Abstract
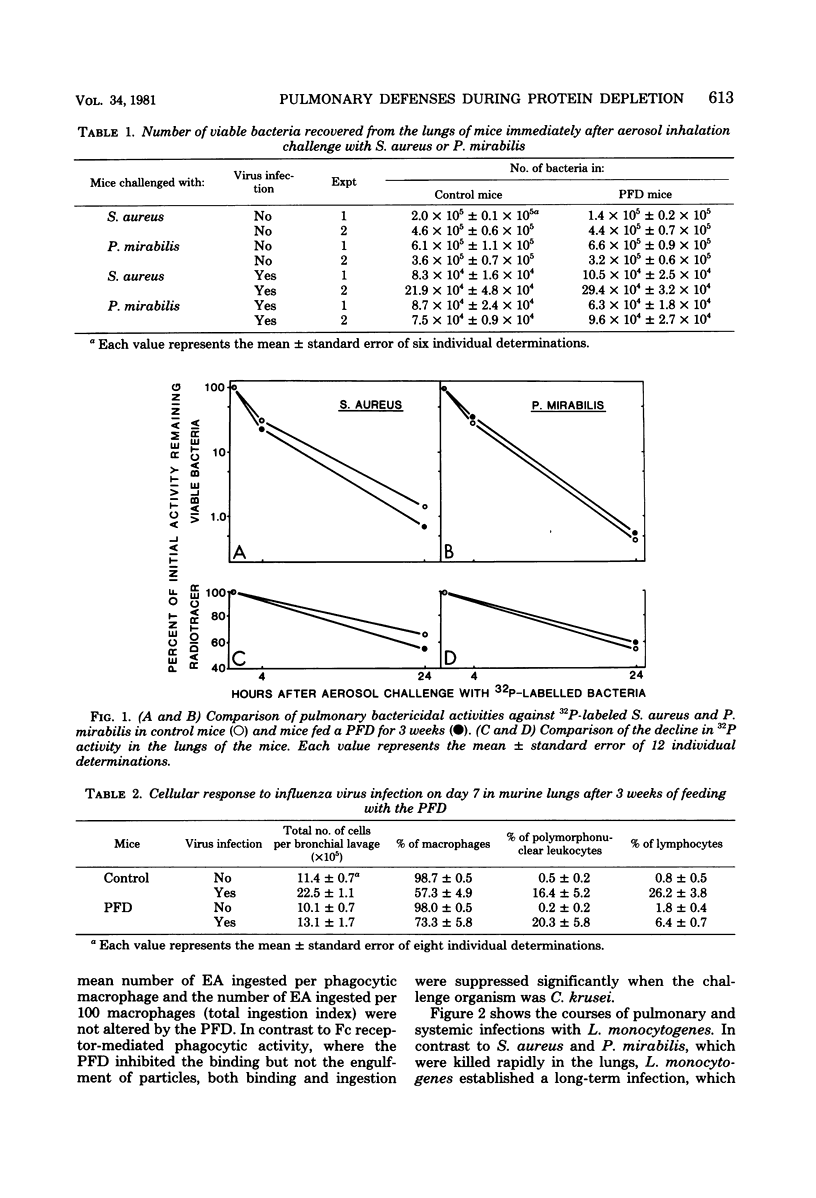
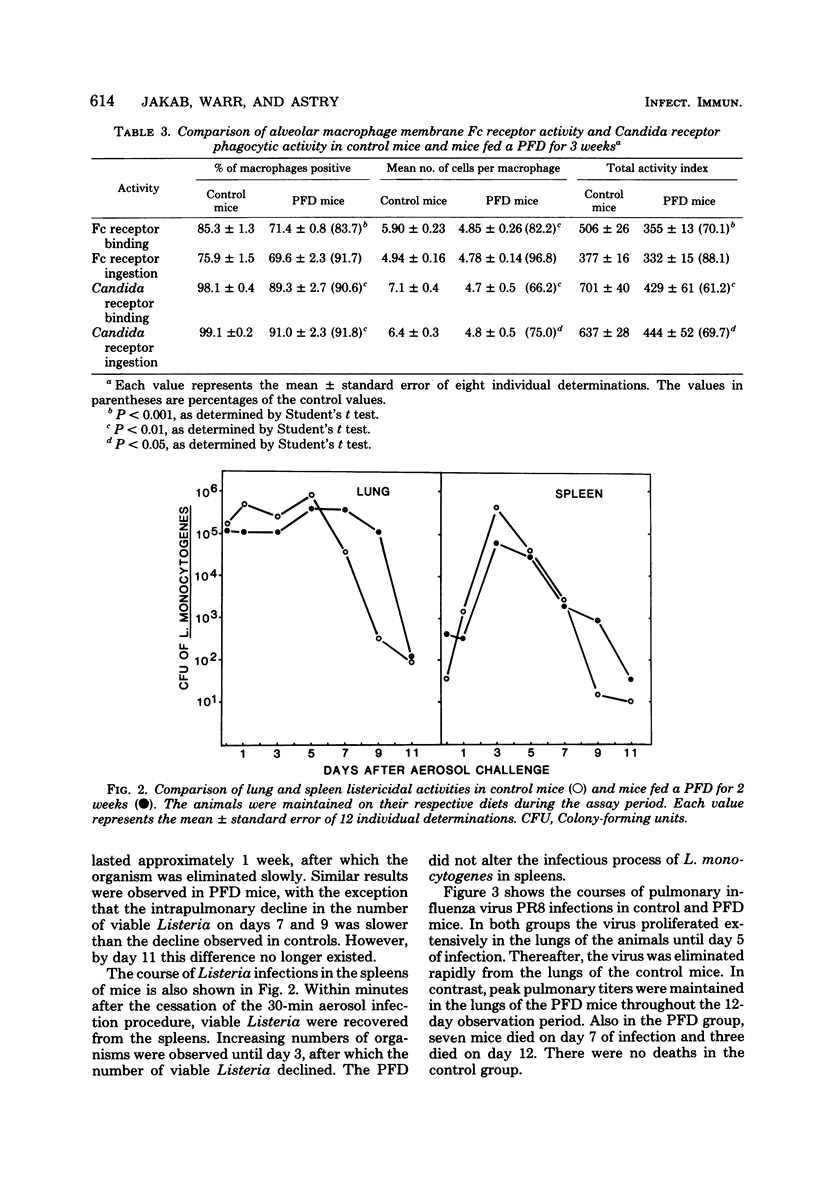
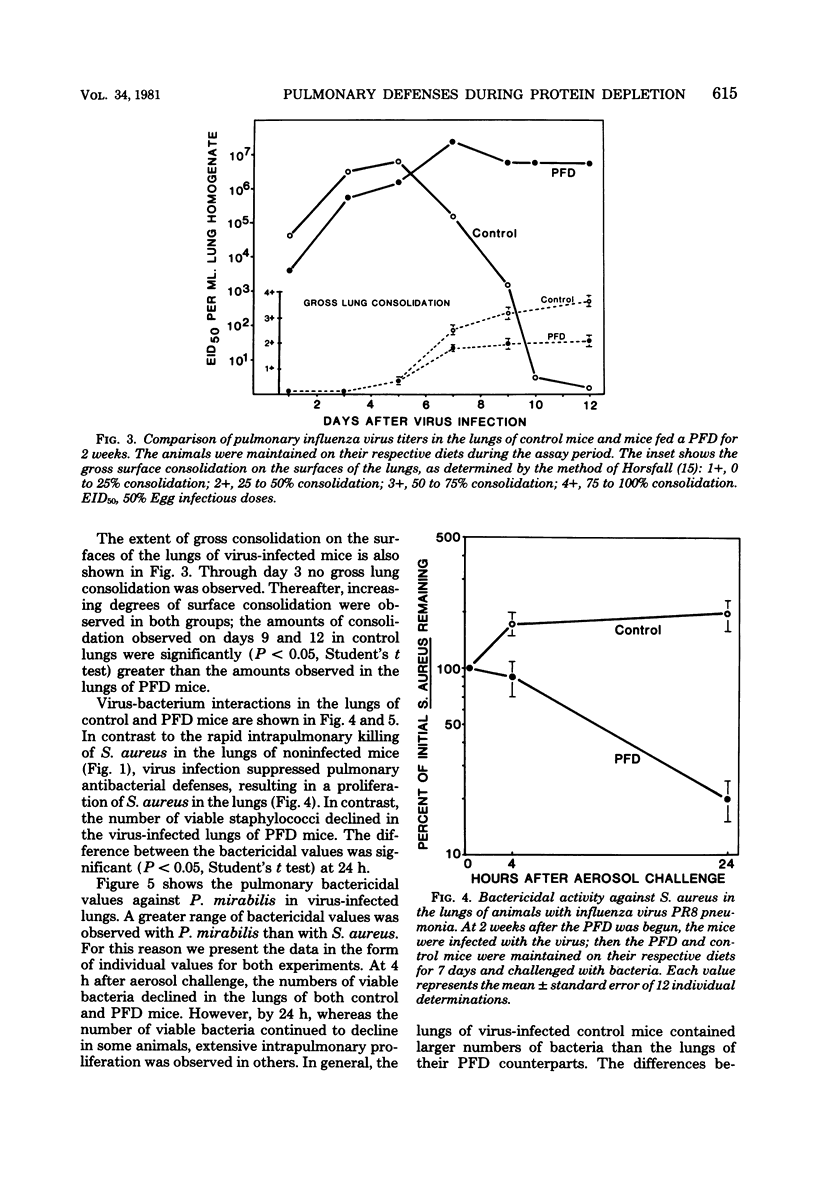
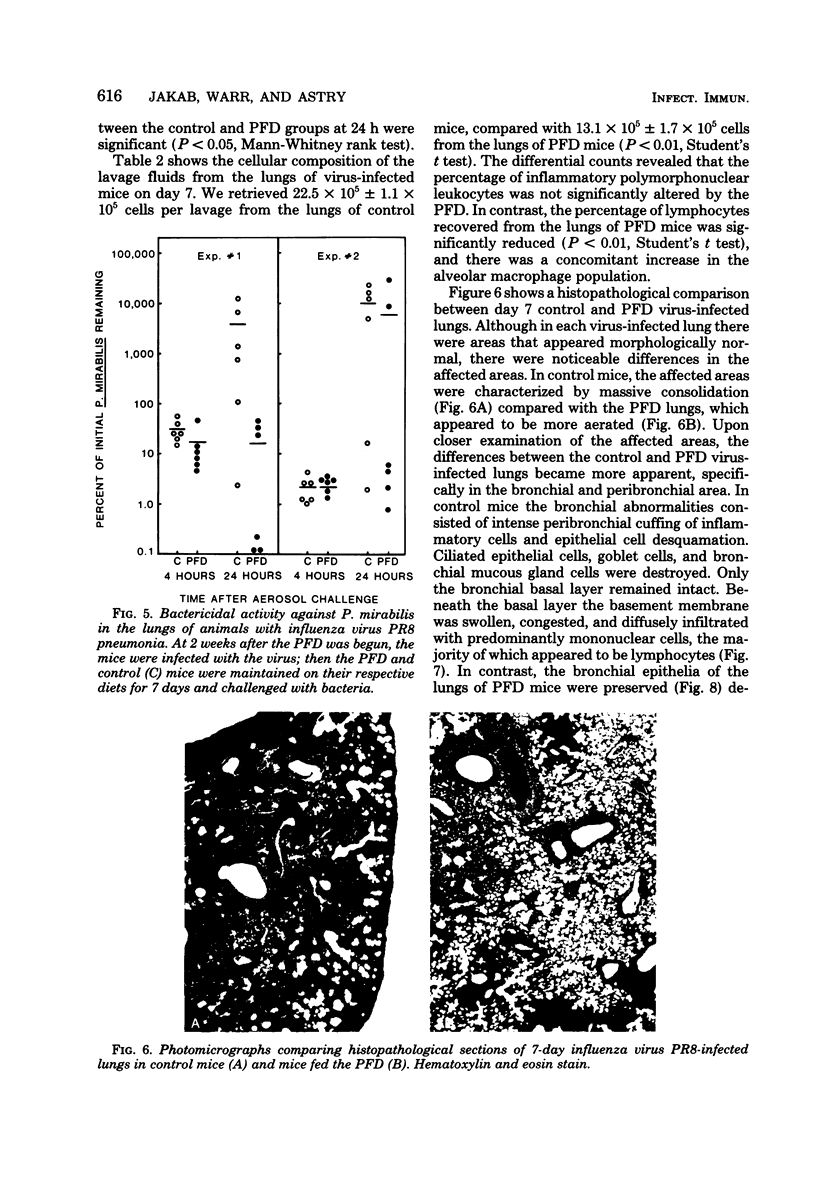
Pulmonary defense mechanisms were quantitated in mice that were fed a protein-free diet (PFD) for periods of 2 and 3 weeks. Despite the severe weight loss and emaciation induced by the diet, the bactericidal mechanisms in their lungs were preserved against aerogenic challenges with staphylococcus aureus, Proteus mirabilis, and Listeria monocytogenes. Phagocytic assays of alveolar macrophages that were retrieved by pulmonary lavage from PFD-fed animals showed a decrease in Fc receptor-mediated binding activity but no alteration in the ingestion of sensitized erythrocytes. In contrast, the PFD induced defects in both the attachment phase and the engulfment phase of the phagocytic process when the challenge organism was Candida krusei. The PFD suppressed the pulmonary inflammatory response after mice were infected with influenza virus strain PR8; such mice also failed to eliminate infectious virus from their lungs. Virus infection in control mice suppressed pulmonary antibacterial defenses against challenges with S. aureus and P. mirabilis, and defect that was ameliorated in the lungs of PFD-fed mice with viral pneumonia. The data demonstrated that pulmonary defense mechanisms were modulated by a PFD but that the observed effect was dependent on the agent used to test host defenses.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berlin B. S., Cochran K. W. Delay of fatal pneumonia in x-irradiated mice inoculated with mouse-adapted influenza virus, PR8 strain. Radiat Res. 1967 Jun;31(2):343–351. [PubMed] [Google Scholar]
- Bhuyan U. N., Ramalingaswami V. Immune responses of the protein-deficient guinea pig to BCG vaccination. Am J Pathol. 1973 Sep;72(3):489–502. [PMC free article] [PubMed] [Google Scholar]
- Blanden R. V., Langman R. E. Cell-mediated immunity to bacterial infection in the mouse. Thymus-derived cells as effectors of acquired resistance to Listeria monocytogenes. Scand J Immunol. 1972;1(4):379–391. doi: 10.1111/j.1365-3083.1972.tb03304.x. [DOI] [PubMed] [Google Scholar]
- Cate T. R., Mold N. G. Increased influenza pneumonia mortality of mice adoptively immunized with node and spleen cells sensitized by inactivated but not live virus. Infect Immun. 1975 May;11(5):908–914. doi: 10.1128/iai.11.5.908-914.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C., Kongshavn P. A., Skamene E. Enhanced primary resistance to Listeria monocytogenes in T cell-deprived mice. Immunology. 1977 Apr;32(4):529–537. [PMC free article] [PubMed] [Google Scholar]
- Cheers C., Waller R. Activated macrophages in congenitally athymic "nude mice" and in lethally irradiate mice. J Immunol. 1975 Sep;115(3):844–847. [PubMed] [Google Scholar]
- Cooper W. C., Good R. A., Mariani T. Effects of protein insufficiency on immune responsiveness. Am J Clin Nutr. 1974 Jun;27(6):647–664. doi: 10.1093/ajcn/27.6.647. [DOI] [PubMed] [Google Scholar]
- Elsbach P. Degradation of microorganisms by phagocytic cells. Rev Infect Dis. 1980 Jan-Feb;2(1):106–128. doi: 10.1093/clinids/2.1.106. [DOI] [PubMed] [Google Scholar]
- Emmerling P., Finger H., Hof H. Cell-mediated resistance to infection with Listeria monocytogenes in nude mice. Infect Immun. 1977 Feb;15(2):382–385. doi: 10.1128/iai.15.2.382-385.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLANIGAN C. C., SPRUNT D. H. The effect of malnutrition on the susceptibility of the host to viral infection. J Exp Med. 1956 Nov 1;104(5):687–706. doi: 10.1084/jem.104.5.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galleli A., Le Garrec Y., Chedid L. Increased resistance and depressed delayed-type hypersensitivity to Listeria monocytogenes induced by pretreatment with lipopolysaccharide. Infect Immun. 1981 Jan;31(1):88–94. doi: 10.1128/iai.31.1.88-94.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein E., Lippert W., Warshauer D. Pulmonary alveolar macrophage. Defender against bacterial infection of the lung. J Clin Invest. 1974 Sep;54(3):519–528. doi: 10.1172/JCI107788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green G. M., Goldstein E. A method for quantitating intrapulmonary bacterial inactivation in individual animals. J Lab Clin Med. 1966 Oct;68(4):669–677. [PubMed] [Google Scholar]
- Gross R. L., Newberne P. M. Role of nutrition in immunologic function. Physiol Rev. 1980 Jan;60(1):188–302. doi: 10.1152/physrev.1980.60.1.188. [DOI] [PubMed] [Google Scholar]
- Hurd J., Heath R. B. Effect of cyclophosphamide on infections in mice caused by virulent and avirulent strains of influenza virus. Infect Immun. 1975 May;11(5):886–889. doi: 10.1128/iai.11.5.886-889.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakab G. J., Green G. M. The effect of Sendai virus infection on bactericidal and transport mechanisms of the murine lung. J Clin Invest. 1972 Aug;51(8):1989–1998. doi: 10.1172/JCI107005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakab G. J. Mechanisms of virus-induced bacterial superinfections of the lung. Clin Chest Med. 1981 Jan;2(1):59–66. [PubMed] [Google Scholar]
- Jakab G. J. Pulmonary defense mechanisms and the interaction between viruses and bacteria in acute respiratory infections. Bull Eur Physiopathol Respir. 1977 Jan-Feb;13(1):119–135. [PubMed] [Google Scholar]
- Jakab G. J., Warr G. A. Lung defenses against viral and bacterial challenges during immunosuppression with cyclophosphamide in mice. Am Rev Respir Dis. 1981 May;123(5):524–528. doi: 10.1164/arrd.1981.123.5.524. [DOI] [PubMed] [Google Scholar]
- Jones T. C. Macrophages and intracellular parasitism. J Reticuloendothel Soc. 1974 May;15(5):439–450. [PubMed] [Google Scholar]
- Keusch G. T., Douglas S. D., Braden K., Geller S. A. Antibacterial functions of macrophages in experimental protein-calorie malnutrition. I. Description of the model, morphologic observations, and macrophage surface IgG receptors. J Infect Dis. 1978 Aug;138(2):125–133. doi: 10.1093/infdis/138.2.125. [DOI] [PubMed] [Google Scholar]
- Keusch G. T., Douglas S. D., Hammer G., Braden K. Antibacterial functions of macrophages in experimental protein-calorie malnutrition. II. Cellular and humoral factors for chemotaxis, phagocytosis, and intracellular bactericidal activity. J Infect Dis. 1978 Aug;138(2):134–142. doi: 10.1093/infdis/138.2.134. [DOI] [PubMed] [Google Scholar]
- LaForce F. M., Kelly W. J., Huber G. L. Inactivation of staphylococci by alveolar macrophages with preliminary observations on the importance of alveolar lining material. Am Rev Respir Dis. 1973 Oct;108(4):784–790. doi: 10.1164/arrd.1973.108.4.784. [DOI] [PubMed] [Google Scholar]
- Li C. Y., Lam K. W., Yam L. T. Esterases in human leukocytes. J Histochem Cytochem. 1973 Jan;21(1):1–12. doi: 10.1177/21.1.1. [DOI] [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson D., Franco D., Arbeter A., Velez H., Vitale J. J. Serum levels of immunoglobulins, cell-mediated immunity, and phagocytosis in protein-calorie malnutrition. Am J Clin Nutr. 1974 Jun;27(6):625–628. doi: 10.1093/ajcn/27.6.625. [DOI] [PubMed] [Google Scholar]
- Nickol A. D., Bonventre P. F. Anomalous high native resistance to athymic mice to bacterial pathogens. Infect Immun. 1977 Dec;18(3):636–645. doi: 10.1128/iai.18.3.636-645.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson L. C., Sisk D. R., Izsak E. Protein-calorie malnutrition impairs the anti-viral function of macrophages. Proc Soc Exp Biol Med. 1978 Oct;159(1):84–87. doi: 10.3181/00379727-159-40289. [DOI] [PubMed] [Google Scholar]
- Patterson-Delafield J., Lehrer R. I. A simple microscopic method for identifying and quantitating phagocytic cells in vitro. J Immunol Methods. 1977;18(3-4):377–379. doi: 10.1016/0022-1759(77)90191-0. [DOI] [PubMed] [Google Scholar]
- Robinson T. W., Cureton R. J., Heath R. B. The effect of cyclophosphamide on Sendai virus infection of mice. J Med Microbiol. 1969 May;2(2):137–145. doi: 10.1099/00222615-2-2-137. [DOI] [PubMed] [Google Scholar]
- Ruppert D., Jakab G. J., Sylwester D. L., Green G. M. Sources of variance in the measurement of intrapulmonary killing of bacteria. J Lab Clin Med. 1976 Mar;87(3):544–558. [PubMed] [Google Scholar]
- Singer S. H., Noguchi P., Kirschstein R. L. Respiratory diseases in cyclophosphamide-treated mice. II. Decreased virulence of PR8 influenza virus. Infect Immun. 1972 Jun;5(6):957–960. doi: 10.1128/iai.5.6.957-960.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan J. L., Mayner R. E., Barry D. W., Ennis F. A. Influenza virus infection in nude mice. J Infect Dis. 1976 Jan;133(1):91–94. doi: 10.1093/infdis/133.1.91. [DOI] [PubMed] [Google Scholar]
- Suzuki F., Oya J., Ishida N. Effect of antilymphocyte serum on influenza virus infection in mice. Proc Soc Exp Biol Med. 1974 May;146(1):78–84. doi: 10.3181/00379727-146-38047. [DOI] [PubMed] [Google Scholar]
- Toews G. B., Gross G. N., Pierce A. K. The relationship of inoculum size to lung bacterial clearance and phagocytic cell response in mice. Am Rev Respir Dis. 1979 Sep;120(3):559–566. doi: 10.1164/arrd.1979.120.3.559. [DOI] [PubMed] [Google Scholar]
- Warr G. A. A macrophage receptor for (mannose/glucosamine)-glycoproteins of potential importance in phagocytic activity. Biochem Biophys Res Commun. 1980 Apr 14;93(3):737–745. doi: 10.1016/0006-291x(80)91139-0. [DOI] [PubMed] [Google Scholar]
- Warr G. A., Jakab G. J., Hearst J. E. Alterations in lung macrophage immune receptor(s) activity associated with viral pneumonia. J Reticuloendothel Soc. 1979 Oct;26(4):357–366. [PubMed] [Google Scholar]
- Warshauer D., Goldstein E., Akers T., Lippert W., Kim M. Effect of influenza viral infection on the ingestion and killing of bacteria by alveolar macrophages. Am Rev Respir Dis. 1977 Feb;115(2):269–277. doi: 10.1164/arrd.1977.115.2.269. [DOI] [PubMed] [Google Scholar]
- Wells M. A., Albrecht P., Ennis F. A. Recovery from a viral respiratory infection. I. Influenza pneumonia in normal and T-deficient mice. J Immunol. 1981 Mar;126(3):1036–1041. [PubMed] [Google Scholar]
- Wing E. J., Young J. B. Acute starvation protects mice against Listeria monocytogenes. Infect Immun. 1980 Jun;28(3):771–776. doi: 10.1128/iai.28.3.771-776.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff J. F. The influence of quantitated post-weaning undernutrition on coxsackievirus B3 infection of adult mice. II. Alteration of host defense mechanisms. J Infect Dis. 1970 Feb;121(2):164–181. doi: 10.1093/infdis/121.2.164. [DOI] [PubMed] [Google Scholar]
- Woodruff J. F., Woodruff J. J. Modification of severe coxsackievirus B 3 infection in marasmic mice by transfer of immune lymphoid cells. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2108–2111. doi: 10.1073/pnas.68.9.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyde P. R., Cate T. R. Cellular changes in lungs of mice infected with influenza virus: characterization of the cytotoxic responses. Infect Immun. 1978 Nov;22(2):423–429. doi: 10.1128/iai.22.2.423-429.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyde P. R., Peavy D. L., Cate T. R. Morphological and cytochemical characterization of cells infiltrating mouse lungs after influenza infection. Infect Immun. 1978 Jul;21(1):140–146. doi: 10.1128/iai.21.1.140-146.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]





