Abstract
Scaffolding proteins are involved in the incorporation, anchoring, maintenance, and removal of AMPA receptors (AMPARs) at synapses, either through a direct interaction with AMPARs or via indirect association through auxiliary subunits of transmembrane AMPAR regulatory proteins (TARPs). Synaptic scaffolding molecule (S-SCAM) is a newly characterized member of the scaffolding proteins critical for the regulation and maintenance of AMPAR levels at synapses, and directly binds to TARPs through a PDZ interaction. However, the functional significance of S-SCAM–TARP interaction in the regulation of AMPARs has not been tested. Here we show that overexpression of the C-terminal peptide of TARP-γ2 fused to EGFP abolished the S-SCAM-mediated enhancement of surface GluA2 expression. Conversely, the deletion of the PDZ-5 domain of S-SCAM that binds TARPs greatly attenuated the S-SCAM-induced increase of surface GluA2 expression. In contrast, the deletion of the guanylate kinase domain of S-SCAM did not show a significant effect on the regulation of AMPARs. Together, these results suggest that S-SCAM is regulating AMPARs through TARPs.
Keywords: AMPA receptor, scaffolding proteins, TARP, S-SCAM, PDZ interaction
Introduction
AMPARs are glutamate-gated ion channels that mediate the majority of excitatory synaptic transmission,1 and are crucial substrates for the expression of synaptic plasticity. For example, rapid trafficking of AMPARs into and out of excitatory synapses is responsible for long-term potentiation and long-term depression, which underlies learning and memory.2-5 Furthermore, synaptic scaling, which provides homeostatic mechanisms for the stabilization of neural network integrity, is also mediated by activity-dependent changes in the levels of synaptic AMPARs.6 During the dynamic trafficking of AMPARs, scaffolding proteins play a crucial role by bringing together other proteins required for the transport, insertion, anchorage/stabilization, and removal of AMPARs. PSD-95 and related proteins of the membrane-associated guanylate kinase (MAGUK) family represent such synaptic scaffolds involved in AMPAR regulation at the postsynaptic density.7,8 S-SCAM is a new and the latest member of scaffolding proteins involved in the regulation of synaptic AMPARs.9 In contrast to PSD-95 that regulates plasticity-involved AMPARs, S-SCAM was found to be critical for the regulation of the maintenance pool of AMPARs, which is characterized by NSF-sensitive, GluA2-containing AMPARs.9 Intriguingly, MAGUKs, except for SAP-97, do not bind directly to AMPARs, but indirectly through TARPs.10 S-SCAM (also called MAGI-211 and AIP112) contains multiple PDZ domains,13 and binds to TARP through a PDZ interaction, like PSD-95.14 Here we addressed the mechanism by which S-SCAM interacts with AMPARs and the contribution of TARPs in this process.
Results and Discussion
Analyses of S-SCAM PDZ interactions based on the known examples revealed that all six PDZ domains of S-SCAM are involved in class I PDZ interaction (Table 1), which bind to the C-terminal amino acid sequence of X-S/T-X-V/L (where X stands for any amino acids).15 In contrast, C-terminals of GluA2 and GluA3 possess class II PDZ interaction motifs (S-V-K-I; belongs to X-Ψ-X-Ψ consensus where Ψ represents hydrophobic amino acids). Thus, given the class specificity of PDZ-ligand interaction, it is unlikely that S-SCAM directly binds to GluA2 or GluA3. Consistent with this prediction, microarray assays showed negative interaction between the PDZ domains (PDZ-1, -4, -5) of S-SCAM and GluA2 or GluA3.16 Furthermore, in the same assay, GluA1 also failed to show a positive interaction, even though it possesses the C-terminal sequence for the class I interaction (A-T-G-L), while it showed a good interaction with PDZ domains of SAP-97.16 Therefore, S-SCAM is highly likely to interact with AMPARs indirectly through other mediator protein(s). TARP has the class I PDZ interaction motif of T-T-A-V, and is the best candidate for this role as it has the ability to directly bind both AMPARs and S-SCAM.14
Table 1. PDZ domain Interactions of S-SCAM.
|
Domain |
Protein |
PDZ Class |
Interacting C-terminal a.a. sequence |
Microarray test with GluAs* |
References |
| PDZ-0 |
|
|
|
|
|
| |
|
|
|
|
|
| PDZ-1 |
ErbB4 |
Class I |
NTVV |
Negative |
24
|
| |
TARP |
|
TTPV |
|
14
|
| |
Rap-GEF |
|
VSAV |
|
25
|
| |
Kif1Bα |
|
ETTV |
|
26
|
| |
Neuroligin 1/2/3 |
|
TTRV |
|
27
|
| |
NR1 |
|
STVV |
|
13
|
| |
|
|
|
|
|
| PDZ-2 |
PTEN1 |
Class I |
ITKV |
|
11
|
| |
|
|
|
|
|
| PDZ-3 |
TARP |
Class I |
TTPV |
|
14
|
| |
|
|
|
|
|
| PDZ-4 |
|
|
|
Negative |
|
| |
|
|
|
|
|
| PDZ-5 |
TARP |
Class I |
TTPV |
Negative |
14
|
| |
NR2A/2B |
|
ESDV |
|
13
|
| |
NR2C |
|
ESEV |
|
13
|
| |
β-Catenin |
|
DTDL |
|
28
|
| |
ActivinRIIA |
|
ESSL |
|
29
|
| |
|
|
|
|
|
| |
* Ref. 16 |
|
Class I PDZ: |
X-S/T-X-Ψ |
|
| |
|
|
Class II PDZ: |
X-Ψ-X-Ψ |
|
| Class III PDZ: | X-D/E-X-Ψ |
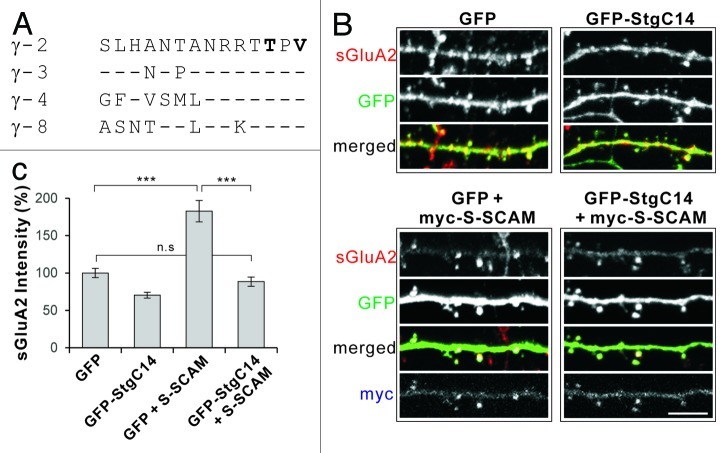
To address the role of TARPs in S-SCAM-mediated regulation of AMPARs, we examined the effect of preventing the S-SCAM—TARP interaction on surface AMPAR levels. To achieve this, we generated an EGFP fusion protein that has a C-terminal addition of the last 14 amino acids of TARP γ-2 (designated GFP-StgC14), whose sequence is highly conserved in all type I TARP family of proteins, especially for the critical last four amino acids (Fig. 1A). C-terminal peptides of PDZ ligands (typically 10–15 amino acids long, either as forms of synthetic peptides or fusion protein to other carrier proteins) serve as competitive inhibitors effective for preventing the PDZ-ligand interaction. This strategy has been successfully used to demonstrate the role of specific PDZ-ligand interaction, including AMPARs, PSD-95, and TARPs.17-20
Figure 1. Overexpression of Stargazin C-terminal peptides (GFP-StgC14) blocks the S-SCAM-induced increase of surface AMPA receptor levels in hippocampal neurons. (A) Sequence alignment of the last 14 amino acids of various TARPs involved in the PDZ interaction. (B) Representative images of surface GluA2 staining in dendritic segments of neurons transfected with GFP alone (control), GFP-StgC14 alone, GFP + myc-S-SCAM, or GFP-StgC14 + myc-S-SCAM. (C) Quantification of the effect of Stg-C14 and S-SCAM on surface GluA2. Scale bar represents 5 µm. ***p < 0.001; n.s, not significant; n = 30 per condition.
Overexpression of GFP-StgC14 alone in cultured hippocampal neurons reduced surface GluA2 (sGluA2) level significantly (100 ± 6 vs 70 ± 4%, GFP Control vs GFP-StgC14, p < 0.001), indicating that GFP-StgC14 indeed prevented the function of endogenous TARPs (Fig. 1B and C). In contrast, S-SCAM overexpression increased s GluA2 level by > 1.8-fold (p < 0.001). However, co-expression of GFP-StgC14 with S-SCAM completely abolished the S-SCAM-induced increase of sGluA2 levels (183 ± 14 vs 88 ± 6%, S-SCAM vs S-SCAM + GFP-StgC14, p < 0.001; compared with GFP control, p = 0.57; Fig. 1B and C). These results strongly suggest that S-SCAM depends on the PDZ interaction with TARPs for the regulation of AMPARs.
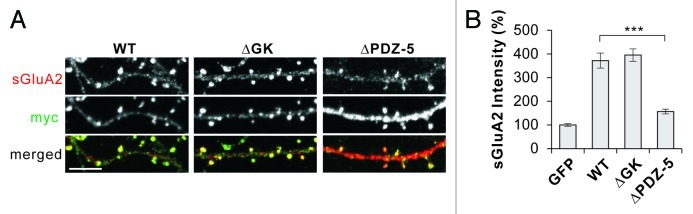
To further corroborate the results, we tested a mutant S-SCAM that has a deletion of the last PDZ domain (designated ΔPDZ-5), which is one of the three PDZ domains shown to interact with TARPs in vitro.14 Remarkably, as shown in Figure 2A and B, the deletion of PDZ-5 greatly impaired S-SCAM’s ability to increase sGluA2 levels (372 ± 32 vs 156 ± 10%, S-SCAM WT vs ΔPDZ-5, p < 0.001), indicating the importance of the PDZ-5 domain in the S-SCAM-mediated regulation of AMPARs. This is in sharp contrast to the previous results showing that the deletion of PDZ-0 did not impair S-SCAM’s ability to increase sGluA2 at all.9 In addition, the deletion of the guanylate kinase (GK) domain of S-SCAM did not produce any detrimental effect on the ability of S-SCAM to increase sGluA2 (372 ± 32 vs 395 ± 27%, S-SCAM WT vs ΔGK, p = 0.56), further demonstrating the specificity of the PDZ-5 domain deletion. Both ΔGK and ΔPDZ-5 mutants showed good dendritic spine targeting indistinguishable from WT (see Fig. 2A). Furthermore, all S-SCAM proteins including the ΔPDZ-5 mutant showed comparable expression levels in heterologous COS cells (data not shown). Therefore, the diminished effectiveness of ΔPDZ-5 in the AMPAR modulation is not likely due to poor expression of the protein or poor synaptic targeting. Taken together, these results suggest that TARP plays an important role in the control of AMPARs by S-SCAM. It is remarkable that the deletion of PDZ-5 had a such huge impact on the AMPAR regulation mediated by S-SCAM, since the mutant still has two other PDZ domains that can interact with TARPs, at least in in vitro pull-down assays.14 The small but significant increase of sGluA2 levels (~50%; compared with GFP control, p < 0.01) by ΔPDZ-5 might be mediated by the remaining PDZ-1 and -3 domains. All in all, these data suggest that PDZ5 is the most important PDZ domain for the regualtion of AMPARs.
Figure 2. PDZ-5 domain of S-SCAM is required for the S-SCAM-mediated regulation of surface GluA2 levels in hippocampal neurons. (A) Representative images of surface GluA2 staining in neurons transfected with myc-S-SCAM (WT), myc-S-SCAM (ΔGK), or myc-S-SCAM (ΔPDZ-5). (B) Quantification of the effect of S-SCAM WT, ΔGK, and ΔPDZ5 mutant on surface GluA2 levels. ***p < 0.001, n = 30 per condition. Scale bar represents 5 µm.
Our results unveil a remarkably similar mechanism by which PSD-95 and S-SCAM regulate AMPARs through auxiliary subunits TARPs. However, this is very different from the mechanism in Caenorhabditis elegans, in which AMPA-like receptor GLR-1/2 directly binds to MAGI-1L through PDZ interaction.21 What would be the advantage of making an indirect interaction with AMPARs through TARPs? One obvious benefit is that it allows S-SCAM (and PSD-95) to interact with AMPARs in a subunit-independent manner; i.e., confers the ability to interact with all AMPAR species. This may be important for S-SCAM and PSD-95 MAGUKs to serve as AMPAR-immobilizers (or “slots”)22 at synapses. In addition, by freeing-up the cytoplasmic PDZ ligands, GluA subunits can also interact with proteins such as PICK1, GRIP/ABP, and SAP97. This allows AMPARs to make GluA subunit-specific interactions that enable complex differential trafficking that is necessary for the expression of various types of synaptic plasticity.
Materials and Methods
Expression constructs
Myc-tagged S-SCAM WT and ΔPDZ-5 (S-SCAM-8) expression constructs were gifts from Dr Y. Hata (Tokyo Medical and Dental University).23 The ΔGK construct has a deletion in amino acids sequence between 165D and 293T and was made by site-directed mutagenesis. GFP-StgC14 was constructed by inserting the following annealed oligonucleotides between the EcoRI and BamHI sites of pEGFP-C1 (Clontech): Top strand 5′-AATTCTCTCCACGCCAACACAGCCAACCGCCGGACCACGCCCGTATGAG -3′; Bottom strand 5′-TATCCTCATACGGGCGTGGTCCGGCGGTTGGCAGTGTTGGCGTGGAGAG-3′.
Neuron transfection and immunocytochemistry
Dissociated hippocampal neurons grown on top of coverslips that were pre-coated with 37.5 μg/ml poly-d-lysine (BD) and 2.5 μg/ml laminin (BD) in a standard 12-well tissue culture plate (Corning). Hippocampi were isolated from E18–19 rat embryos and dissociated with trypsin and trituration. Dissociated neurons were plated at the density of 75,000 cells per coverslip and grown in Neurobasal media (Life Technologies) supplemented with B27 (Life Technologies). Neurons were transfected at DIV 14 using Lipofectamine 2000 reagent (Life Technologies) as described previously.9 After 2 d post-transfection, neurons were fixed in 4% formaldehyde/4% sucrose/1 × PBS for 5 min at room temperature. Surface GluA2 staining was performed by incubating fixed neurons with mouse anti-GluA2 (MAB397, Millipore) diluted at 5 μg/ml in ADB (4% normal horse serum/0.1% BSA/1 × PBS). After permeabilization by incubation in cold (-20°C) methanol for 2 min, neurons were further incubated with rabbit anti-myc antibody (1:100 dilution; Cell Signaling Technology) diluted in ADB. Bound antibodies were visualized by incubating with anti-mouse Cy3 (1:500 dilution; Jackson Immunoresearch) and anti-rabbit Alexa Fluor 488 (1:250 dilution; Life Technologies). Imunofluorescence images were acquired by confocal microscopy and analyzed as described previously.9 A one-way ANOVA with a Tukey’s post hoc test was used for the statitical analyses of the data.
Acknowledgments
This work was supported by the NIH grant MH078135 (to S.H.L.).
Glossary
Abbreviations:
- ABP
AMPA receptor binding protein
- AIP1
atrophin-1 interacting protein
- AMPAR
alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
- EGFP
enhanced green fluorescent protein
- GRIP
glutamate receptor-interacting protein
- MAGUK
membrane associated guanylate kinase
- MAGI-2
membrane associated guanylate kinase inverted-2
- PDZ
postsynaptic density-95/disc large/zonula occludens-1
- PICK-1
protein interacting with protein kinase C
- S-SCAM
synaptic scaffolding molecule
- TARP
transmembrane AMPAR regulatory protein
Footnotes
Previously published online: www.landesbioscience.com/journals/channels/article/21301
References
- 1.Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, et al. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 2010;62:405–96. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collingridge GL, Isaac JT, Wang YT. Receptor trafficking and synaptic plasticity. Nat Rev Neurosci. 2004;5:952–62. doi: 10.1038/nrn1556. [DOI] [PubMed] [Google Scholar]
- 3.Derkach VA, Oh MC, Guire ES, Soderling TR. Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat Rev Neurosci. 2007;8:101–13. doi: 10.1038/nrn2055. [DOI] [PubMed] [Google Scholar]
- 4.Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol. 2007;23:613–43. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- 5.Kessels HW, Malinow R. Synaptic AMPA receptor plasticity and behavior. Neuron. 2009;61:340–50. doi: 10.1016/j.neuron.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turrigiano GG. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell. 2008;135:422–35. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elias GM, Nicoll RA. Synaptic trafficking of glutamate receptors by MAGUK scaffolding proteins. Trends Cell Biol. 2007;17:343–52. doi: 10.1016/j.tcb.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Xu W. PSD-95-like membrane associated guanylate kinases (PSD-MAGUKs) and synaptic plasticity. Curr Opin Neurobiol. 2011;21:306–12. doi: 10.1016/j.conb.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danielson E, Zhang N, Metallo J, Kaleka K, Shin SM, Gerges N, et al. S-SCAM/MAGI-2 is an essential synaptic scaffolding molecule for the GluA2-containing maintenance pool of AMPA receptors. J Neurosci. 2012;32:6967–80. doi: 10.1523/JNEUROSCI.0025-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackson AC, Nicoll RA. The expanding social network of ionotropic glutamate receptors: TARPs and other transmembrane auxiliary subunits. Neuron. 2011;70:178–99. doi: 10.1016/j.neuron.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu X, Hepner K, Castelino-Prabhu S, Do D, Kaye MB, Yuan XJ, et al. Evidence for regulation of the PTEN tumor suppressor by a membrane-localized multi-PDZ domain containing scaffold protein MAGI-2. Proc Natl Acad Sci U S A. 2000;97:4233–8. doi: 10.1073/pnas.97.8.4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wood JD, Yuan J, Margolis RL, Colomer V, Duan K, Kushi J, et al. Atrophin-1, the DRPLA gene product, interacts with two families of WW domain-containing proteins. Mol Cell Neurosci. 1998;11:149–60. doi: 10.1006/mcne.1998.0677. [DOI] [PubMed] [Google Scholar]
- 13.Hirao K, Hata Y, Ide N, Takeuchi M, Irie M, Yao I, et al. A novel multiple PDZ domain-containing molecule interacting with N-methyl-D-aspartate receptors and neuronal cell adhesion proteins. J Biol Chem. 1998;273:21105–10. doi: 10.1074/jbc.273.33.21105. [DOI] [PubMed] [Google Scholar]
- 14.Deng F, Price MG, Davis CF, Mori M, Burgess DL. Stargazin and other transmembrane AMPA receptor regulating proteins interact with synaptic scaffolding protein MAGI-2 in brain. J Neurosci. 2006;26:7875–84. doi: 10.1523/JNEUROSCI.1851-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nourry C, Grant SG, Borg JP. PDZ domain proteins: plug and play! Sci STKE. 2003;179:RE7. doi: 10.1126/stke.2003.179.re7. [DOI] [PubMed] [Google Scholar]
- 16.Stiffler MA, Chen JR, Grantcharova VP, Lei Y, Fuchs D, Allen JE, et al. PDZ domain binding selectivity is optimized across the mouse proteome. Science. 2007;317:364–9. doi: 10.1126/science.1144592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi S, Hayashi Y, Esteban JA, Malinow R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell. 2001;105:331–43. doi: 10.1016/S0092-8674(01)00321-X. [DOI] [PubMed] [Google Scholar]
- 18.Osten P, Srivastava S, Inman GJ, Vilim FS, Khatri L, Lee LM, et al. The AMPA receptor GluR2 C terminus can mediate a reversible, ATP-dependent interaction with NSF and alpha- and beta-SNAPs. Neuron. 1998;21:99–110. doi: 10.1016/S0896-6273(00)80518-8. [DOI] [PubMed] [Google Scholar]
- 19.Sainlos M, Tigaret C, Poujol C, Olivier NB, Bard L, Breillat C, et al. Biomimetic divalent ligands for the acute disruption of synaptic AMPAR stabilization. Nat Chem Biol. 2011;7:81–91. doi: 10.1038/nchembio.498. [DOI] [PubMed] [Google Scholar]
- 20.Passafaro M, Sala C, Niethammer M, Sheng M. Microtubule binding by CRIPT and its potential role in the synaptic clustering of PSD-95. Nat Neurosci. 1999;2:1063–9. doi: 10.1038/15990. [DOI] [PubMed] [Google Scholar]
- 21.Emtage L, Chang H, Tiver R, Rongo C. MAGI-1 modulates AMPA receptor synaptic localization and behavioral plasticity in response to prior experience. PLoS One. 2009;4:e4613. doi: 10.1371/journal.pone.0004613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Opazo P, Sainlos M, Choquet D. Regulation of AMPA receptor surface diffusion by PSD-95 slots. Curr Opin Neurobiol. 2011;22:453–60. doi: 10.1016/j.conb.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 23.Hirao K, Hata Y, Deguchi M, Yao I, Ogura M, Rokukawa C, et al. Association of synapse-associated protein 90/ postsynaptic density-95-associated protein (SAPAP) with neurofilaments. Genes Cells. 2000;5:203–10. doi: 10.1046/j.1365-2443.2000.00318.x. [DOI] [PubMed] [Google Scholar]
- 24.Buxbaum JD, Georgieva L, Young JJ, Plescia C, Kajiwara Y, Jiang Y, et al. Molecular dissection of NRG1-ERBB4 signaling implicates PTPRZ1 as a potential schizophrenia susceptibility gene. Mol Psychiatry. 2008;13:162–72. doi: 10.1038/sj.mp.4001991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohtsuka T, Hata Y, Ide N, Yasuda T, Inoue E, Inoue T, et al. nRap GEP: a novel neural GDP/GTP exchange protein for rap1 small G protein that interacts with synaptic scaffolding molecule (S-SCAM) Biochem Biophys Res Commun. 1999;265:38–44. doi: 10.1006/bbrc.1999.1619. [DOI] [PubMed] [Google Scholar]
- 26.Mok H, Shin H, Kim S, Lee JR, Yoon J, Kim E. Association of the kinesin superfamily motor protein KIF1Balpha with postsynaptic density-95 (PSD-95), synapse-associated protein-97, and synaptic scaffolding molecule PSD-95/discs large/zona occludens-1 proteins. J Neurosci. 2002;22:5253–8. doi: 10.1523/JNEUROSCI.22-13-05253.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iida J, Hirabayashi S, Sato Y, Hata Y. Synaptic scaffolding molecule is involved in the synaptic clustering of neuroligin. Mol Cell Neurosci. 2004;27:497–508. doi: 10.1016/j.mcn.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 28.Nishimura W, Yao I, Iida J, Tanaka N, Hata Y. Interaction of synaptic scaffolding molecule and Beta -catenin. J Neurosci. 2002;22:757–65. doi: 10.1523/JNEUROSCI.22-03-00757.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shoji H, Tsuchida K, Kishi H, Yamakawa N, Matsuzaki T, Liu Z, et al. Identification and characterization of a PDZ protein that interacts with activin type II receptors. J Biol Chem. 2000;275:5485–92. doi: 10.1074/jbc.275.8.5485. [DOI] [PubMed] [Google Scholar]