Abstract
Background
The mechanism(s) responsible for the acquisition of maternal antibody isotypes other than IgG are not fully understood.
Objective
To define the ability of the neonatal Fc receptor for IgG uptake (FcRn) to mediate intestinal absorption of IgG1 anti-IgE/IgE immune complexes.
Methods
C57BL/6 allergic ovalbumin (OVA)-immune foster mothers were generated to nurse naïve FcRn+/− or FcRn−/− progeny. At the time of weaning, serum levels of OVA-specific antibodies and IgG1 anti-IgE/IgE immune complexes were determined in allergic foster mothers and FcRn+/+, FcRn+/−, or FcRn−/− breastfed offspring. In separate experiments, FcRn+/− or FcRn−/− neonatal mice were gavage fed TNP-specific IgE as IgG1 anti-IgE/IgE immune complexes, IgG1 isotype control and IgE, or IgE alone. Mice were sacrificed 2 hours after feeding to determine serum levels and biologic activity of absorbed TNP-specific IgE.
Results
As expected, the absorption of maternal OVA-specific IgG1 in FcRn−/− offspring was at levels 103–104 less than observed in FcRn+/+ or FcRn+/− offspring. Surprisingly, FcRn expression also influenced the absorption of maternal IgE. OVA-specific IgE was detected in FcRn+/+ and FcRn+/− offspring, but not in FcRn−/− offspring. IgG1 anti-IgE/IgE immune complexes were detected in allergic foster mothers and correlated strongly with levels in FcRn+/+ and FcRn+/− offspring (rho=0.88, P <0.0001). Furthermore, FcRn expression was required for neonatal mice to absorb TNP-specific IgE when fed as IgG1 anti-IgE/IgE immune complexes. When immune complexes were generated with IgG1 anti-IgE directed against the Cε4 domain, the absorbed IgE was able to function in antigen-dependent basophil degranulation.
Conclusions and Clinical Relevance
These data demonstrate a novel mechanism by which FcRn may facilitate absorption of maternal antibodies other than IgG. These findings are clinically relevant because FcRn mediates the transplacental passage of maternal IgG to the fetus. This raises the possibility that FcRn could mediate the transplacental passage of maternal IgE as IgG anti-IgE/IgE immune complexes.
Keywords: Allergy, sensitization, autoantibodies, placenta, maternal transmission
INTRODUCTION
The acquisition of maternal IgG provides offspring with short-term protective immunity during a period of neonatal immune deficiency and maturation. The specific receptor mediating the transport of maternal IgG is the neonatal Fc receptor (FcRn) [1], a β2-microglobulin-associated MHC-class-I like molecule [2] that also regulates IgG catabolism through its expression on the vascular endothelium [3;4] and possibly several other cell types [5;6]. In humans and rodents, FcRn mediates the transport of maternally derived IgG in ingested milk across the epithelial-cell layer of the proximal small intestine [7–11]. A substantial amount of maternal IgG is also transported in utero, with receptors localized to the syncytiotrophoblast of the human placenta [12;13] and the yolk sac endoderm in mice [14].
Recently, it has been demonstrated that murine FcRn mediates the bidirectional transport of IgG across epithelial barriers [15;16]. This finding has extended FcRn’s role in immunity, as the bidirectional transport of IgG confers the ability to retrieve intestinal luminal antigens complexed with IgG and deposit them into the intestinal mucosa for processing by local immune cells [16;17]. It is unclear if FcRn localized to the syncytiotrophoblast of the placental villi exhibits similar transport properties, however several studies suggest the transplacental passage of several antigens may be facilitated by IgG [18;19]. Using an ex vivo placental perfusion model, Szepfalusi et al. demonstrated the placental transport of inhalant and nutritive allergens is increased in the presence of human immunoglobulin [20]. In addition, the transplacental passage of exogenous insulin from mother to fetus is associated with the presence of anti-insulin antibodies, suggesting in this situation that insulin can cross the placental barrier as IgG-insulin immune complexes [21].
Despite the finding that all 5 classes of antibodies are variably present in the serum of newborns [22;23], the mechanism(s) responsible for acquisition of maternal antibody isotypes other than IgG are not fully understood. Using a murine model of ovalbumin (OVA)-induced allergic airway disease (AAD) we previously demonstrated that allergen-specific IgG1 and IgE are absorbed from the neonatal gastrointestinal tract into the systemic circulation of naïve mice nursed by allergic mothers [24;25]. In this report, we demonstrate the absorption of allergen-specific IgE by breastfed offspring was dependent on offspring FcRn expression. Because it is generally thought that FcRn does not bind IgE [7;26], we hypothesized that IgE could be absorbed from the milk of allergic mothers as IgG anti-IgE/IgE immune complexes. To investigate this possibility we demonstrated that IgG1 anti-IgE/IgE immune complexes were present in the serum of allergic mothers and correlated strongly with the serum concentration of IgG1 anti-IgE/IgE immune complexes in breastfed offspring. Furthermore, in neonatal mice fed IgG1 anti-IgE/IgE immune complexes, the ability to absorb IgE into the systemic circulation was dependent on FcRn. Our results suggest a mechanism by which FcRn may facilitate the absorption of maternal antibodies other than IgG.
METHODS
Animals
C57BL/6J-wildtype or -FcRn-deficient (FcRn−/−) mice were obtained from Jackson Laboratories (Bar Harbor, ME) or bred in our colony at the University of CT Health Center. All mice were fed sterile food and water, and housed in microisolators under pathogen-free conditions. Their care was in accordance with institutional and Office of Laboratory Animal Welfare guidelines. To distinguish FcRn+/+, FcRn+/−, and FcRn−/− mice, genomic DNA was isolated from tail pieces and PCR was performed as described [27].
Generation of allergic foster mothers
Maternal AAD was generated in 5–6 week old female C57BL/6J wildtype mice with two weekly immunizations by intraperitoneal (i.p.) injection of 8 μg or 0.32 μg/gram body weight OVA (grade V, Sigma Chemical Co., St. Louis, MO) adsorbed to 2 mg or 0.08 mg/gram body weight Al(OH)3. Seven to 19 days following the second immunization, animals were exposed daily to aerosolized antigen generated from 1% OVA in normal saline with a Bioaerosol Nebulizing Generator (BANG, CH Technologies, Inc., Westwood, NJ). Exposures were 1 hour for 7 consecutive days delivered via a nose-only inhalation exposure chamber (In-Tox Products, Moriarty, NM). Fifty-two to 63 days after the primary aerosol exposure, females were bred with naïve C57BL/6J males. Pregnant mice were subjected to a secondary challenge with aerosolized OVA daily, during embryonic days (E) 11–17 of pregnancy (duration of pregnancy being 19–20 days). Allergic foster mothers were screened for responsiveness to secondary aerosol challenge with OVA and only those with serum concentrations of OVA-specific IgE ≥ 3000 ng/ml were used in the final analysis. This ensured all breastfed offspring ingested significant amounts of OVA-specific antibodies in the breast milk.
Feeding or intraperitoneal injection of IgG1 anti-IgE/IgE immune complexes
Sixteen to 18 day old FcRn+/− naïve neonatal mice were gavage fed 100 μg mouse TNP-specific IgE (C38-2) (BD Pharmingen, San Diego, CA) in equal molar amounts (79 μg) of rat IgG1 anti-mouse IgE (R35-72) (BD Pharmingen) or rat IgG1 isotype control (eBRG1) (eBioscience, San Diego, CA), or alone in PBS. As a positive control for the systemic absorption of IgE, FcRn+/− naïve neonatal mice were injected i.p. with 100 μg mouse TNP-specific IgE in equal molar amounts of rat IgG1 anti-mouse IgE (R35-72), or alone in PBS. Prior to administration, antibodies were dialyzed in molecularporous membrane tubing (12–14,000 MWCO, Spectrum Laboratories, Rancho Dominuez, CA) against PBS to remove sodium azide and concentrated by centrifugation at 3,000 × g in Centriplus Centrifugal Filter Devices (10,000 MWCO, Millipore, Billerica, MA). Antibody mixtures were incubated for 1 hour at room temperature to allow formation of IgG1 anti-IgE/IgE immune complexes. Formation of immune complexes was confirmed by Western blot analysis using goat anti-mouse IgG Horseradish Peroxidase Conjugate (H2708) (Southern Biotech, Birmingham, AL) essentially as described [28]. In some experiments, 11 to 12 day old FcRn+/− or FcRn−/− naïve neonatal mice were gavage fed 50 μg tracer mouse OVA-specific IgG1 (3G3A7, obtained through Dr. Lester Kobzik, Harvard School of Public Health, Boston, MA; produced by Bio X Cell, West Lebanon, NH) at the same time as IgG1 anti-IgE/IgE immune complexes.
To determine the biologic activity of absorbed IgE, rat IgG1 anti-IgE (B1E3, kind gift from Dr. Dan Conrad, Virginia Commonwealth University, Richmond, VA) directed against the Cε4 domain, a region not involved in the binding of IgE to FcεRI [29], was used to generate IgG1 anti-IgE/IgE immune complexes exactly as described above. Thirteen day old C57BL/6J mice were gavage fed 200 μg mouse TNP-specific IgE in equal molar amounts (158 μg) of rat IgG1 anti-IgE (B1E3) or IgG1 isotype control (eBRG1), or fed 158 μg of rat IgG1 anti-IgE (B1E3) alone. Two hours following feeding or injection of antibodies, mice were sacrificed to determine serum concentrations of antibodies or to prepare serum for use in the basophil mediator release assay.
Determination of antigen-specific Ig levels
Serum OVA-specific Ig levels were measured by ELISA as previously described [27]. Limits of detection for OVA-specific IgG1 and IgE in the serum were 60 ng/ml and 20 ng/ml respectively. Serum TNP-specific IgE levels were measured by ELISA using BD Falcon Microtest™ plates (BD Falcon, Franklin Lakes, NJ) coated with TNP(14)-OVA (Bioresearch Technologies, Novato, CA), at 2 μg/ml in 0.1 M carbonate (pH 9.5) for 16 hours at 4 °C. After blocking non-specific binding, TNP-specific antibodies were captured in duplicate, as 3–4, two-fold serial dilutions of serum. Detection of IgE antibodies was with goat anti-mouse IgE-HRP (Southern Biotech). Development was with the TMB microwell peroxidase substrate system (Kirkegaard & Perry Laboratories, Gaithersburg, MD) and A450 measured with a Biorad Model 480 microplate reader (Hercules, CA). The limit of detection for TNP-specific IgE in the serum was 4 ng/ml.
Assay of IgG1 anti-IgE/IgE immune complexes
BD Falcon Microtest™ plates were coated with goat anti-mouse IgE (Southern Biotech) at 2 μg/ml in 0.1 M Carbonate (pH 9.5) for 16 hours at 4 °C. After blocking non-specific binding, IgE antibodies were captured in duplicate, as 3–4, two-fold serial dilutions of serum. Detection of IgG1 anti-IgE/IgE immune complexes was with Biotin-SP-conjugated goat anti-mouse IgG Fcγ Subclass 1 Specific (Jackson ImmunoResearch, West Grove, PA) followed by Avidin-Horseradish Peroxidase (BD Pharmingen). Plates were developed with the TMB microwell peroxidase substrate system and measured with a microplate reader as above. As a reference standard, adjacent wells on the same plates were coated with OVA at 10 μg/ml in PBS. After blocking non-specific binding, predetermined amounts of murine IgG1 anti-OVA were added as 2–3, two-fold serial dilutions in duplicate. The remainder of the assay was performed exactly as above. Serum concentrations of IgG1 anti-IgE/IgE immune complexes were calculated based on A450 relative to the reference standard. The limit of detection was 30 ng/ml.
Determination of IgG1 anti-IgE levels
BD Falcon Microtest™ plates were coated with mouse monoclonal IgE (E-G5) (Chondrex, Redmond, WA) at 10 μg/ml in 0.1 M Carbonate (pH 9.5) for 16 hours at 4 °C. After blocking non-specific binding, IgG1 anti-IgE antibodies were captured in duplicate, as 3–4, two-fold serial dilutions of serum. Detection of IgG1 anti-IgE was with Biotin-SP-conjugated goat anti-mouse IgG Fcγ Subclass 1 Specific (Jackson ImmunoResearch) followed by Avidin-Horseradish Peroxidase (BD Pharmingen). Similar to the immune complex assay, adjacent wells on the same plates were coated with OVA at 10 μg/ml in PBS and predetermined amounts of murine IgG1 anti-OVA were used as a reference standard.
Rat basophil mediator release assay
RBL-2H3, rat basophil leukemia cells were purchased from the American Type Culture Collection (ATCC) and maintained in Eagle’s MEM with 10% fetal calf serum at 37°C in 5% CO2. For β-hexosaminidase release assays, RBL cells were cultured in 96-well plates at a density of 1 × 105 cells/well. Following overnight culture, the media was removed and cells were preincubated for 1 hour at 37°C with serial dilutions of serum obtained from mice previously fed IgG1 anti-IgE (B1E3)/IgE immune complexes, IgG1 isotype control and IgE, or IgG1 anti-IgE (B1E3) alone. As a positive control, RBL cells were sensitized with mouse TNP-specific IgE (1 μg/ml) diluted in culture media. The cells were washed four times with Tyrode’s salt solution (Sigma-Aldrich, St. Louis, MO) and degranulation was induced by adding TNP-OVA (5 μg/ml) in Tyrode’s salt solution for 1 hour at 37°C. Spontaneous release of β-hexosaminidase was determined by adding naïve serum in similar dilutions as experimental specimens to the RBL cells followed by TNP-OVA, or by adding TNP-OVA in the absence of serum or IgE. Total release was obtained by adding 1% Triton X-100 (Sigma-Aldrich) to the buffer. Enzymatic activity of released β-hexosaminidase was measured by mixing 30 μl of culture supernatant with 50 μl of p-nitrophenyl-N-acetyl β-D-glucosamide (Sigma-Aldrich) (1.3 mg/ml in 0.1 M citric acid buffer, pH 4.5). Following one hour of incubation at 37°C, the reaction was stopped with the addition of 100 μl of 0.2 M glycine pH 10.7 and absorbance was measured at 415 nm. After subtraction of the spontaneous release, results were expressed as percentage of the total release.
Statistical analysis
Results are expressed as mean ± standard error of the mean (SEM). Differences in antibody levels between groups were determined using nonparametric Mann-Whitney or Kruskal-Wallis tests. When no antigen-specific antibodies were detected in serum samples, values corresponding to the assay’s limit of detection were used in the statistical analysis. Correlation analysis was performed using Spearman’s rank correlation. Comparisons in the ability of neonatal serum to sensitize RBL cells and promote β-hexosaminidase release were performed using ANOVA and Tukey’s post-test. All statistical comparisons were performed with Prism 4 (GraphPad Software, San Diego, CA). Statistical significance was defined as a P value ≤ 0.05.
RESULTS
Adoptive nursing strategy
The strategy to determine the role of offspring FcRn in the ability to absorb allergen-specific Igs from the milk of allergic mothers has been previously described [27]. Naive C57BL/6J-FcRn+/− females were mated to C57BL/6J-FcRn−/− males, generating FcRn+/− or FcRn−/− progeny. Within 24 hours of delivery, pups with or without FcRn were adoptively nursed by OVA-immune (allergic) foster mothers. Using this strategy where all fostered pups were born to naïve mothers, acquisition of maternal allergen-specific Igs is restricted to breast milk. In this experiment, FcRn−/− offspring were expected to have reduced systemic levels of OVA-specific IgG1 as a consequence of decreased absorption of maternal IgG from the lumen of the neonatal gastrointestinal tract [10;27].
Absorption of maternal OVA-specific IgE was dependent on offspring FcRn
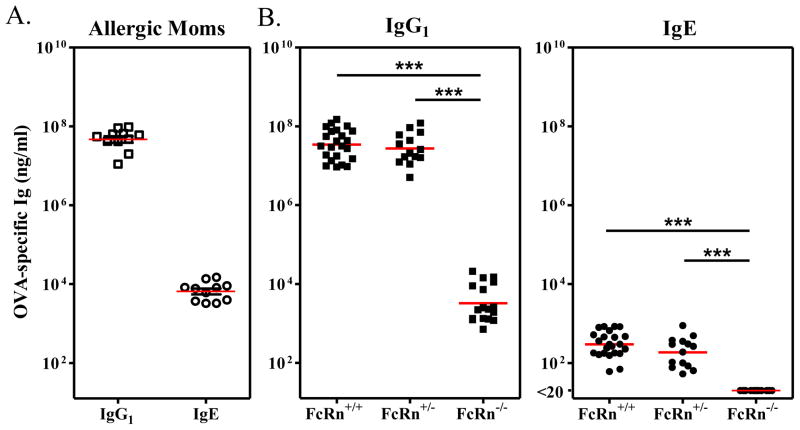
As anticipated at weaning, FcRn+/+ or FcRn+/− pups had similar OVA-specific IgG1 serum concentrations (4.9 ± 0.8 × 107 ng/ml and 3.9 ± 0.9 × 107 ng/ml respectively) as their allergic foster mothers (5.4 ± 0.8 × 107 ng/ml). In contrast, FcRn−/− pups displayed significantly reduced OVA-specific IgG1 serum concentrations (<6.0 ± 1.6 × 103 ng/ml; P < 0.001) (Fig. 1). These findings were consistent with our previous report which demonstrates that there is decreased absorption of maternal IgG across the neonatal intestinal tract in FcRn−/− mice [27]. However, to our surprise, FcRn expression also influenced the ability of offspring to absorb maternal OVA-specific IgE. While FcRn+/+ and FcRn+/− offspring had similar OVA-specific IgE serum concentrations at weaning (380 ± 54 ng/ml and 261 ± 61 ng/ml respectively), OVA-specific IgE was not detected in the serum of FcRn−/− offspring (P < 0.001), even though they were foster nursed by the same allergic mothers. Thus, the transfer of antigen-specific antibodies (irrespective of Ig isotype [IgG1 or IgE]) from allergic mothers to breastfed offspring was dependent on offspring FcRn expression.
Figure 1.
Absorption of OVA-specific maternal antibodies was dependent on offspring FcRn. Serum concentrations of OVA-specific IgG1 or IgE antibodies in (A) allergic foster mothers and (B) FcRn+/+, FcRn+/−, or FcRn−/− breastfed offspring are shown. Serum concentrations of OVA-specific IgG1 were significantly lower in FcRn−/− offspring when compared to FcRn+/+ or FcRn+/− offspring. OVA-specific IgE was detected in the serum of FcRn+/+ and FcRn+/− offspring, but not in the serum of FcRn−/− offspring. Similar results were obtained in 2 additional experiments. *** P < 0.001.
Correlation between serum levels of IgG1 anti-IgE/IgE immune complexes in allergic foster mothers and FcRn-sufficient breastfed offspring
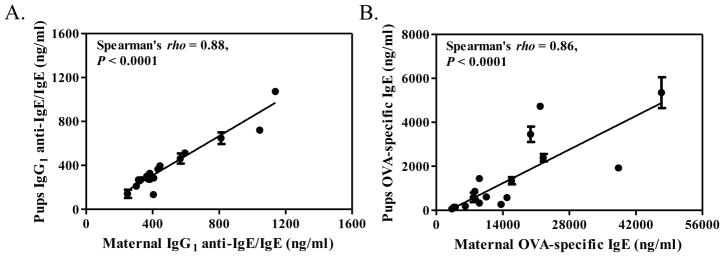
Because the mechanism by which FcRn-sufficient offspring absorbed maternal IgE was unclear, we investigated for the presence of maternal autoantibodies directed against IgE. Interestingly, there were significant quantities of IgG1 anti-IgE autoantibodies in the serum of allergic foster mothers, whereas no IgG1 anti-IgE was detected in the serum of naïve adult mice (data not shown). This suggested the possibility that maternal IgE was absorbed from the milk as IgG1-anti IgE/IgE immune complexes. To further investigate this possibility, we determined serum concentrations of IgG1 anti-IgE/IgE immune complexes in allergic foster mothers and FcRn-sufficient (FcRn+/+ and FcRn+/−) breastfed offspring. As shown in Fig. 2A, at weaning, levels of IgG1 anti-IgE/IgE in the serum of allergic foster mothers strongly correlated with levels of IgG1 anti-IgE/IgE in the serum of FcRn-sufficient breastfed offspring (rho = 0.88, P < 0.0001). Similarly, serum concentrations of OVA-specific IgE in allergic foster mothers correlated with serum concentrations of OVA-specific IgE in FcRn-sufficient breastfed offspring (rho = 0.86, P < 0.0001) (Fig. 2B).
Figure 2.
Correlation between serum concentrations of (A) IgG1 anti-IgE/IgE immune complexes and (B) OVA-specific IgE in allergic foster mothers and FcRn-sufficient breastfed offspring. Data represents results obtained at weaning in 18 allergic foster mothers and 54 FcRn-sufficient breastfed offspring. Results for offspring are grouped per litter and expressed as mean ± SEM.
Neonatal mice fed IgG1 anti-IgE/IgE immune complexes absorb IgE into the systemic circulation
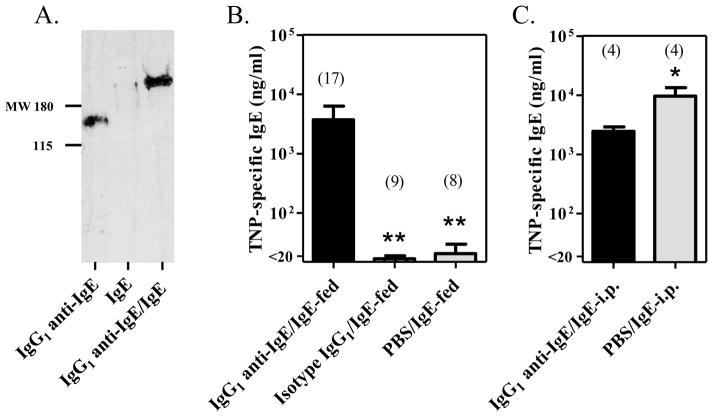
To determine the ability of FcRn-sufficient offspring to absorb IgE in the form of IgG1 anti-IgE/IgE immune complexes, 16–18 day old FcRn+/− naïve neonatal mice were gavage fed with TNP-specific IgE as IgG1 anti-IgE/IgE immune complexes, IgG1 isotype control and IgE, or IgE alone. As a positive control for the systemic absorption of IgE, FcRn+/− naïve neonatal mice were injected i.p. with TNP-specific IgE as IgG1 anti-IgE/IgE immune complexes or IgE alone. Western blot analysis confirmed the formation of immune complexes in antibody mixtures in vitro with IgG1 anti-IgE/IgE demonstrating a band of higher molecular weight than observed for IgG1 anti-IgE alone (Fig. 3A). Mice were bled 2 hours after administering the antibody mixtures to determine serum concentrations of TNP-specific IgE. As shown in Fig. 3B, FcRn+/− neonatal mice fed IgG1 anti-IgE/IgE complexes absorbed TNP-specific IgE efficiently into the systemic circulation (3733 ng/ml ± 2600 ng/ml), while those fed IgG1 isotype control and IgE, or IgE alone (17 ng/ml ± 2 ng/ml and 21 ng/ml ± 9 ng/ml respectively) demonstrated 100–200 fold lower absorption (P < 0.01). Thus, when IgE was introduced into the neonatal intestine via gavage feeding, it was absorbed most efficiently when complexed to IgG1 anti-IgE. In contrast, mice injected i.p. were able to absorb TNP-specific IgE efficiently into the systemic circulation when injected as IgE alone (9559 ng/ml ± 3780 ng/ml) or as IgG1 anti-IgE/IgE immune complexes (2448 ng/ml ± 452 ng/ml) (Fig. 3C).
Figure 3.
FcRn-sufficient neonatal mice absorbed allergen-specific IgE when fed as IgG1 anti-IgE/IgE immune complexes. (A) Western blot analysis of antibody mixtures confirming the formation of immune complexes. Molecular weight markers are in kDa. (B) Neonatal mice fed IgG1 anti-IgE/IgE immune complexes absorbed TNP-specific IgE systemically. (C) Neonatal mice injected with IgG1 anti-IgE/IgE immune complexes or IgE alone absorbed TNP-specific IgE systemically. The number of mice in each group is included. * P < 0.05, ** P ≤ 0.01.
Intestinal absorption of IgG1 anti-IgE/IgE immune complexes was dependent on offspring FcRn
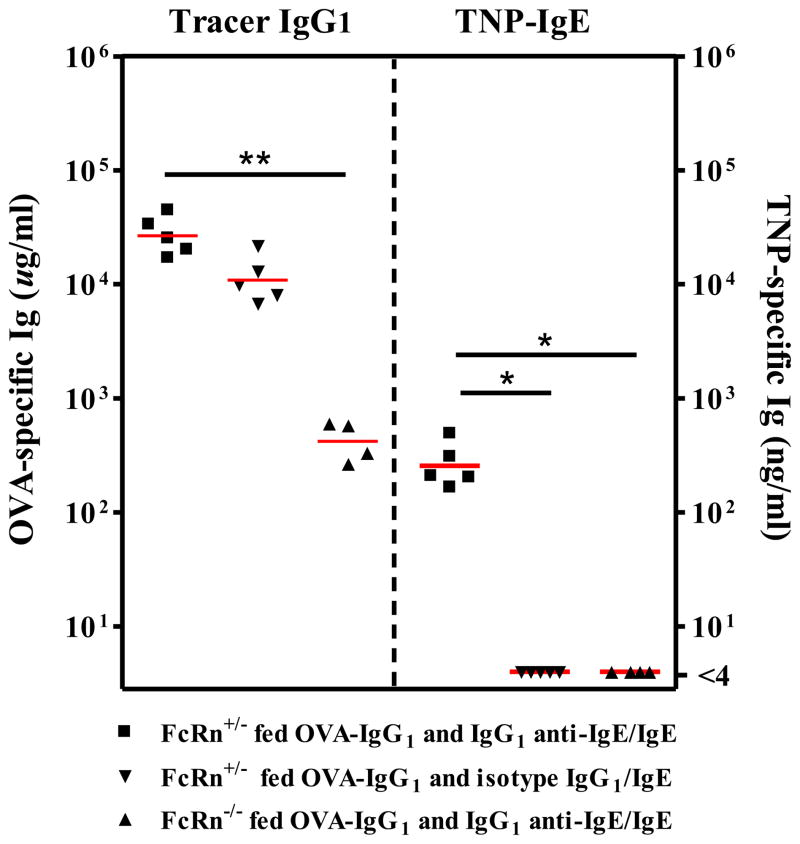
To determine the role of offspring FcRn in the ability of neonatal mice to absorb fed IgG1 anti-IgE/IgE, 11–12 day old FcRn+/− or FcRn−/− naïve neonatal mice were fed TNP-specific IgE as IgG1 anti-IgE/IgE immune complexes, or IgG1 isotype control and IgE. At the same time, mice were fed tracer OVA-specific IgG1 as a marker for intestinal IgG transport. Mice were bled 2 hours after feeding to determine serum concentrations of OVA-specific IgG1 and TNP-specific IgE. As expected, tracer OVA-specific IgG1 was absorbed efficiently into the systemic circulation of FcRn+/− neonates [those fed either IgG1 anti-IgE/IgE, or IgG1 isotype control and IgE (2.8 ± 0.5 × 104 μg/ml and 1.2 ± 0.3 × 104 μg/ml respectively)]; whereas significantly less was absorbed into the systemic circulation of FcRn−/− neonatal mice (4.4 ± 0.9 × 102 μg/ml; P < 0.01) (Fig. 4). When neonatal mice were evaluated for the ability to absorb fed TNP-specific IgE, only FcRn+/− mice fed IgG1 anti-IgE/IgE immune complexes absorbed TNP-specific IgE into the systemic circulation (277 ng/ml ± 59 ng/ml). No TNP-specific IgE was detected in the serum of FcRn+/− neonates fed IgG1 isotype control and IgE, or FcRn−/− neonates fed IgG1 anti-IgE/IgE immune complexes (P < 0.05).
Figure 4.
The ability to absorb IgE when fed as IgG1 anti-IgE/IgE immune complexes was dependent on FcRn. Tracer OVA-specific IgG1 was absorbed more efficiently into the systemic circulation of FcRn+/− neonatal mice as compared to FcRn−/− neonatal mice. FcRn expression was required for neonatal mice to absorb TNP-specific IgE when fed as IgG1 anti-IgE/IgE immune complexes. * P < 0.05, ** P ≤ 0.01. Similar results were obtained in one additional experiment.
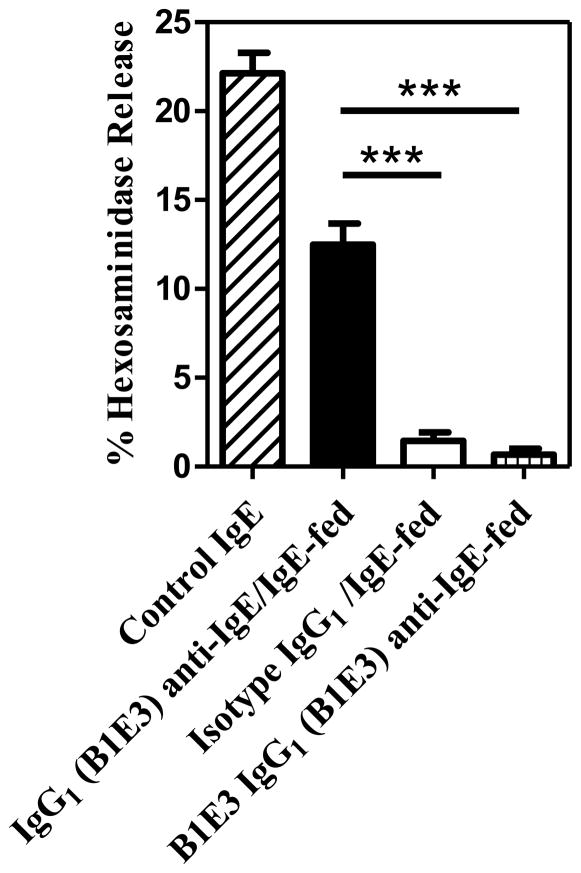
IgE absorbed as IgG1 anti-IgE/IgE immune complexes was biologically active RBL cells were preincubated with serum generated from neonatal mice previously fed IgG1 anti-IgE (B1E3)/IgE immune complexes, IgG1 isotype control and IgE, or IgG1 anti-IgE (B1E3) alone. Serum levels of TNP-specific IgE in neonatal mice fed IgG1 anti-IgE (B1E3)/IgE immune complexes were 356 ± 148 ng/ml, while those fed IgG1 isotype control and IgE, or IgG1 anti-IgE (B1E3) alone were undetectable (data not shown). As a positive control, cells were preincubated with TNP-specific IgE diluted in culture media. Degranulation of RBL cells was induced by the addition of TNP-OVA. Figure 5 demonstrates preincubation of RBL cells with a four-fold dilution of serum obtained from neonatal mice fed IgG1 anti-IgE (B1E3)/IgE immune complexes resulted in the passive sensitization of cells as indicted by the release of β-hexosaminidase following the addition of TNP-OVA. In contrast, the same dilution of serum obtained from mice fed IgG1 isotype control and IgE, or IgG1 anti-IgE (B1E3) alone resulted in little release of β-hexosaminidase following the addition of TNP-OVA, indicating no sensitization of RBL cells. There was no difference in spontaneous release of β-hexosaminidase in RBL cells preincubated with naïve serum and exposed to TNP-OVA or RBL cells exposed to TNP-OVA in the absence of serum or IgE (data not shown).
Figure 5.
Serum obtained from neonatal mice previously fed IgG1 anti-IgE (B1E3)/IgE immune complexes passively sensitized RBL cells. RBL cells on the same plate were preincubated with control IgE or serum obtained from 3 neonatal mice per group. Degranulation was induced by the addition of TNP-OVA. RBL cells preincubated with serum obtained from neonatal mice fed IgG1 anti-IgE (B1E3)/IgE immune complexes released significantly greater amounts of β-hexosaminidase than those preincubated with serum obtained from neonatal mice fed isotype control and IgE, or IgG1 anti-IgE (B1E3) alone. *** P < 0.001. Similar results were obtained in one additional experiment.
DISCUSSION
The mechanism(s) responsible for the transport of maternal IgE to offspring remain poorly defined. The importance of characterizing this process is exhibited by the increased risk for development of allergic disease in infants with elevated levels of IgE in their serum at birth [30–33]. An improved understanding of the origin of antigen-specific IgE in neonates could aid in the design and implementation of allergy prevention strategies during pregnancy or early infancy. We previously demonstrated in a murine model of OVA-induced allergic airway disease that allergen-specific IgG1 and IgE are absorbed from the neonatal gastrointestinal tract into the systemic circulation of naïve mice nursed by allergic mothers [24;25]. In the present study, we demonstrated the absorption of allergen-specific IgE by breastfed offspring was dependent on offspring FcRn expression. Although it is generally thought that FcRn does not bind IgE [7;26], our data provides compelling evidence that FcRn plays a pivotal role in the absorption of maternal IgE. These findings are relevant to humans because FcRn mediates the active transplacental passage of IgG to the fetus. This raises the possibility that FcRn could mediate the transplacental passage of IgE in humans.
The finding that FcRn was required for offspring to absorb IgE from the gastrointestinal tract was initially surprising; however further investigation suggested a potential role for maternal IgG1 anti-IgE autoantibodies in facilitating the process via the formation of IgG1 anti-IgE/IgE immune complexes. IgG1 anti-IgE autoantibodies were detected in the serum of allergic foster mothers but were absent from the serum of naïve adult mice. Furthermore, serum concentrations of IgG1 anti-IgE/IgE immune complexes in allergic foster mothers were equivalent to serum concentrations of IgG1 anti-IgE/IgE immune complexes in FcRn-sufficient breastfed offspring. Because all fostered pups were born to naïve mothers, acquisition of IgG1 anti-IgE/IgE immune complexes and OVA-specific IgE was restricted to the breast milk of allergic foster mothers. We have previously demonstrated FcRn−/− mice subjected to OVA-sensitization and OVA aerosol challenge develop equivalent OVA-specific-IgE and -IgG1 responses as wildtype mice [27]. Thus, it is unlikely that OVA-specific IgE detected in the serum of FcRn-sufficient offspring was the result of OVA transfer and sensitization.
Interestingly, the relationship between maternal and offspring IgG1 anti-IgE/IgE immune complexes was 1:1, whereas that between maternal and offspring IgE was 10:1. It is known by binding FcRn, IgG is protected from degradation and has an extended serum half-life as compared to other antibody isotypes [34]. Thus, the differences in ratios may reflect a role for FcRn in protecting IgG1 anti-IgE/IgE immune complexes from catabolism. Additional support for the ability of FcRn to facilitate absorption of IgG1 anti-IgE/IgE immune complexes was provided by gavage feeding studies, where offspring FcRn expression was required for neonatal mice to absorb TNP-specific IgE after being fed IgG1 anti-IgE/IgE immune complexes. While our study does not negate other potential mechanisms involved in the intestinal absorption of IgE, these data suggest a previously undescribed mechanism by which FcRn facilitates the absorption of maternal IgE, as IgG1 anti-IgE/IgE immune complexes.
The existence of autoantibodies to IgG was first described by Waaler et al. [34], and since then autoantibodies against all other antibody isotypes have been described [35]. Of particular interest, is the finding that circulating IgG anti-IgE exist in a large proportion of patients with atopic disease [36–38]. Up to 70–95% of patients with allergic asthma have detectable levels of IgG anti-IgE compared to 32% of non-allergic healthy individuals [39;40]. Similarly, IgG anti-IgE is present in the serum of up to 86% of patients with atopic dermatitis and exists mainly as immune complexes with self-IgE [41].
Although the finding of anti-IgE antibodies in humans initially evoked considerable interest, subsequent studies have failed to demonstrate consistent physiologic benefits or detriment associated with their presence. In some patients, circulating fractions of IgG anti-IgE appear directed against the Cε2–Cε3 interdomain region within the IgE molecule [42]. This region is believed to contain at least part of the FcεRI binding site, and antibodies with this specificity could potentially block IgE binding to FcεRI. However, other specificities of circulating IgG anti-IgE may facilitate crosslinking of IgE bound to the surface of basophils or mast cells, thereby triggering histamine release and exacerbation of allergic symptoms [43]. Hence, the ability of maternal IgG anti-IgE/IgE immune complexes to influence fetal or neonatal T cell priming may depend on the binding site within the IgE molecule and whether IgE (bound by IgG anti-IgE) retains the capacity to bind FcεRI. Our results from the rat basophil mediator release assay were supportive of this concept. The ability of absorbed IgE to function in antigen-dependent basophil degranulation was dependent on the epitope specificity of the IgG anti-IgE. After gavage feeding immune complexes generated using IgG1 anti-IgE directed against the Cε4 domain, a region not involved in IgE binding to FcεRI [29], the absorbed IgE in the neonatal serum passively sensitized RBL cells and induced degranulation upon antigen exposure. It is also known that binding of allergen-specific IgE to the surface of antigen-presenting cells (APCs) via FcεRI, optimizes the capacity for allergen presentation and the ability of APCs to elicit T cell responses [44;45]. Given that antigen presentation is functionally immature in neonatal life [46–48]; maternal IgE that is transferred to offspring in the form of IgG anti-IgE/IgE immune complexes may bind to the surface of neonatal APCs and provide a stronger adjuvant stimulus to initiate T cell priming [49].
The acquisition of maternal IgG is generally regarded as beneficial to the neonate [27;50]; however several circumstances demonstrate undesirable effects mediated by maternal IgG. Congenital heart block, neonatal myasthenia Gravis, and erythroblastosis fetalis are disorders of the fetal and neonatal periods mediated by pathogenic maternal IgG [51–53]. In such instances, the apparent inability of FcRn to discriminate between pathogenic IgG and those mediating protective immunity results in the transfer of IgG with binding specificity directed against endogenous antigens expressed by fetal and neonatal cells. From a therapeutic standpoint, inhibition of FcRn-mediated transfer of pathogenic maternal IgG could prevent specific disease processes in infants and children.
The idea that FcRn can transport IgG-antigen immune complexes is not new [16;17]; however, the concept that FcRn can mediate the absorption of maternal IgG anti-IgE/IgE immune complexes is novel. Importantly, because FcRn is expressed within the human placenta, our findings raise the possibility that FcRn may mediate the transplacental passage of IgG anti-IgE/IgE immune complexes. Although it is generally accepted that IgG is the only maternal antibody isotype capable of crossing the placental barrier, our results imply that in situations where high levels of maternal IgE and IgG anti-IgE coexist, the formation of IgG anti-IgE/IgE immune complexes may occur with the potential for transplacental passage. A similar situation has been reported for the transplacental passage of exogenous insulin from mother to fetus, mediated by IgG anti-insulin/insulin immune complexes [21]. These findings are particularly relevant because like free IgE, the human placenta is impermeable to free insulin [54]. Furthermore, the insulin transferred to the fetus retains its biologic activity, as cord blood insulin concentrations correlate with development of fetal macrosomia [21]. Given that IgG anti-IgE/IgE immune complexes are found in a large percentage of atopic adults [41], we speculate a similar mechanism exists for the transplacental passage of maternal IgE, mediated via FcRn. Additional studies are needed to substantiate this hypothesis, however if confirmed may provide the basis for subsequent efforts focused on reducing maternal IgE transmission as a means of allergy prevention.
Acknowledgments
The authors thank Elizabeth G. Lingenheld and Li Zhu for their assistance. We are grateful to Roger Thrall for his critical evaluation of the manuscript, and to Michelle Cloutier for her helpful advice. This work was funded by National Institutes of Health grants K08AI071918 (to Adam P. Matson) and R01HL080508 and R21AI092060 (to Lynn Puddington).
Footnotes
CONFLICT OF INTEREST
The authors have no conflict of interest with any of the material reported in this manuscript.
References
- 1.Simister NE, Rees AR. Isolation and characterization of an Fc receptor from neonatal rat small intestine. Eur J Immunol. 1985;15:733–8. doi: 10.1002/eji.1830150718. [DOI] [PubMed] [Google Scholar]
- 2.Simister NE, Mostov KE. An Fc receptor structurally related to MHC class I antigens. Nature. 1989;337:184–7. doi: 10.1038/337184a0. [DOI] [PubMed] [Google Scholar]
- 3.Ward ES, Zhou J, Ghetie V, Ober RJ. Evidence to support the cellular mechanism involved in serum IgG homeostasis in humans. Int Immunol. 2003;15:187–95. doi: 10.1093/intimm/dxg018. [DOI] [PubMed] [Google Scholar]
- 4.Borvak J, Richardson J, Medesan C, Antohe F, Radu C, Simionescu M, Ghetie V, Ward ES. Functional expression of the MHC class I-related receptor, FcRn, in endothelial cells of mice. Int Immunol. 1998;10:1289–98. doi: 10.1093/intimm/10.9.1289. [DOI] [PubMed] [Google Scholar]
- 5.Akilesh S, Christianson GJ, Roopenian DC, Shaw AS. Neonatal FcR expression in bone marrow-derived cells functions to protect serum IgG from catabolism. J Immunol. 2007;179:4580–8. doi: 10.4049/jimmunol.179.7.4580. [DOI] [PubMed] [Google Scholar]
- 6.Haymann JP, Levraud JP, Bouet S, Kappes V, Hagege J, Nguyen G, Xu Y, Rondeau E, Sraer JD. Characterization and localization of the neonatal Fc receptor in adult human kidney. J Am Soc Nephrol. 2000;11:632–9. doi: 10.1681/ASN.V114632. [DOI] [PubMed] [Google Scholar]
- 7.Jones EA, Waldmann TA. The mechanism of intestinal uptake and transcellular transport of IgG in the neonatal rat. J Clin Invest. 1972;51:2916–27. doi: 10.1172/JCI107116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodewald R, Kraehenbuhl JP. Receptor-mediated transport of IgG. J Cell Biol. 1984;99:159s–64s. doi: 10.1083/jcb.99.1.159s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Israel EJ, Taylor S, Wu Z, Mizoguchi E, Blumberg RS, Bhan A, Simister NE. Expression of the neonatal Fc receptor, FcRn, on human intestinal epithelial cells. Immunology. 1997;92:69–74. doi: 10.1046/j.1365-2567.1997.00326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roopenian DC, Christianson GJ, Sproule TJ, Brown AC, Akilesh S, Jung N, Petkova S, Avanessian L, Choi EY, Shaffer DJ, Eden PA, Anderson CL. The MHC class I-like IgG receptor controls perinatal IgG transport, IgG homeostasis, and fate of IgG-Fc-coupled drugs. J Immunol. 2003;170:3528–33. doi: 10.4049/jimmunol.170.7.3528. [DOI] [PubMed] [Google Scholar]
- 11.He W, Ladinsky MS, Huey-Tubman KE, Jensen GJ, McIntosh JR, Bjorkman PJ. FcRn-mediated antibody transport across epithelial cells revealed by electron tomography. Nature. 2008;455:542–6. doi: 10.1038/nature07255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simister NE, Story CM, Chen HL, Hunt JS. An IgG-transporting Fc receptor expressed in the syncytiotrophoblast of human placenta. Eur J Immunol. 1996;26:1527–31. doi: 10.1002/eji.1830260718. [DOI] [PubMed] [Google Scholar]
- 13.Leach JL, Sedmak DD, Osborne JM, Rahill B, Lairmore MD, Anderson CL. Isolation from human placenta of the IgG transporter, FcRn, and localization to the syncytiotrophoblast: implications for maternal-fetal antibody transport. J Immunol. 1996;157:3317–22. [PubMed] [Google Scholar]
- 14.Kim J, Mohanty S, Ganesan LP, Hua K, Jarjoura D, Hayton WL, Robinson JM, Anderson CL. FcRn in the yolk sac endoderm of mouse is required for IgG transport to fetus. J Immunol. 2009;182:2583–9. doi: 10.4049/jimmunol.0803247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Claypool SM, Dickinson BL, Wagner JS, Johansen FE, Venu N, Borawski JA, Lencer WI, Blumberg RS. Bidirectional transepithelial IgG transport by a strongly polarized basolateral membrane Fcgamma-receptor. Mol Biol Cell. 2004;15:1746–59. doi: 10.1091/mbc.E03-11-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshida M, Claypool SM, Wagner JS, Mizoguchi E, Mizoguchi A, Roopenian DC, Lencer WI, Blumberg RS. Human neonatal Fc receptor mediates transport of IgG into luminal secretions for delivery of antigens to mucosal dendritic cells. Immunity. 2004;20:769–83. doi: 10.1016/j.immuni.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 17.Yoshida M, Kobayashi K, Kuo TT, Bry L, Glickman JN, Claypool SM, Kaser A, Nagaishi T, Higgins DE, Mizoguchi E, Wakatsuki Y, Roopenian DC, Mizoguchi A, Lencer WI, Blumberg RS. Neonatal Fc receptor for IgG regulates mucosal immune responses to luminal bacteria. J Clin Invest. 2006;116:2142–51. doi: 10.1172/JCI27821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maidji E, McDonagh S, Genbacev O, Tabata T, Pereira L. Maternal antibodies enhance or prevent cytomegalovirus infection in the placenta by neonatal Fc receptor-mediated transcytosis. Am J Pathol. 2006;168:1210–26. doi: 10.2353/ajpath.2006.050482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.May K, Grube M, Malhotra I, Long CA, Singh S, Mandaliya K, Siegmund W, Fusch C, Schneider H, King CL. Antibody-dependent transplacental transfer of malaria blood-stage antigen using a human ex vivo placental perfusion model. PLoS One. 2009;4:e7986. doi: 10.1371/journal.pone.0007986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szepfalusi Z, Loibichler C, Pichler J, Reisenberger K, Ebner C, Urbanek R. Direct evidence for transplacental allergen transfer. Pediatr Res. 2000;48:404–7. doi: 10.1203/00006450-200009000-00024. [DOI] [PubMed] [Google Scholar]
- 21.Menon RK, Cohen RM, Sperling MA, Cutfield WS, Mimouni F, Khoury JC. Transplacental passage of insulin in pregnant women with insulin-dependent diabetes mellitus. Its role in fetal macrosomia. N Engl J Med. 1990;323:309–15. doi: 10.1056/NEJM199008023230505. [DOI] [PubMed] [Google Scholar]
- 22.Buckley RH, Dees SC, O’Fallon WM. Serum immunoglobulins. I. Levels in normal children and in uncomplicated childhood allergy. Pediatrics. 1968;41:600–11. [PubMed] [Google Scholar]
- 23.Avrech OM, Samra Z, Lazarovich Z, Caspi E, Jacobovich A, Sompolinsky D. Efficacy of the placental barrier for immunoglobulins: correlations between maternal, paternal and fetal immunoglobulin levels. Int Arch Allergy Immunol. 1994;103:160–5. doi: 10.1159/000236622. [DOI] [PubMed] [Google Scholar]
- 24.Matson AP, Zhu L, Lingenheld EG, Schramm CM, Clark RB, Selander DM, Thrall RS, Breen E, Puddington L. Maternal transmission of resistance to development of allergic airway disease. J Immunol. 2007;179:1282–91. doi: 10.4049/jimmunol.179.2.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matson AP, Thrall RS, Rafti E, Puddington L. Breastmilk from allergic mothers can protect offspring from allergic airway inflammation. Breastfeed Med. 2009;4:167–74. doi: 10.1089/bfm.2008.0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shields RL, Namenuk AK, Hong K, Meng YG, Rae J, Briggs J, Xie D, Lai J, Stadlen A, Li B, Fox JA, Presta LG. High resolution mapping of the binding site on human IgG1 for Fc gamma RI, Fc gamma RII, Fc gamma RIII, and FcRn and design of IgG1 variants with improved binding to the Fc gamma R. J Biol Chem. 2001;276:6591–604. doi: 10.1074/jbc.M009483200. [DOI] [PubMed] [Google Scholar]
- 27.Matson AP, Thrall RS, Rafti E, Lingenheld EG, Puddington L. IgG transmitted from allergic mothers decreases allergic sensitization in breastfed offspring. Clin Mol Allergy. 2010;8:9. doi: 10.1186/1476-7961-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parr EL, Bozzola JJ, Parr MB. Purification and measurement of secretory IgA in mouse milk. J Immunol Methods. 1995;180:147–57. doi: 10.1016/0022-1759(94)00310-s. [DOI] [PubMed] [Google Scholar]
- 29.Keegan AD, Fratazzi C, Shopes B, Baird B, Conrad DH. Characterization of new rat anti-mouse IgE monoclonals and their use along with chimeric IgE to further define the site that interacts with Fc epsilon RII and Fc epsilon RI. Mol Immunol. 1991;28:1149–54. doi: 10.1016/0161-5890(91)90030-n. [DOI] [PubMed] [Google Scholar]
- 30.Tariq SM, Arshad SH, Matthews SM, Hakim EA. Elevated cord serum IgE increases the risk of aeroallergen sensitization without increasing respiratory allergic symptoms in early childhood. Clin Exp Allergy. 1999;29:1042–8. doi: 10.1046/j.1365-2222.1999.00594.x. [DOI] [PubMed] [Google Scholar]
- 31.Odelram H, Bjorksten B, Leander E, Kjellman NI. Predictors of atopy in newborn babies. Allergy. 1995;50:585–92. doi: 10.1111/j.1398-9995.1995.tb01204.x. [DOI] [PubMed] [Google Scholar]
- 32.Hansen LG, Halken S, Host A, Moller K, Osterballe O. Prediction of allergy from family history and cord blood IgE levels. A follow-up at the age of 5 years. Cord blood IgE. IV. Pediatr Allergy Immunol. 1993;4:34–40. doi: 10.1111/j.1399-3038.1993.tb00063.x. [DOI] [PubMed] [Google Scholar]
- 33.Edenharter G, Bergmann RL, Bergmann KE, Wahn V, Forster J, Zepp F, Wahn U. Cord blood-IgE as risk factor and predictor for atopic diseases. Clin Exp Allergy. 1998;28:671–8. doi: 10.1046/j.1365-2222.1998.00241.x. [DOI] [PubMed] [Google Scholar]
- 34.Waaler E. On the occurrence of a factor in human serum activating the specific agglutintion of sheep blood corpuscles. 1939. APMIS. 2007;115:422–38. doi: 10.1111/j.1600-0463.2007.apm_682a.x. [DOI] [PubMed] [Google Scholar]
- 35.Johansson SG. Anti-IgE antibodies in human serum. J Allergy Clin Immunol. 1986;77:555–7. doi: 10.1016/0091-6749(86)90344-1. [DOI] [PubMed] [Google Scholar]
- 36.Boluda L, Berrens L. Do IgE-IgG complexes occur in the circulation? Clin Exp Immunol. 1995;100:145–50. doi: 10.1111/j.1365-2249.1995.tb03616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shakib F, Sihoe J, Smith SJ, Wilding P, Clark MM, Knox A. Circulating levels of IgG1 and IgG4 anti-IgE antibodies and asthma severity. Allergy. 1994;49:192–5. doi: 10.1111/j.1398-9995.1994.tb00825.x. [DOI] [PubMed] [Google Scholar]
- 38.Shakib F, Smith SJ, Pritchard DI. Do autoantibodies to IgE play a role in IgE-mediated events? Immunol Cell Biol. 1995;73:109–12. doi: 10.1038/icb.1995.18. [DOI] [PubMed] [Google Scholar]
- 39.Inganas M, Johansson SG, Bennich H. Anti-IgE antibodies in human serum: occurrence and specificity. Int Arch Allergy Appl Immunol. 1981;65:51–61. doi: 10.1159/000232737. [DOI] [PubMed] [Google Scholar]
- 40.Nawata Y, Koike T, Yanagisawa T, Iwamoto I, Itaya T, Yoshida S, Tomioka H. Anti-IgE autoantibody in patients with bronchial asthma. Clin Exp Immunol. 1984;58:348–56. [PMC free article] [PubMed] [Google Scholar]
- 41.Nawata Y, Koike T, Hosokawa H, Tomioka H, Yoshida S. Anti-IgE autoantibody in patients with atopic dermatitis. J Immunol. 1985;135:478–82. [PubMed] [Google Scholar]
- 42.Shakib F, Powell-Richards A. Elucidation of the epitope locations of human autoanti-IgE: recognition of two epitopes located within the C epsilon 2 and the C epsilon 4 domains. Int Arch Allergy Appl Immunol. 1991;95:102–8. doi: 10.1159/000235413. [DOI] [PubMed] [Google Scholar]
- 43.Shakib F, Smith SJ. In vitro basophil histamine-releasing activity of circulating IgG1 and IgG4 autoanti-IgE antibodies from asthma patients and the demonstration that anti-IgE modulates allergen-induced basophil activation. Clin Exp Allergy. 1994;24:270–5. doi: 10.1111/j.1365-2222.1994.tb00230.x. [DOI] [PubMed] [Google Scholar]
- 44.Maurer D, Ebner C, Reininger B, Fiebiger E, Kraft D, Kinet JP, Stingl G. The high affinity IgE receptor (Fc epsilon RI) mediates IgE-dependent allergen presentation. J Immunol. 1995;154:6285–90. [PubMed] [Google Scholar]
- 45.Bieber T. The pro- and anti-inflammatory properties of human antigen-presenting cells expressing the high affinity receptor for IgE (Fc epsilon RI) Immunobiology. 2007;212:499–503. doi: 10.1016/j.imbio.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 46.Petty RE, Hunt DW. Neonatal dendritic cells. Vaccine. 1998;16:1378–82. doi: 10.1016/s0264-410x(98)00095-4. [DOI] [PubMed] [Google Scholar]
- 47.Katamura K, Tabata Y, Oshima Y, Shintaku N, Yamauchi Y, Mayumi M. Selective induction of interleukin-4- and interferon-y-producing T cells from cord blood naive T cells. Effects of costimulatory signaling through CD28. Int Arch Allergy Immunol. 1995;106:101–6. doi: 10.1159/000236828. [DOI] [PubMed] [Google Scholar]
- 48.Adkins B, LeClerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004;4:553–64. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- 49.Holt PG. Prenatal versus postnatal priming of allergen specific immunologic memory: the debate continues. J Allergy Clin Immunol. 2008;122:717–8. doi: 10.1016/j.jaci.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 50.Zinkernagel RM. Maternal antibodies, childhood infections, and autoimmune diseases. N Engl J Med. 2001;345:1331–5. doi: 10.1056/NEJMra012493. [DOI] [PubMed] [Google Scholar]
- 51.Karnabi E, Qu Y, Wadgaonkar R, Mancarella S, Yue Y, Chahine M, Clancy RM, Buyon JP, Boutjdir M. Congenital heart block: identification of autoantibody binding site on the extracellular loop (domain I, S5–S6) of alpha(1D) L-type Ca channel. J Autoimmun. 2010;34:80–6. doi: 10.1016/j.jaut.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Batocchi AP, Majolini L, Evoli A, Lino MM, Minisci C, Tonali P. Course and treatment of myasthenia gravis during pregnancy. Neurology. 1999;52:447–52. doi: 10.1212/wnl.52.3.447. [DOI] [PubMed] [Google Scholar]
- 53.Smits-Wintjens VE, Walther FJ, Lopriore E. Rhesus haemolytic disease of the newborn: Postnatal management, associated morbidity and long-term outcome. Semin Fetal Neonatal Med. 2008;13:265–71. doi: 10.1016/j.siny.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 54.Buse MG, Roberts WJ, Buse J. The role of the human placenta in the transfer and metabolism of insulin. J Clin Invest. 1962;41:29–41. doi: 10.1172/JCI104464. [DOI] [PMC free article] [PubMed] [Google Scholar]