”), whereas the uncleaved a form was detectable in the PAC1 cell lysate (Figure 1C, lane labeled “PAC1-Cthrc1, CL”). CHO cells transduced with the same adenovirus secrete only noncleaved Cthrc1 in the conditioned medium (Figure 1C, lane labeled “CHO-Cthrc1 CM”). We have observed that Cthrc1 sticks to PAC1 cell matrix (not shown), and the c band in the lysate (Figure 1A) may represent plasmin-cleaved Cthrc1 bound to cell associated matrix. Because SMCs express plasminogen activators, these cells will generate plasmin from extracellular plasminogen supplied with the serum. We therefore grew PAC1 cells in the presence of the plasmin inhibitor ε-aminocaproic acid after transduction with the Cthrc1-expressing adenovirus. Immunoblotting with antipro demonstrated increased amounts of unprocessed Cthrc1 in the medium of cultures grown in the presence of ε-aminocaproic acid whereas cell lysates and conditioned medium contained similar levels of total Cthrc1 (Figure 1D). These data indicate that plasmin cleaves the N-terminal propeptide in PAC1 cells.
Cytoplasmic Localization of proCthrc1 in Differentiated Smooth Muscle
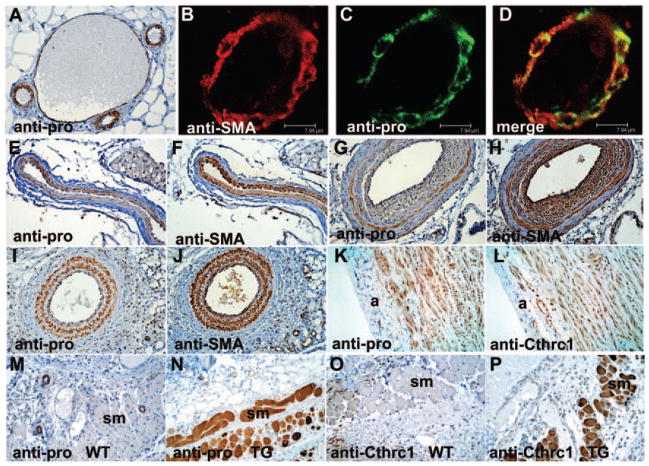
We used the antipro antibody to determine the localization of the propeptide or noncleaved Cthrc1 in blood vessels (Figure 2). Specific immunoreactivity was detected in smooth muscle, including vascular smooth muscle of arterioles and arteries (Figure 2A through 2D), uterine smooth muscle (Figure 3E), smooth muscle of the gastrointestinal tract (Figure 3F), and in myoepithelial cells (Figure 3G). In all cases, the immunoreactivity localized to the cytoplasm. There was partial colocalization of smooth muscle α-actin and antiproCthrc1 immunoreactivity as determined by confocal microscopy (Figure 2B through 2D). In veins, proCthrc1 immunoreactivity was only detected in larger veins (Figure 2A).
Figure 2.
Immunohistochemistry with antipro, anti-Cthrc1, and anti-SMA antibodies. A, proCthrc1 localizes to SMC of arterioles, arteries, and larger veins (center). B–D, Colocalization of SMA and proCthrc1 by confocal microscopy. Serial sections of normal (E–F) and 2-week ligated (G and H) mouse carotid arteries with a decrease of proCthrc1 in the developing neointima. I and J, Lack of dedifferentiation and proliferation of SMCs in Cthrc1 transgenic 2-week ligated carotid arteries correlates with maintenance of proCthrc1 immunoreactivity. K and L, Uncleaved Cthrc1 in SMC of pig aorta; a indicates adventitia. M–P, Two-week-old skin incision with uncleaved Cthrc1 in striated muscle of Cthrc1 transgenic (TG) but not wild-type (WT) mice; myofibroblasts are only anti-Cthrc1 positive (O). Original magnification: A, 400×; B–D, 1000×; E–P 200×.
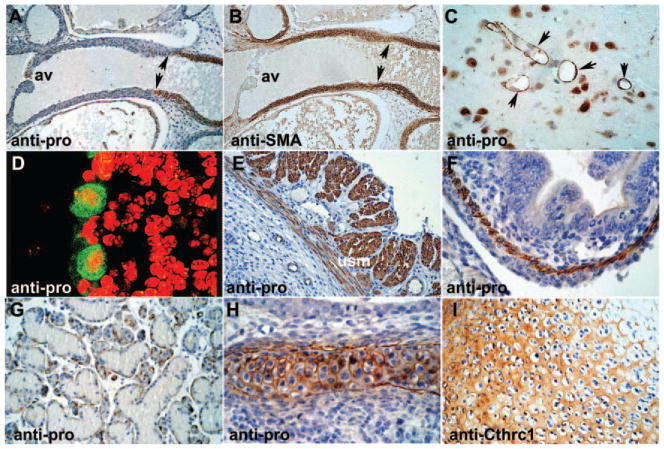
Figure 3.
Immunohistochemistry on wild-type mouse organs. A and B, E16.5 embryo with proCthrc1 in the distal aortic outflow tract (arrows) but not adjacent to the aortic valve (av) while SMA is also in the myocardium (supplemental materials). Neurons (C) and cerebellar Purkinje cells (D) with strong intracellular immunoreactivity; basement membrane type staining in brain vessels (arrows in C). Uterine smooth muscle (usm; E) and gastrointestinal smooth muscle (F) in wild-type E16.5 mouse embryos. G, Myoepithelial cells of salivary glands with antipro immunoreactivity in the basal aspects. H, E18.5 cartilage with distinct pericellular immunoreactivity with antipro. I, Diffuse extracellular matrix staining with anti-Cthrc1. Nuclei stained with hematoxylin, immunoreactivity in brown. Original magnification: A and B, 100×; E and G, 200×; C, F, H, and I, 400×; D, 1000×.
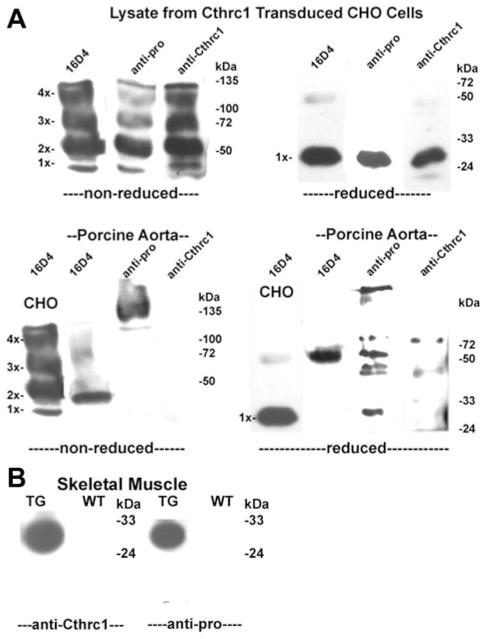
Using the available antibody panel, we examined Cthrc1 expressed in CHO cells after adenoviral transduction under reducing and nonreducing conditions by immunoblotting and compared it side-by-side with lysates prepared from porcine aorta. In nonreduced CHO cell lysate, all antibodies recognized bands with molecular weights consistent with monomer, dimer, trimer, and tetramer (Figure 4A, labeled 1×–4×). Under reducing conditions, the majority of the protein ran as an apparent monomer, which was detected by all 3 antibodies. In the aortic sample, the antipro antibody detected a prominent high molecular weight band with an apparent molecular weight of >130 kDa under nonreducing conditions (Figure 4A), whereas under reducing conditions multiple immunoreactive bands were detected with this antibody and all of them had a higher apparent molecular weight than the uncleaved Cthrc1 from CHO cells (Figure 4A). The antibodies 16D4 and anti-Cthrc1, both raised against the full-length aggregating Cthrc1, did not recognize the high molecular weight Cthrc1 complex (Figure 4A); however, some of the smaller bands recognized by the antipro antibody were also detected by 16D4 and anti-Cthrc1 after longer exposure times (Figure 4A). 16D4 specifically recognized a band in the 50 kDa range under both reduced and nonreducing conditions. These results are consistent with the explanation that unprocessed Cthrc1 resides intracellularly in smooth muscle in form of a complex with 1 or more other proteins resulting in epitope masking and poor detection by antibodies raised against aggregating or multimeric Cthrc1. In addition, the propeptide remains accessible in this complex and is detected by the antipro antibody.
Figure 4.
A, Immunoblotting with indicated antibodies. Note the oligomerization of Cthrc1 under nonreducing conditions and the antipro immunoreactive high-molecular-weight band in the pig aorta. B, Accumulation of noncleaved Cthrc1 in Cthrc1 transgenic (TG) but absence in wild-type (WT) skeletal muscle.
Cthrc1 Expressed Ectopically in Skeletal Muscle Remains Intracellular
We observed that CMV-driven full-length Cthrc1 expression in mice leads to highly efficient transgene expression in skeletal muscle, predominantly the a form (Figure 4B). Skeletal muscle of wild-type mice contains no detectable endogenous Cthrc1 (Figure 4B). These immunoblot data agreed with the immunohistochemical staining performed on skin wounds from wild-type and Cthrc1 transgenic mice. These specimens contain striated muscle from the cutaneous panniculus carnosus that stains strongly with both antipro and anti-Cthrc1, but only in the transgenic mice (Figure 2M through 2P). These observations provide additional evidence that Cthrc1 localizes to cytoplasmic components despite its signal peptide. The skin wounds also showed scattered myofibroblasts with surrounding anti-Cthrc1 immunoreactivity (Figure 2O) but no antipro immunoreactivity was detected in these cells (Figure 2M).
Loss of proCthrc1 Immunoreactivity Correlates With Dedifferentiation of SMC In Vivo
On injury, vascular SMCs dedifferentiate and adopt the synthetic phenotype. This coincides with a transient reduction in contractile filaments and other differentiation markers. Compared to normal vessels, antipro immunoreactivity decreases sharply in dedifferentiated SMCs of the developing neointima and adjacent inner layer of the media of 2-week injured carotid arteries (Figures 5A, 5B, and 2G). At 2 weeks after injury, these SMCs reexpressed abundant levels of SMA (Figure 2H). Intracellular antipro immunoreactivity in SMCs was detectable again at 4 weeks after injury after the remodeling process is completed (Figure 5C). Unlike SMCs in normal arteries we consider all SMCs in vitro dedifferentiated to a certain extent, and this may be one reason why a similar staining pattern in vitro is limited to few cells in postconfluent cultures (data not shown). We have generated transgenic mice overexpressing Cthrc1 under CMV promoter control,1 which are resistant to developing neointimal lesions. The lack of significant neointima formation and SMC dedifferentiation correlates with the retained presence of antipro immunoreactivity in SMCs and strong SMA immunoreactivity (Figure 2G through 2J). Immunohistochemistry performed on pig aorta sections with the antipro antibody detected Cthrc1 immunoreactivity in a pattern similar to SMA (Figure 2K). After antigen retrieval with reducing agents, we were ultimately able to detect a similar immunoreactivity pattern also with the anti-Cthrc1 antibody (Figure 2L).
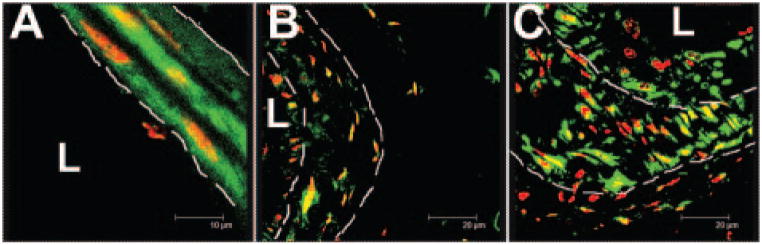
Figure 5.
Time course of proCthrc1 immunolocalization after mouse carotid artery ligation as assessed by confocal microscopy. A, Granular cytoplasmic localization in SMCs of normal vessels. B, Decreased proCthrc1 at 2 weeks after ligation and restored levels 4 weeks after ligation C, Dashed lines mark internal and external elastic laminae, L-lumen. Immunoreactivity in green, nuclei in red. Original magnification 1000×.
Cthrc1 in the Aortic Outflow Tract
By immunohistochemistry, we were unable to detect Cthrc1 in embryonic and postnatal cardiac muscle (supplemental materials, available online at http://atvb.ahajournals.org). Examination of the aortic outflow tract revealed antipro immunoreactivity in the aortic valve endothelium, whereas the muscle of the surrounding downstream outflow tract showed no detectable Cthrc1 expression (Figure 3A). As the outflow tract transitioned into the ascending aorta, there was a distinct transition to Cthrc1-expressing SMCs as detected by the antipro antibody (Figure 3A and 3B). This is in contrast to SMA, which is present throughout the embryonic myocardium including the proximal aortic outflow tract (Figure 3A versus 3B, and supplemental materials). Overexpression of Cthrc1 in SMC did not increase levels of SMA (supplemental materials).
Localization of Cthrc1 in Nonvascular Smooth Muscle and Other Tissues
A striking intracellular localization of Cthrc1 was also reported by us for some neurons, Purkinje cells, parafollicular cells of the thyroid gland, and certain neuronal populations.7 In addition to these earlier observations, the antipro antibody localized intracellular immunoreactivity in smooth muscle of the uterus (Figure 3C and 3D) and gastrointestinal tract (Figure 3F), and in the basal portion of myoepithelial cells of secretory glands (Figure 3G). The basal aspects of these myoepithelial cells are typically SMA-positive (not shown). Certain neurons (Figure 3C and 3D) showed strong intracellular antipro immunoreactivity. Similar to SMCs, Cthrc1 in these cells was distributed throughout the cytoplasm in a granular pattern (Figures 3D and 5A). In brain microvessels, antipro localization appeared associated with the basement membrane or pericytes (Figure 3C). Cartilage of developing bones was previously shown to exhibit diffuse extracellular Cthrc1 immunoreactivity with anti-Cthrc1 (Figure 3I).7 Immunostaining of cartilage with antipro, however, revealed distinctly pericellular staining, consistent with secretion of unprocessed Cthrc1 from chondrocytes with subsequent propeptide cleavage (Figure 3H).
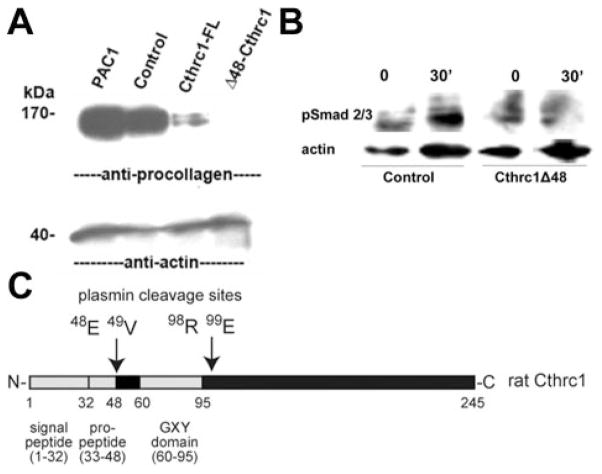
Cthrc1Δ48 Is a Potent Inhibitor of Procollagen Synthesis
We addressed the question whether N-terminal truncation of full-length Cthrc1 resulted in an active or inactive form by generating an expression vector with the N-terminally 48 amino acids deleted (Figure 6C). This truncated form was fused to the IgK signal peptide derived from the pDisplay vector to assure secretion from the cell. An adenovirus expressing this mutant form of Cthrc1 proved to be more potent in inhibiting procollagen synthesis in PAC1 cells than the adenovirus expressing full-length Cthrc1 (Figure 6A). This was not attributable to differences in expression levels of the overexpressed Cthrc1 (data not shown). The truncated Cthrc1Δ48 also inhibited phosphorylation of Smad2/3 in response to stimulation with TGF-β (Figure 6B).
Figure 6.
Enhanced effect of Cthrc1Δ48 on procollagen production. A, Procollagen levels in PAC1 cells after transduction with no virus (PAC1), β-galactosidase (Control), full-length Cthrc1 (Cthrc1-FL), or Cthrc1Δ48 (Δ48-Cthrc1) adenovirus. B, Reduced TGF-β–induced Smad2/3 phosphorylation in PAC1 cells transduced with Cthrc1Δ48 adenovirus. C, Schematic of rat Cthrc1 domains.
Discussion
The initial characterization of Cthrc1 highlighted its importance during vascular injury; the present study has broadened this scope suggesting that Cthrc1 may play a role in the maintenance of a differentiated phenotype in SMCs. Using epitope-specific antibodies we demonstrated the intracellular localization of unprocessed Cthrc1 in several cell types. The intracellular localization of uncleaved Cthrc1 is not the result of ongoing protein synthesis, which would have revealed an ER-Golgi type staining pattern in the presence of detectable mRNA. This is supported by previous work which demonstrated that in uninjured arteries, Cthrc1 mRNA levels were not detectable by Northern blot analysis or in situ hybridization.1 In addition, the immunohistochemical staining pattern observed with the antipro antibody was similar to the distribution of SMA in smooth muscle and similar to contractile fiber localization when ectopically expressed in skeletal muscle (Figure 2N and 2P). Furthermore, these results also suggest that Cthrc1 in uninjured smooth muscle has a prolonged half life.
Our study brings up the intriguing question of the function of intracellular Cthrc1 and its potential cytoplasmic binding partners. We anticipate that the high molecular weight band detected by the antipro antibody on immunoblots under nonreducing conditions is composed of multiple components. It appears that at least some of the interactions of Cthrc1 with these components involve covalent bonds because all immunoreactive bands after reduction have higher apparent molecular weights than monomeric Cthrc1 secreted from CHO cells. Potential differences in posttranslational modifications are less likely to account for such dramatic differences in molecular weight.
Previous work demonstrated that the leucine rich N terminus of Cthrc1 functions as a signal peptide allowing for secretion into the conditioned medium of transfected CHO cells, and that secreted Cthrc1 is posttranslationally modified by glycosylatation.1 As shown here, secreted Cthrc1 self-aggregates, and this has hampered efforts to crystallize the molecule (data not shown). We are not aware of another example where a protein with a signal peptide remains intracellular as opposed to being secreted from the cell or being located in the cell membrane. This raises the question of whether proCthrc1 in uninjured smooth muscle has trafficked through the cellular secretion pathway and whether it is posttranslationally modified. We are not aware of any alternatively spliced forms of Cthrc1 in the species examined here that could give rise to forms of Cthrc1 lacking a signal peptide.
After vascular injury, Cthrc1 mRNA levels are detectable in neointimal SMCs, and immunoreactive Cthrc1 is detected in the extracellular matrix.1 These findings suggest that under situations of increased Cthrc1 synthesis, Cthrc1 is released efficiently from SMCs into the extracellular space. This view is supported by the lack of antipro immunoreactivity in the dedifferentiated SMCs of the neointima (Figures 2G and 5B). Even though these intimal SMCs reexpressed SMA (Figure 2H), antipro immunoreactivity was negligible in these cells (Figures 2G and 5B), whereas SMCs of the outer media, which do not typically participate in the proliferative response of the mouse carotid ligation model (Figures 2G and 5B), retained their antipro immunoreactivity. On completion of the remodeling process at 4 weeks after injury, localization of proCthrc1 is reestablished in cells of the neointima (Figure 5C). There are several scenarios that could explain the low levels of antipro immunoreactivity in neointimal SMCs. One is that the levels of the intracellular protein(s) binding Cthrc1 in uninjured SMCs are low in dedifferentiated SMCs. This in turn, in combination with increased Cthrc1 expression, could favor oligomerization of Cthrc1 and secretion of Cthrc1 from the cell. With respect to SMCs after injury, we currently do not know whether cleavage of the Cthrc1 propeptide occurs intracellularly or after release from the cell. Although Cthrc1 does not contain a predictable consensus propeptide cleavage site, we have direct and indirect evidence that Cthrc1 is secreted from various cells in an uncleaved form. The majority of Cthrc1 secreted into the medium by CHO cells contains the propeptide (Figure 1B), and uncleaved Cthrc1 is detectable in the medium of PAC1 cells in the presence of a plasmin inhibitor (Figure 1D). In addition, in chondrocytes antipro immunoreactivity is distinctly pericellular, which is in contrast to the diffuse anti-Cthrc1 immunoreactivity seen in the cartilage matrix (Figure 3H and 3I). This finding suggests that cleavage of the propeptide may occur in the vicinity of the cell membrane in these cells. As many cell types express plasminogen activators including SMCs of injured arteries,8 generation of plasmin from ubiquitous plasminogen renders plasmin cleavage of Cthrc1 a relevant mechanism in the activation of Cthrc1. For SMCs of injured arteries, propeptide cleavage of Cthrc1 appears to occur very efficiently because very little antipro immunoreactivity was detectable in the neointima (Figure 5B). As we show here, the N-terminally truncated from of Cthrc1 generated by plasmin was more potent in inhibiting procollagen synthesis in the PAC1 cell line than full-length Cthrc1. There is abundant extracellular collagenous matrix synthesis in the developing intima despite the increased expression of Cthrc1,9 which raises questions about the biological significance of the released Cthrc1. One possibility is that in the absence of Cthrc1, collagenous matrix synthesis would be even more pronounced. We will be able to address this issue in the future with genetic loss-of-function models.
In many tissues we report the presence of uncleaved Cthrc1 in the vicinity of organelles with contractile function where expression of SMA typically occurs. This includes smooth muscle present in a variety of organs (vasculature, gastrointestinal tract, uterus, etc), myoepithelial cells as shown here for the salivary gland (Figure 3G), and pericytes of small brain vessels (Figure 3C). During embryonic development Cthrc1 is expressed in the dorsal aorta at E9.5,10 which corresponds to the time when the SMC marker SMA is also turned (see supplemental materials). However, in the aortic outflow tract of the developing heart, localization of proCthrc1 is more specific to SMCs than SMA because it is not present in the myocardium and the associated proximal part of the aortic outflow tract (Figure 4A versus 4B). There are, however, several examples of cell types that do not express SMA but exhibit intracellular localization of uncleaved Cthrc1. These include a variety of neurons (Figure 3C and 3D) and also the parafollicular cells of the thyroid gland.7 We also observed many smaller veins that were immmunoreactive for SMA but did not exhibit antipro immunoreactivity (data not shown). Therefore, Cthrc1 is not a SMC-specific marker, but in certain situations its presence in SMCs is more representative of the differentiated SMC phenotype than SMA (Figures 2 and 5).
In summary, the present study identifies Cthrc1 as a protein that under normal conditions resides in the cytoplasm of SMCs as well as other cell types in unprocessed form despite the presence of a signal peptide. The function of Cthrc1 residing intracellularly remains to be determined. Vascular injury is associated with release of Cthrc1 from cells and cleavage of an N-terminal propeptide, which results in a molecule with increased ability to inhibit collagen matrix deposition.
Supplementary Material
Acknowledgments
Generation of the antipro antisera was generously supported by Novartis.
Sources of Funding
This publication was made possible by NIH Grants HL69182 (to V.L.) and P20 RR-15555 (to V.L. and Dr Robert Friesel) from the National Center for Research Resources. R.J.L. is supported by Grant Number F32HL091615 from the National Heart, Lung, and Blood Institute. The Histopathology Core Facility was supported by P20RR181789 (Director Dr Don Wojchowski).
Footnotes
Disclosures
None.
References
- 1.LeClair RJ, Durmus T, Wang Q, Pyagay P, Terzic A, Lindner V. Cthrc1 is a novel inhibitor of transforming growth factor-beta signaling and neointimal lesion formation. Circ Res. 2007;100:826–833. doi: 10.1161/01.RES.0000260806.99307.72. [DOI] [PubMed] [Google Scholar]
- 2.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 3.Majesky MW, Lindner V, Twardzik DR, Schwartz SM, Reidy MA. Production of transforming growth factor beta 1 during repair of arterial injury. J Clin Invest. 1991;88:904–910. doi: 10.1172/JCI115393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lindner V. Role of basic fibroblast growth factor and platelet-derived growth factor (B-chain) in neointima formation after arterial injury. Z Kardiol. 1995;84(Suppl 4):137–144. [PubMed] [Google Scholar]
- 5.Kumar A, Lindner V. Remodeling with neointima formation in the mouse carotid artery after cessation of blood flow. Arterioscler Thromb Vasc Biol. 1997;17:2238–2244. doi: 10.1161/01.atv.17.10.2238. [DOI] [PubMed] [Google Scholar]
- 6.Flaumenhaft R, Abe M, Sato Y, Miyazono K, Harpel J, Heldin CH, Rifkin DB. Role of the latent TGF-beta binding protein in the activation of latent TGF-beta by co-cultures of endothelial and smooth muscle cells. J Cell Biol. 1993;120:995–1002. doi: 10.1083/jcb.120.4.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durmus T, Leclair RJ, Park KS, Terzic A, Yoon JK, Lindner V. Expression analysis of the novel gene collagen triple helix repeat containing-1 (Cthrc1) Gene Expr Patterns. 2006;6:935–940. doi: 10.1016/j.modgep.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Jackson CL, Reidy MA. The role of plasminogen activation in smooth muscle cell migration after arterial injury. Ann N Y Acad Sci. 1992;667:141–150. doi: 10.1111/j.1749-6632.1992.tb51606.x. [DOI] [PubMed] [Google Scholar]
- 9.Pyagay P, Heroult M, Wang Q, Lehnert W, Belden J, Liaw L, Friesel RE, Lindner V. Collagen triple helix repeat containing 1, a novel secreted protein in injured and diseased arteries, inhibits collagen expression and promotes cell migration. Circ Res. 2005;96:261–268. doi: 10.1161/01.RES.0000154262.07264.12. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi Y, Imanaka T, Takano T. Spatial and temporal pattern of smooth muscle cell differentiation during development of the vascular system in the mouse embryo. Anat Embryol (Berl) 1996;194:515–526. doi: 10.1007/BF00185997. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.