Preeclampsia and other hypertensive disorders of pregnancy continue to be major contributors to maternal mortality and morbidity worldwide (1). Currently, primary treatment for preeclampsia centers on management of symptoms until delivery is indicated; hence, early onset preeclampsia is strongly associated with fetal growth restriction and is one of the leading causes of pre-term birth (1). A wide variety of putative factors and pathways have been implicated in development of this pregnancy-specific syndrome (1). Indeed, characterization of biomarkers for identification of patients at risk for developing preeclampsia continues to be an important endeavor. The complement system is a humoral immune amplification system, and products of complement system activation have been implicated both as biomarkers for the disease as well as factors important in development of preeclampsia. The study by Buurma et al. (2) in the current issue of Hypertension takes a critical step towards identifying the important pathway of complement activation leading to excessive local complement activation in the preeclamptic placenta as well as demonstrating key complement regulatory proteins that are increased in preeclampsia presumably to limit activation of the pathophysiologic pathway.
The original discovery of the complement system focused on its ability to ‘complement’ the actions of antibody in host defense and bacterial killing. As part of innate immunity, the complement system contributes to survival, and components are found in species as ancient as horseshoe crabs. While basal complement activity is controlled by numerous regulators, excessive activation contributes to autoimmune diseases including arthritis, Systemic Lupus Erythematosus (SLE) and antiphospholipid syndrome. Interestingly, individuals with SLE and antiphospholipid syndrome are also at increased risk for adverse fetal outcomes and hypertension during pregnancy.
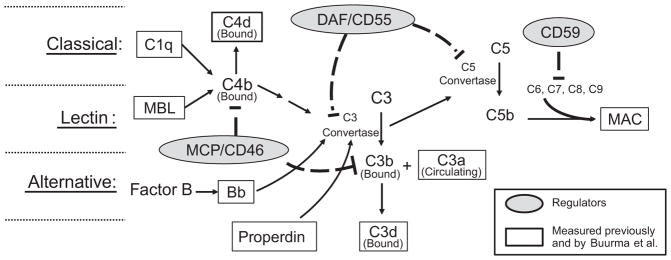
A brief overview of the most relevant aspects of the complement system is illustrated in Figure 1. Central to all 3 pathways of complement activation (classical, lectin and alternative) is the enzyme C3 convertase which cleaves C3 to generate C3a with covalent binding of C3b fragment to the invader and/or neighboring tissue components. The C3b ‘tag’ marks the surface for attack by phagocytes and also serves as nucleus for forming C5 convertase for cleavage of C5. Following C5 cleavage, C5b inserts in the surrounding membrane, and subsequent protein-protein interactions of C5b with C6, C7, C8 and C9 leads to formation of the Membrane Attack Complex (MAC) that can lyse cells. The classical pathway is initiated by antigen antibody interactions with C1q and the lectin pathway by polysaccharide interactions with mannose binding lectin (MBL). In both cases, C4 is cleaved with covalent binding of C4b to surfaces and C4b participation as part of C3 convertase. Alternative pathway is more promiscuous and is activated by endotoxin and many surfaces including negatively charged particles. Cleavage product of Factor B (Bb) is an alternative pathway marker that does not covalently bind to surfaces, but forms a complex with C3b to form an alternative pathway C3 convertase. Properdin stabilizes alternative pathway C3 convertase and thus promotes continued C3 cleavage. Bound C3b and C4b can be further degraded to C3d and C4d, respectively, which still signal phagocytes, but do not serve as a nucleus for formation of C5 convertase.
Figure 1.
Simplistic schematic of complement system activation depicting regulators and complement components measured by Buurma and others. The complement system is activated via three pathways: Classical, lectin and alternative. All three pathways result in formation of C3 convertase to cleave C3 into a covalently bound C3b fragment and a fluid phase C3a fragment. C1q is indicative of classical pathway involvement, and mannose binding lectin (MBL) indicates lectin pathway involvement. With activation of either the classical or lectin pathway, C4b covalently binds locally to invader or self surfaces. C4b either participates in formation of C3 convertase, or is degraded to bound C4d. With alternative pathway activation, Factor B is cleaved to form Bb which participates in formation of the alternative pathway C3 convertase. Properdin stabilizes C3 convertase formed with alternative pathway activation. C3b can either participate as part of the C5 convertase complex to cleave C5 or be degraded to bound C3d. C5b, C6, C7, C8 and C9 assemble in cell membranes to form membrane attack complex (MAC) which deteriorates membrane potential and induces cell lysis. Regulators present on the trophoblast membrane as well as numerous cells in mother and fetus include MCP (CD46), DAF (CD55), and CD59. MCP, or membrane cofactor protein, facilitates degradation of C3b and C4b so they do not continue as part of the C3 convertase. DAF, or decay-accelerating factor, facilitates decay of convertases assembled from activation of any of the pathways, and CD59 hampers assembly of MAC.
Components of the complement system are important for normal placentation and delicate regulation of complement activation is critical for a successful pregnancy. While some level of placental complement activation is normal in pregnancy, previous studies suggest that excessive activity may contribute to pathology of preeclampsia (3–5). Indeed, animal models show that excessive complement activation is associated with adverse pregnancy outcomes such as oxidative stress and placental dysfunction (6) and hypertension (7) and inhibiting complement activation ameliorates these features of preeclampsia, respectively, in a mouse and rat model (6;7).
In transplantation, tissue bound C4d indicates classical pathway activation and antibody dependent allograft rejection. Likening pregnancy to a transplant, studies of Cohen and Buurma et al (8) demonstrated more C4d at the maternal-fetal interface in placenta of patients with SLE and antiphospholipid syndrome compared to controls, and C4d was associated with adverse fetal outcomes. Since no differences were seen in MBL and properdin, the pathological complement activation was most likely occurring via classical pathway (8). A subset of patients in this recent study had coexisting preeclampsia and set the stage for the current study in Hypertension looking for excessive complement activation in preeclamptic placentas.
In the current study by Buurma et al. (2) the presence of C4d and the lack of MBL and properdin provide evidence of local classical pathway activation at the feto-maternal interface. An association was noted with classical complement system activation and the severity of adverse fetal outcome, but no association was seen with increased blood pressure. An upregulation of endogenous complement regulators and no difference in C3 deposition, suggested the presence of a feto-protective feedback mechanism regulating C3 activation. These data suggest excessive complement activation does not progress beyond C3 in this patient population because of increased mRNA for trophoblastic membrane bound regulators. The Buurma study differs from earlier work reporting increased C1q, C3d as well as C9 (3;5) in placentas from preeclamptic vs. normal pregnancies, indicating that excessive local activation of complement in the preeclamptic placenta went beyond C3 to the MAC. Investigating complement activation locally in the placenta is an excellent strategy to determine events occurring at the feto-maternal interface and is clearly limited by heterogeneity of the patient population.
Systemically, increased circulating concentrations of C3a (produced by all pathways) and Bb (alternative pathway) have been documented as early markers of complement activation in the maternal circulation of women who developed preeclampsia compared to normal pregnancies (9), with the presence of increased Bb suggesting alternative pathway activation is important in the pathogenesis. Studies of others (4) also provide evidence that excessive complement activation is present systemically at time of delivery of the fetus. Besides the placenta, adipose tissue may also contribute to the complement story. Adipose tissue is a source of acylation-stimulating protein (ASP) which is identical to C3a without its carboxy terminal arginine (C3a des arg). Women with pre-pregnancy obesity and high concentrations of C3a or Bb early in pregnancy have the highest risk of developing preeclampsia (10).
The idea that complement system and inflammation are involved in cardiovascular disease and associated hypertension is not new. Previously, elevations in C3 have been associated with hypertension. In pregnancy, recent work demonstrates that pregnant C1q deficient mice develop features of preeclampsia including hypertension, angiogenic imbalance and fetal distress (11) pointing to the physiological role of C1q in maintaining normal pregnancy. Moreover, recent studies by our group and others are demonstrating that inhibition of complement activation mitigates characteristics of preeclampsia in different animal models (6;7). Taken together, there is accumulating evidence that the complement system plays a role in development and/or maintenance of hypertension associated with preeclampsia.
The present work by Buurma and colleagues raises many new questions. Perhaps the foremost question is whether complement activation contributes to preeclampsia in a causal way or is a consequence of one or more aspects of the syndrome? It is unclear if complement is activated by AT1-auto antibodies, or if complement activation plays a role in the development of an angiogenic imbalance, both having been implicated in the pathogenesis of preeclampsia. Further studies are also of interest to clarify mechanistic connections between obesity and preeclampsia given that both are associated with the complement system. Lastly, the question specific to both local and systemic complement activation remains; is it excessive activation, inadequate control or both. Studies in our laboratories and others are working towards answering these questions with hopes of identifying significant new therapeutic targets to advance treatment of preeclamptic patients and minimize the necessity for pre-term delivery of the fetus.
Acknowledgments
Source of Funding
JSG is supported in part by funding from AHA 10SDG2600040, NIH HL114096 and NIH HL109843. VLK is a consultant on NIH HL114096. JFR is supported in part by funding from HL109843.
Footnotes
Disclosures
None
References
- 1.Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. The Lancet. 2010;376:631–644. doi: 10.1016/S0140-6736(10)60279-6. [DOI] [PubMed] [Google Scholar]
- 2.Buurma A, Cohen D, Veraar K, Schonkeren D, Claas FHJ, Bruijin J, Bloemenkamp K, Baelde H. Preeclampsia Is Characterized By Placental Complement Dysregulation. Hypertension. 2012;xx:xxx. doi: 10.1161/HYPERTENSIONAHA.112.194324. [DOI] [PubMed] [Google Scholar]
- 3.Tedesco F, Radillo O, Candussi G, Nazzaro A, Mollnes TE, Pecorari D. Immunohistochemical detection of terminal complement complex and S protein in normal and pre-eclamptic placentae. Clin Exp Immunol. 1990;80:236–240. doi: 10.1111/j.1365-2249.1990.tb05240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Derzsy Z, Prohaszka Z, Rigo J, Jr, Fust G, Molvarec A. Activation of the complement system in normal pregnancy and preeclampsia. Mol Immunol. 2010;47:1500–1506. doi: 10.1016/j.molimm.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 5.Sinha D, Wells M, Faulk WP. Immunological studies of human placentae: complement components in pre-eclamptic chorionic villi. Clin Exp Immunol. 1984;56:175–184. [PMC free article] [PubMed] [Google Scholar]
- 6.Qing X, Redecha PB, Burmeister MA, Tomlinson S, D’Agati VD, Davisson RL, Salmon JE. Targeted inhibition of complement activation prevents features of preeclampsia in mice. Kidney Int. 2011;79:331–339. doi: 10.1038/ki.2010.393. [DOI] [PubMed] [Google Scholar]
- 7.Lillegard KE, Johnson AC, Lojovich SJ, Bauer AJ, Marsh H, Gilbert JS, Regal JF. Inhibitor of complement activation attenuates placental ischemia-induced hypertension in rat. The FASEB Journal. 2012;26:1097. [Google Scholar]
- 8.Cohen D, Buurma A, Goemaere NN, Girardi G, le Cessie S, Scherjon S, Bloemenkamp KW, de Heer E, Bruijn JA, Bajema IM. Classical complement activation as a footprint for murine and human antiphospholipid antibody-induced fetal loss. J Pathol. 2011;225:502–511. doi: 10.1002/path.2893. [DOI] [PubMed] [Google Scholar]
- 9.Lynch AM, Gibbs RS, Murphy JR, Giclas PC, Salmon JE, Holers VM. Early elevations of the complement activation fragment C3a and adverse pregnancy outcomes. Obstet Gynecol. 2011;117:75–83. doi: 10.1097/AOG.0b013e3181fc3afa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lynch AM, Eckel RH, Murphy JR, Gibbs RS, West NA, Giclas PC, Salmon JE, Holers VM. Prepregnancy obesity and complement system activation in early pregnancy and the subsequent development of preeclampsia. Am J Obstet Gynecol. 2012;206:428. doi: 10.1016/j.ajog.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh J, Ahmed A, Girardi G. Role of Complement Component C1q in the Onset of Preeclampsia in Mice. Hypertension. 2011;58:716–724. doi: 10.1161/HYPERTENSIONAHA.111.175919. [DOI] [PubMed] [Google Scholar]