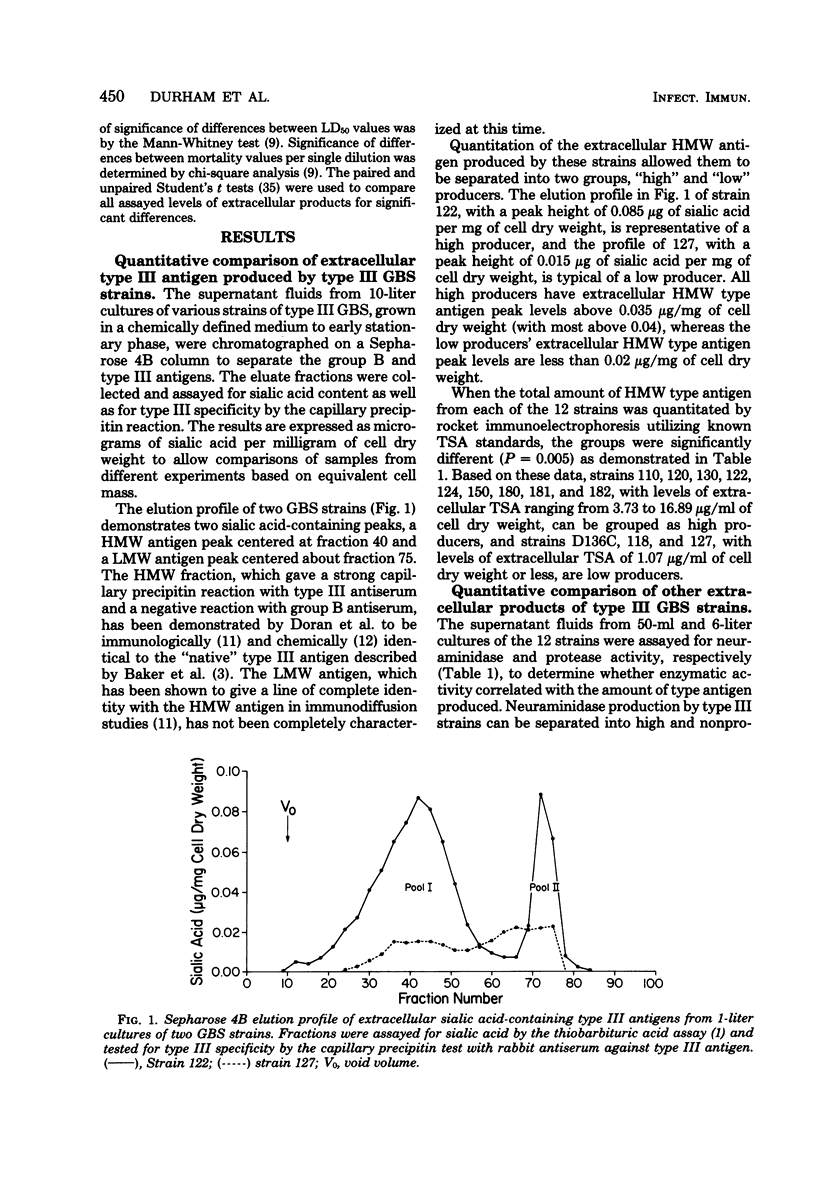
Abstract
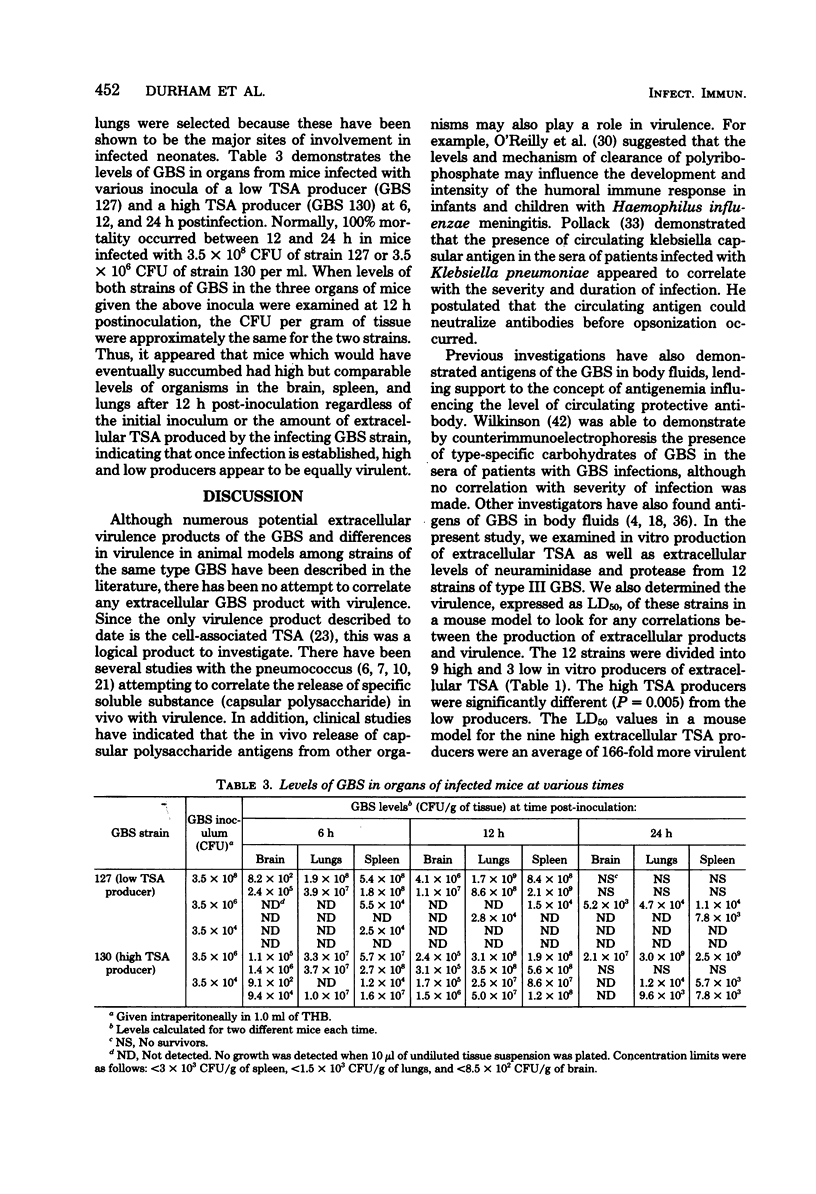
Twelve strains of serotype III group B streptococci (8 isolated from cases of neonatal disease, 3 isolated from asymptomatically colonized infants, and 1 laboratory reference strain) were examined for the vitro production of three potential extracellular virulence products: type-specific antigen, neuraminidase, and protease. In addition, virulence in a mouse model, expressed as 50% lethal dose, was determined for the 12 strains to determine whether a relationship existed between the production of any of the three extracellular products and virulence. Only production of extracellular type-specific antigen showed a correlation with virulence in the mouse model. The high producers of extracellular type-specific antigen were an average of 166-fold more virulent for mice than low producers of the same component. There was no correlation between virulence and either neuraminidase or protease production, nor was there a correlation between either of these two extracellular products and the levels of extracellular type-specific antigen. When levels of group B streptococci of each type (a high and low producer of extracellular type-specific antigen) in organs of infected mice were examined, comparable levels of organisms were found in the brain, spleen, and lungs of mice near death regardless of the initial inoculum. However, the high producer of extracellular type-specific antigen caused death in mice with a 2 to 3 log lower inoculum than the low producer, suggesting that these strains may be more invasive.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMINOFF D. Methods for the quantitative estimation of N-acetylneuraminic acid and their application to hydrolysates of sialomucoids. Biochem J. 1961 Nov;81:384–392. doi: 10.1042/bj0810384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker C. J., Kasper D. L., Davis C. E. Immunochemical characterization of the "native" type III polysaccharide of group B Streptococcus. J Exp Med. 1976 Feb 1;143(2):258–270. doi: 10.1084/jem.143.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker C. J., Webb B. J., Jackson C. V., Edwards M. S. Countercurrent immunoelectrophoresis in the evaluation of infants with group B streptococcal disease. Pediatrics. 1980 Jun;65(6):1110–1114. [PubMed] [Google Scholar]
- Carey R. B., Eisenstein T. K., Shockman G. D., Greber T. F., Swenson R. M. Soluble group- and type-specific antigens from type III group B Streptococcus. Infect Immun. 1980 Apr;28(1):195–203. doi: 10.1128/iai.28.1.195-203.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dochez A. R., Avery O. T. THE ELABORATION OF SPECIFIC SOLUBLE SUBSTANCE BY PNEUMOCOCCUS DURING GROWTH. J Exp Med. 1917 Oct 1;26(4):477–493. doi: 10.1084/jem.26.4.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran T. I., Straus D. C., Mattingly S. J. Extracellular antigens of serotype III group B streptococci. Infect Immun. 1980 Dec;30(3):890–893. doi: 10.1128/iai.30.3.890-893.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran T. I., Straus D. C., Mattingly S. J. Factors influencing release of type III antigens by group B streptococci. Infect Immun. 1981 Feb;31(2):615–623. doi: 10.1128/iai.31.2.615-623.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrieri P., Burke B., Nelson J. Production of bacteremia and meningitis in infant rats with group B streptococcal serotypes. Infect Immun. 1980 Mar;27(3):1023–1032. doi: 10.1128/iai.27.3.1023-1032.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrieri P., Gray E. D., Wannamaker L. W. Biochemical and immunological characterization of the extracellular nucleases of group B streptococci. J Exp Med. 1980 Jan 1;151(1):56–68. doi: 10.1084/jem.151.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutschik E., Møller S., Christensen N. Experimental endocarditis in rabbits. 3. Significance of the proteolytic capacity of the infecting strains of Streptococcus faecalis. Acta Pathol Microbiol Scand B. 1979 Dec;87(6):353–362. [PubMed] [Google Scholar]
- Hayano S., Tanaka A., Okuyama Y. Distribution and serological specificity of sialidase produced by various groups of streptococci. J Bacteriol. 1969 Oct;100(1):354–357. doi: 10.1128/jb.100.1.354-357.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayano S., Tanaka A. Sialidase-like enzymes produced by group A, B, C, G, and L streptococci and by Streptococcus sanguis. J Bacteriol. 1969 Mar;97(3):1328–1333. doi: 10.1128/jb.97.3.1328-1333.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder I. A., Haidaris C. G. Experimental studies of the pathogenesis of infections due to Pseudomonas aeruginosa: extracellular protease and elastase as in vivo virulence factors. Can J Microbiol. 1979 May;25(5):593–599. doi: 10.1139/m79-085. [DOI] [PubMed] [Google Scholar]
- Kato N., Kato O., Nakashima I., Naito S., Asai J. Effect of capsular polysaccharide of Klebsiella pneumoniae on host resistance to bacterial infections. III. Further study of its effects on interactions between peritoneal leukocytes and virulent Salmonella enteritidis. Microbiol Immunol. 1979;23(5):369–382. doi: 10.1111/j.1348-0421.1979.tb00474.x. [DOI] [PubMed] [Google Scholar]
- Kenny G. E., Wentworth B. B., Beasley R. P., Foy H. M. Correlation of circulating capsular polysaccharide with bacteremia in pneumococcal pneumonia. Infect Immun. 1972 Oct;6(4):431–437. doi: 10.1128/iai.6.4.431-437.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjems E., Perch B., Henrichsen J. Serotypes of group B streptococci and their relation to hyaluronidase production and hydrolysis of salicin. J Clin Microbiol. 1980 Feb;11(2):111–113. doi: 10.1128/jcm.11.2.111-113.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancefield R. C. TWO SEROLOGICAL TYPES OF GROUP B HEMOLYTIC STREPTOCOCCI WITH RELATED, BUT NOT IDENTICAL, TYPE-SPECIFIC SUBSTANCES. J Exp Med. 1938 Jan 1;67(1):25–40. doi: 10.1084/jem.67.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattingly S. J., Milligan T. W., Pierpont A. A., Straus D. C. Extracellular neuraminidase production by clinical isolates of group B streptococci from infected neonates. J Clin Microbiol. 1980 Oct;12(4):633–635. doi: 10.1128/jcm.12.4.633-635.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan T. W., Baker C. J., Straus D. C., Mattingly S. J. Association of elevated levels of extracellular neuraminidase with clinical isolates of type III group B streptococci. Infect Immun. 1978 Sep;21(3):738–746. doi: 10.1128/iai.21.3.738-746.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan T. W., Doran T. I., Straus D. C., Mattingly S. J. Growth and amino acid requirements of various strains of group B streptococci. J Clin Microbiol. 1978 Jan;7(1):28–33. doi: 10.1128/jcm.7.1.28-33.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan T. W., Straus D. C., Mattingly S. J. Extracellular neuraminidase production by group B streptococci. Infect Immun. 1977 Oct;18(1):189–195. doi: 10.1128/iai.18.1.189-195.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. C., Kline L. F. Activation of the classical and properdin pathways of complement by bacterial lipopolysaccharides (LPS). J Immunol. 1977 Jan;118(1):362–368. [PubMed] [Google Scholar]
- O'Reilly R. J., Anderson P., Ingram D. L., Peter G., Smith D. H. Circulating polyribophosphate in Hemophilus influenzae, type b meningitis. Correlation with clinical course and antibody response. J Clin Invest. 1975 Oct;56(4):1012–1022. doi: 10.1172/JCI108148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovskis O. R., Wretlind B. Assessment of protease (elastase) as a Pseudomonas aeruginosa virulence factor in experimental mouse burn infection. Infect Immun. 1979 Apr;24(1):181–187. doi: 10.1128/iai.24.1.181-187.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack M. Significance of circulating capsular antigen in Klebsiella infections. Infect Immun. 1976 Jun;13(6):1543–1548. doi: 10.1128/iai.13.6.1543-1548.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stechenberg B. W., Schreiner R. L., Grass S. M., Shackelford P. G. Countercurrent immunoelectrophoresis in group B streptococcal disease. Pediatrics. 1979 Nov;64(5):632–634. [PubMed] [Google Scholar]
- Straus D. C., Mattingly S. J., Milligan T. W., Doran T. I., Nealon T. J. Protease production by clinical isolates of type III group B streptococci. J Clin Microbiol. 1980 Sep;12(3):421–423. doi: 10.1128/jcm.12.3.421-423.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summary of the workshop on perinatal infections due to group B Streptococcus. J Infect Dis. 1977 Jul;136(1):137–152. doi: 10.1093/infdis/136.1.137. [DOI] [PubMed] [Google Scholar]
- TOENNIES G., GALLANT D. L. The relation between photometric turbidity and bacterial concentration. Growth. 1949 Mar;13(1):7–20. [PubMed] [Google Scholar]
- Tieffenberg J., Vogel L., Kretschmer R. R., Padnos D., Gotoff S. P. Chicken embryo model for type III group B beta-hemolytic streptococcal septicemia. Infect Immun. 1978 Feb;19(2):481–485. doi: 10.1128/iai.19.2.481-485.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeke B. A manual of quantitative immunoelectrophoresis. Methods and applications. 1. General remarks on principles, equipment, reagents and procedures. Scand J Immunol Suppl. 1973;1:15–35. doi: 10.1111/j.1365-3083.1973.tb03776.x. [DOI] [PubMed] [Google Scholar]
- Wilkinson H. W. Detection of group B streptococcal antibodies in human sera by radioimmunoassay: concentrations of type-specific antibodies in sera of adults and infants infected with group B streptococci. J Clin Microbiol. 1978 Feb;7(2):194–201. doi: 10.1128/jcm.7.2.194-201.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokochi T., Nakashima I., Kato N. Effect of capsular polysaccharide of Klebsiella pneumoniae on the differentiation and functional capacity of macrophages cultured in vitro. Microbiol Immunol. 1977 Oct 20;21(10):601–610. doi: 10.1111/j.1348-0421.1977.tb00328.x. [DOI] [PubMed] [Google Scholar]
- Yokochi T., Nakashima I., Kato N. Further studies on generation of macrophages in in vitro cultures of mouse spleen cells and its inhibition by the capsular polysaccharide of Klebsiella pneumoniae. Microbiol Immunol. 1979;23(6):487–499. doi: 10.1111/j.1348-0421.1979.tb00488.x. [DOI] [PubMed] [Google Scholar]


