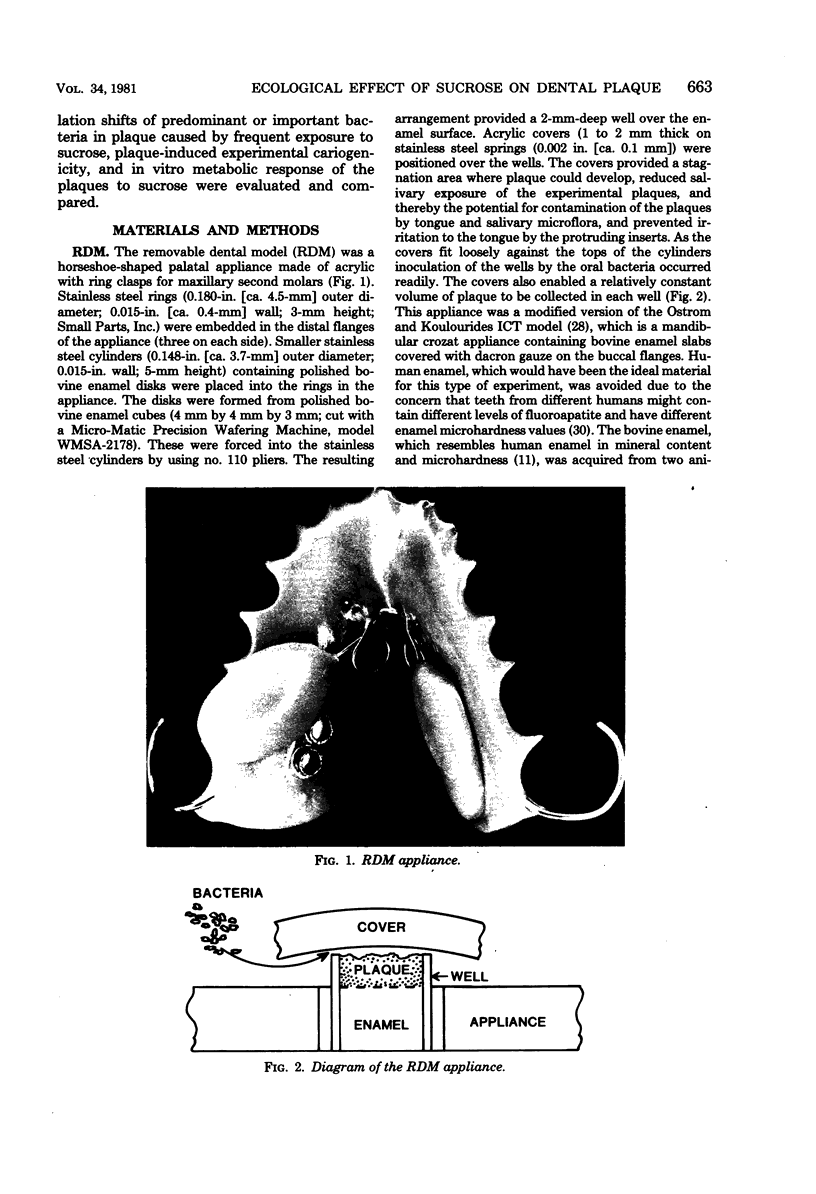
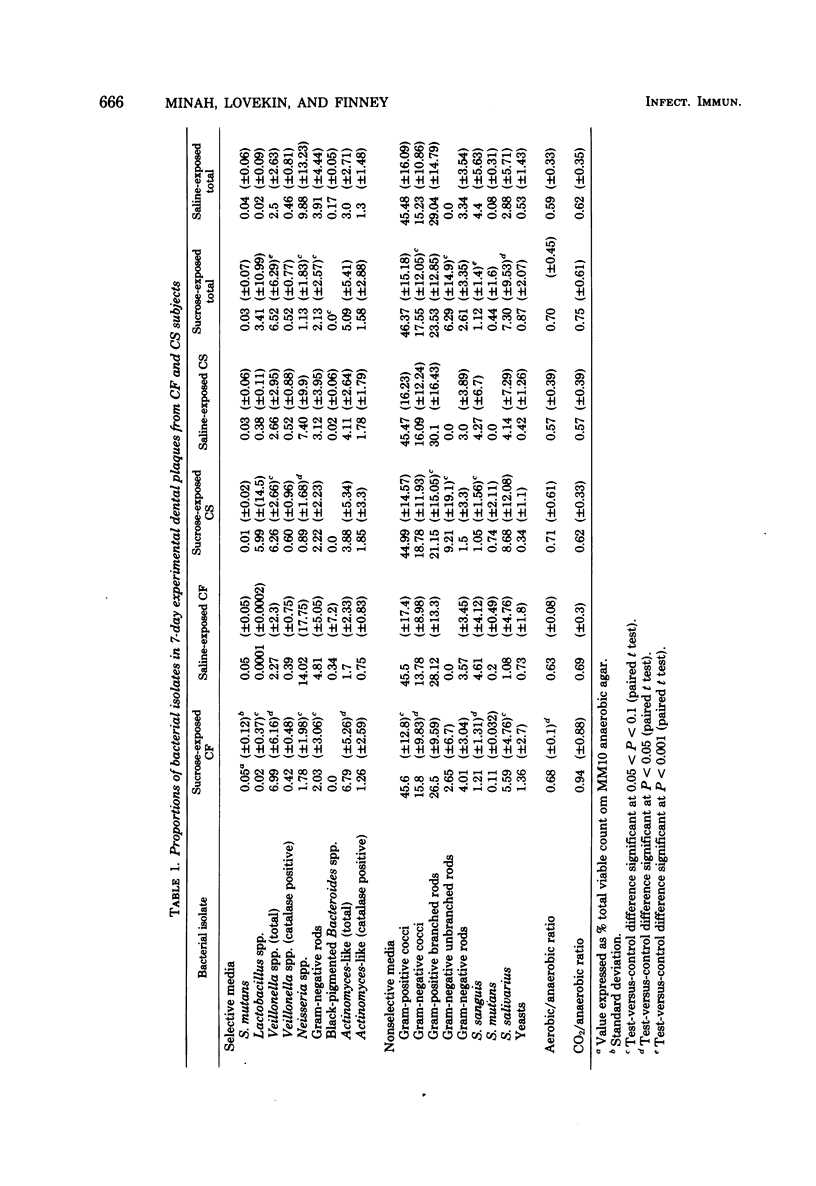
Abstract
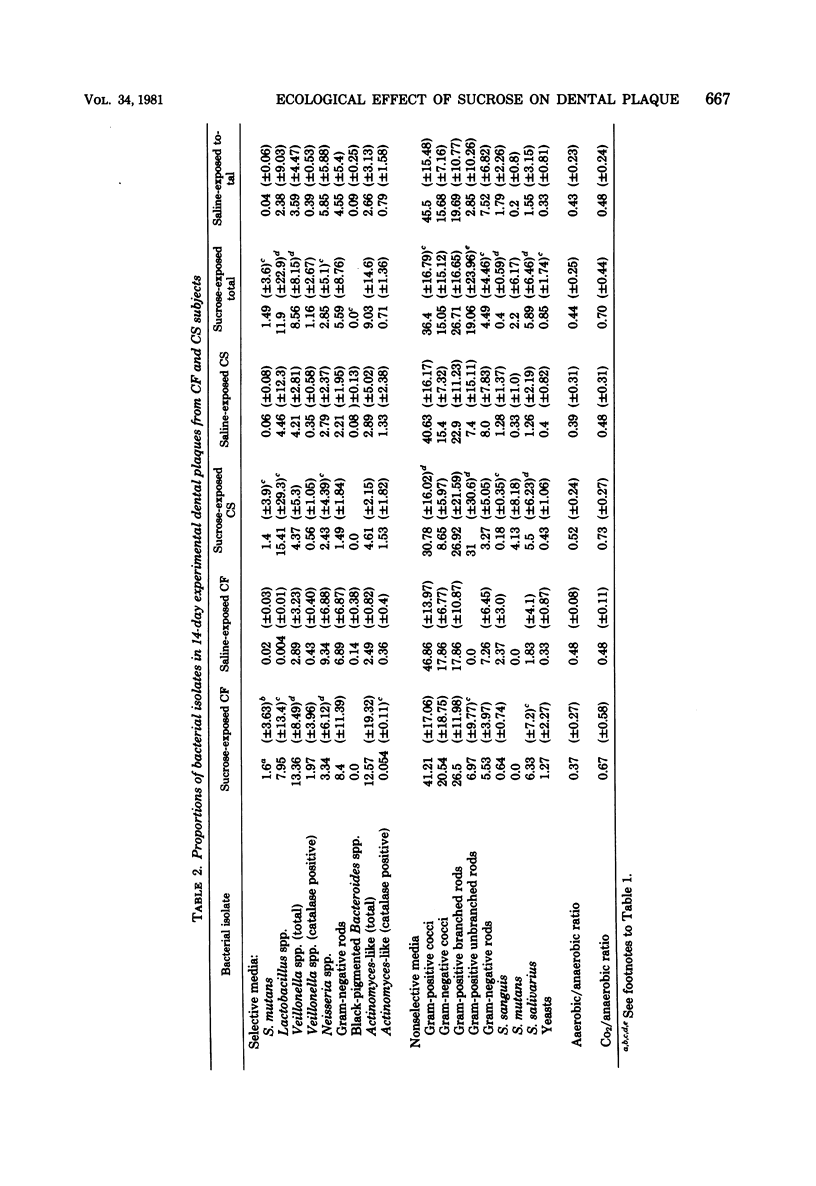
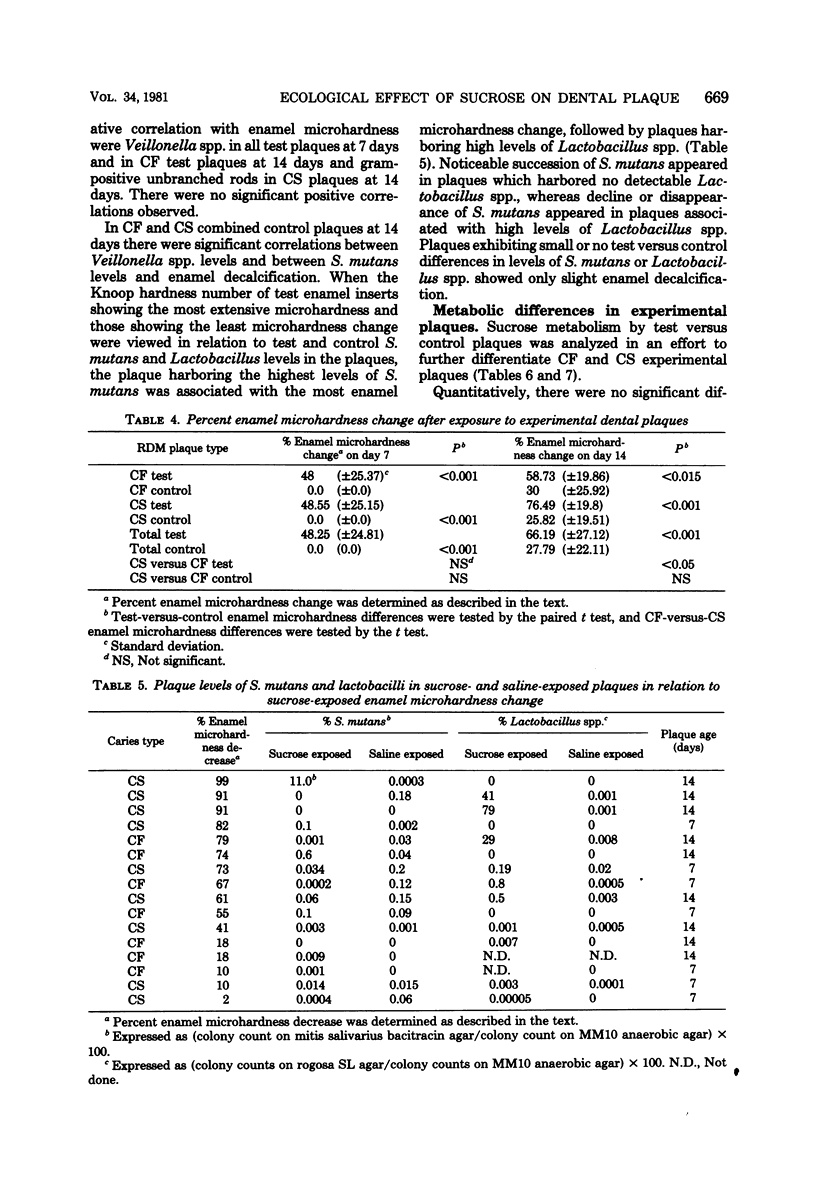
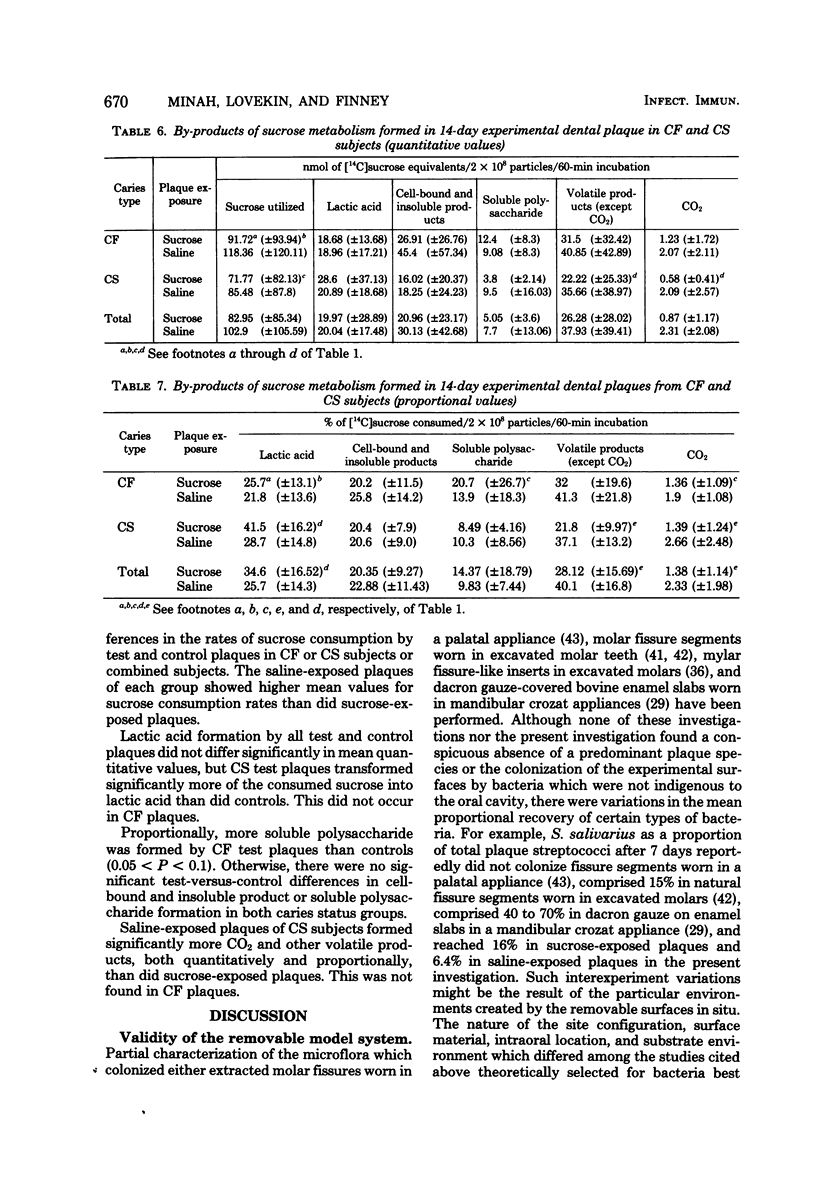
Microbial succession, experimental cariogenicity, and sucrose metabolism were examined in dental plaques which developed on sterile bovine enamel inserts in acrylic palatal appliances. The appliances were worn for a period of 14 days by 10 caries-free and 10 caries-susceptible human volunteers. Three of six enamel inserts on each appliance were exposed extraorally to 10% sucrose in 0.85% saline six times a day, and three were exposed simultaneously to 0.85% saline as a control environment. The responses of the plaques to the high-sucrose environment in both caries status populations were compared. In all plaques, exposure to 10% sucrose stimulated the succession of Veillonella spp., Lactobacillus spp., Streptococcus salivarius, and, to a lesser extent, Streptococcus mutans and a decline in levels of Streptococcus sanguis, Neisseria spp., and gram-negative anaerobic rods. Plaques from caries-free mouths, in contrast to those from caries-susceptible mouths, harbored higher levels of Veillonella spp., gram-negative anaerobic rods, and Neisseria spp. and lower levels of Lactobacillus spp. Sucrose-exposed plaques from caries-free mouths also induced less enamel microhardness changes and formed less lactic acid from [14C]sucrose during a 60-min incubation at 37 degrees C than did comparable plaques from caries-susceptible mouths. The experiments revealed consistent differences in the ecological response to a cariogenic substrate environment in plaques from the two populations, with plaques from caries-free subjects exhibiting less cariogenic potential than those from caries-susceptible subjects.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowden G. H., Hardie J. M., Slack G. L. Microbial variations in approximal dental plaque. Caries Res. 1975;9(4):253–277. doi: 10.1159/000260162. [DOI] [PubMed] [Google Scholar]
- Brown L. R., Dreizen S., Handler S., Johnston D. A. Effect of radiation-induced xerostomia on human oral microflora. J Dent Res. 1975 Jul-Aug;54(4):740–750. doi: 10.1177/00220345750540040801. [DOI] [PubMed] [Google Scholar]
- De Stoppelaar J. D., Van Houte J., Backer DIRKS O. The effect of carbohydrate restriction on the presence of Streptococcus mutans, Streptococcus sanguis and iodophilic polysaccharide-producing bacteria in human dental plaque. Caries Res. 1970;4(2):114–123. doi: 10.1159/000259633. [DOI] [PubMed] [Google Scholar]
- Dennis D. A., Gawronski T. H., Sudo S. Z., Harris R. S., Folke L. E. Variations in microbial and biochemical components of four-day plaque during a four-week controlled diet period. J Dent Res. 1975 Jul-Aug;54(4):716–722. doi: 10.1177/00220345750540040401. [DOI] [PubMed] [Google Scholar]
- Duany L. F., Zinner D. D., Jablon J. M. Epidemiologic studies of caries-free and caries-active students. II. Diet, dental plaque, and oral hygiene. J Dent Res. 1972 May-Jun;51(3):727–733. doi: 10.1177/00220345720510030701. [DOI] [PubMed] [Google Scholar]
- Duchin S., van Houte J. Relationship of Streptococcus mutans and lactobacilli to incipient smooth surface dental caries in man. Arch Oral Biol. 1978;23(9):779–786. doi: 10.1016/0003-9969(78)90155-3. [DOI] [PubMed] [Google Scholar]
- Ellen R. P., Balcerzak-Raczkowski I. B. Differential medium for detecting dental plaque bacteria resembling Actinomyces viscosus and Actinomyces naeslundii. J Clin Microbiol. 1975 Oct;2(4):305–310. doi: 10.1128/jcm.2.4.305-310.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feagin F., Koulourides T., Pigman W. The characterization of enamel surface demineralization, remineralization, and associated hardness changes in human and bovine material. Arch Oral Biol. 1969 Dec;14(12):1407–1417. doi: 10.1016/0003-9969(69)90258-1. [DOI] [PubMed] [Google Scholar]
- Folke L. E., Gawronski T. H., Staat R. H., Harris R. S. Effect of dietary sucrose on quantity and quality of plaque. Scand J Dent Res. 1972;80(6):529–533. doi: 10.1111/j.1600-0722.1972.tb00325.x. [DOI] [PubMed] [Google Scholar]
- GEORG L. K., AJELLO L., PAPAGEORGE C. Use of cycloheximide in the selective isolation of fungi pathogenic to man. J Lab Clin Med. 1954 Sep;44(3):422–428. [PubMed] [Google Scholar]
- Gold O. G., Jordan H. V., Van Houte J. A selective medium for Streptococcus mutans. Arch Oral Biol. 1973 Nov;18(11):1357–1364. doi: 10.1016/0003-9969(73)90109-x. [DOI] [PubMed] [Google Scholar]
- Ikeda T., Sandham H. J., Bradley E. L., Jr Changes in Streptococcus mutans and lactobacilli in plaque in relation to the initiation of dental caries in Negro children. Arch Oral Biol. 1973 Apr;18(4):555–566. doi: 10.1016/0003-9969(73)90076-9. [DOI] [PubMed] [Google Scholar]
- KOULOURIDES T., VOLKER J. F. CHANGES OF ENAMEL MICROHARDNESS IN THE HUMAN MOUTH. Ala J Med Sci. 1964 Oct;1:435–437. [PubMed] [Google Scholar]
- Keene H. J., Shklair I. L. Relationship of Streptococcus mutans carrier status to the development of carious lesions in initially cariesfree recruits. J Dent Res. 1974 Sep-Oct;53(5):1295–1295. doi: 10.1177/00220345740530053801. [DOI] [PubMed] [Google Scholar]
- Loesche W. J., Hockett R. N., Syed S. A. The predominant cultivable flora of tooth surface plaque removed from institutionalized subjects. Arch Oral Biol. 1972 Sep;17(9):1311–1325. doi: 10.1016/0003-9969(72)90164-1. [DOI] [PubMed] [Google Scholar]
- Loesche W. J., Rowan J., Straffon L. H., Loos P. J. Association of Streptococcus mutants with human dental decay. Infect Immun. 1975 Jun;11(6):1252–1260. doi: 10.1128/iai.11.6.1252-1260.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche W. J., Straffon L. H. Longitudinal investigation of the role of Streptococcus mutans in human fissure decay. Infect Immun. 1979 Nov;26(2):498–507. doi: 10.1128/iai.26.2.498-507.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche W. J., Syed S. A. Bacteriology of human experimental gingivitis: effect of plaque and gingivitis score. Infect Immun. 1978 Sep;21(3):830–839. doi: 10.1128/iai.21.3.830-839.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikx F. H., van der Hoeven J. S., König K. G., Plasschaert A. J., Guggenheim B. Establishment of defined microbial ecosystems in germ-free rats. I. The effect of the interactions of streptococcus mutans or Streptococcus sanguis with Veillonella alcalescens on plaque formation and caries activity. Caries Res. 1972;6(3):211–223. doi: 10.1159/000259801. [DOI] [PubMed] [Google Scholar]
- Minah G. E., Loesche W. J. Sucrose metabolism by prominent members of the flora isolated from cariogenic and non-cariogenic dental plaques. Infect Immun. 1977 Jul;17(1):55–61. doi: 10.1128/iai.17.1.55-61.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minah G. E., Loesche W. J. Sucrose metabolism in resting-cell suspensions of caries associated and non-caries-associated dental plaque. Infect Immun. 1977 Jul;17(1):43–54. doi: 10.1128/iai.17.1.43-54.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrom C. A., Koulourides T., Hickman F., McGhee J. R. Microbial characterization of an experimental cariogenic plaque in man. J Dent Res. 1977 Jun;56(6):550–558. doi: 10.1177/00220345770560060101. [DOI] [PubMed] [Google Scholar]
- Ostrom C. A., Koulourides T. The intraoral cariogenicity test in young subjects. Caries Res. 1976;10(6):442–452. doi: 10.1159/000260236. [DOI] [PubMed] [Google Scholar]
- Purdell-Lewis D. J., Groeneveld A., Arends J. Hardness tests on sound enamel and artificially demineralized white spot lesions. Caries Res. 1976;10(3):201–215. doi: 10.1159/000260202. [DOI] [PubMed] [Google Scholar]
- ROGOSA M., FITZGERALD R. J., MACKINTOSH M. E., BEAMAN A. J. Improved medium for selective isolation of Veillonella. J Bacteriol. 1958 Oct;76(4):455–456. doi: 10.1128/jb.76.4.455-456.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROGOSA M., MITCHELL J. A., WISEMAN R. F. A selective medium for the isolation and enumeration of oral lactobacilli. J Dent Res. 1951 Oct;30(5):682–689. doi: 10.1177/00220345510300051201. [DOI] [PubMed] [Google Scholar]
- ROGOSA M. THE GENUS VEILLONELLA. I. GENERAL CULTURAL, ECOLOGICAL, AND BIOCHEMICAL CONSIDERATIONS. J Bacteriol. 1964 Jan;87:162–170. doi: 10.1128/jb.87.1.162-170.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz H. L. Microbial population shifts in developing human dental plaque. Arch Oral Biol. 1967 Dec;12(12):1561–1568. doi: 10.1016/0003-9969(67)90190-2. [DOI] [PubMed] [Google Scholar]
- Rogosa M., Krichevsky M. I., Bishop F. S. Truncated Glycolytic System in Veillonella. J Bacteriol. 1965 Jul;90(1):164–171. doi: 10.1128/jb.90.1.164-171.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shklair I. L., Keene H. J., Cullen P. The distribution of Streptococcus mutans on the teeth of two groups of naval recruits. Arch Oral Biol. 1974 Feb;19(2):199–202. doi: 10.1016/0003-9969(74)90214-3. [DOI] [PubMed] [Google Scholar]
- Shklair I. L., Keene H. J., Simonson L. G. Distribution and frequency of streptococcus mutants in caries-active individuals. J Dent Res. 1972 May-Jun;51(3):882–882. doi: 10.1177/00220345720510034201. [DOI] [PubMed] [Google Scholar]
- Stevens R. H., Mandel I. D. Streptococcus mutans serotypes in caries-resistant and caries-susceptible adults. J Dent Res. 1977 Sep;56(9):1044–1044. doi: 10.1177/00220345770560090201. [DOI] [PubMed] [Google Scholar]
- Svanberg M., Loesche W. J. The salivary concentration of Streptococci mutans and Streptococci sanguis and their colonization of artificial tooth fissures in man. Arch Oral Biol. 1977;22(7):441–447. doi: 10.1016/0003-9969(77)90125-x. [DOI] [PubMed] [Google Scholar]
- Theilade E., Fejerskov O., Karring T., Theilade J. A microbiological study of old plaque in occlusal fissures of human teeth. Caries Res. 1978;12(6):313–319. doi: 10.1159/000260350. [DOI] [PubMed] [Google Scholar]
- Theilade E., Fejerskov O., Prachyabrued W., Kilian M. Microbiologic study on developing plaque in human fissures. Scand J Dent Res. 1974;82(6):420–427. doi: 10.1111/j.1600-0722.1974.tb00396.x. [DOI] [PubMed] [Google Scholar]
- Thott E. K., Folke L. E., Sveen O. B. A microbiologic study of human fissure plaque. Scand J Dent Res. 1974;82(6):428–436. doi: 10.1111/j.1600-0722.1974.tb00397.x. [DOI] [PubMed] [Google Scholar]
- Wrobel W. L., Catalanotto F. A., Walter R. G. Taste thresholds in caries-free and caries-active naval recruits. Arch Oral Biol. 1978;23(10):881–885. doi: 10.1016/0003-9969(78)90291-1. [DOI] [PubMed] [Google Scholar]
- Zengo A. N., Mandel I. D. Sucrose tasting and dental caries in man. Arch Oral Biol. 1972 Mar;17(3):605–607. doi: 10.1016/0003-9969(72)90079-9. [DOI] [PubMed] [Google Scholar]
- van Houte J. Bacterial specificity in the etiology of dental caries. Int Dent J. 1980 Dec;30(4):305–326. [PubMed] [Google Scholar]