Abstract
Objective
To determine if premenopausal ovarian reserve is associated with susceptibility for atherosclerosis.
Methods
Female cynomologus macaques (n = 66, women’s equivalent age = 45 yrs) consumed an atherogenic diet for ~5 months prior to the measurement of a marker of ovarian reserve (antimüllerian hormone, AMH), plasma lipids, follicular phase estradiol (E2) and body weight (BW). Monkeys were then ovariectomized (OVX, n =17) remained premenopausal (PRE, n=20) or induced to have reduce ovarian reserve (ROR, n=29). After 26 additional months on the diet, atherosclerosis measurements and risk variables were reassessed.
Results
No differences in baseline AMH, plasma lipids, BW, E2 or post-diet lipids and BW, were observed among the groups subsequently assigned to OVX, PRE or ROR conditions. Post-diet measurements of atherosclerosis extent did not differ among the groups. However, analysis of plaque size by tertile of baseline AMH revealed that plaques were largest in monkeys that began the experiment with the lowest baseline AMH, followed by those in the middle and high tertiles (plaque extent mm2: Low AMH = 0.76 ± 0.12, Mid AMH = 0.46 ± 0.1, High AMH = 0.34 ± 0.08, p=0.02). Baseline AMH and plaque size were also correlated negatively (r = −0.31, p = 0.01). Plasma lipids were also correlated significantly with plaque extent (all p’s <0.01), but not with AMH.
Conclusions
We report for the first time an inverse relationship between a marker of ovarian reserve (AMH) and subsequent atherosclerosis risk.
Keywords: Atherosclerosis, antimüllerian hormone, ovariectomy, ovarian reserve, cynomologus, nonhuman primate
Introduction
Cardiovascular disease remains the leading cause of mortality in postmenopausal women 1. Coronary heart disease (CHD) due to atherosclerosis accounts for the majority of those deaths, with greater than 50% of women experiencing sudden cardiac death. Furthermore, many of these women succumb without previously reported symptoms 2. Because the underlying atherosclerosis likely develops over a period of decades, these data emphasize the need for improved methods of assessment that would allow earlier identification of women at risk for CHD. However, gaps in knowledge still exist regarding the initiation and progression of atherosclerosis in this population. For example, the Framingham Risk Score has been reported to underestimate risk in peri- and early postmenopausal women. Hence, data from a group of young (~55yrs), non diabetic, postmenopausal women revealed detectable coronary artery calcium, indicative of subclinical atherosclerosis, despite having a low Framingham risk score 3.
Although evidence is accumulating in support of an interventional “window of opportunity” for estrogen treatment in the reduction of CHD in early postmenopausal women 4, the nature of the association between CHD risk and loss of ovarian function (menopause) is still controversial. For example, in a meta-analysis of 18 studies, no relationship between postmenopausal status and cardiovascular disease was found and only a modest effect of early menopause (surgical > natural) was observed5. Conflicting data on this subject may be due, in part, to the inability to account for the variability in type of “menopause” being investigated, including natural vs. surgical (hysterectomy with or without oophorectomy) and early (premature ovarian failure) vs. later menopause. Further, it has not been possible in most studies to account for differences among women in menopausal transition experiences, such as long (6–10 yrs) vs. short (~2yrs) menopausal transitions, or to account for differences in premenopausal reproductive dysfunction prior to the menopausal transition and menopause. Data from women and nonhuman primates suggest that premenopausal ovarian function may affect risk for chronic disease postmenopausally 6, 7. More precise estimations of ovarian reserve using antimüllerian hormone (AMH), along with application of improved clinical staging of reproductive aging using criteria such as those from STRAW criteria 8 for the classification of women in menopausal transition, may increase understanding of the aforementioned relationships.
AMH is a protein hormone in the transforming growth factor-β family and is produced by granulosa cells of small growing (preantral and small antral) follicles in the ovary of rodents, nonhuman and human primates. AMH is positively correlated with primordial follicle numbers (ovarian reserve) in both women 9, 10 and Old World nonhuman primates 11 (r >0.7 for both). Declines in AMH parallel follicle loss as women approach menopause 12 and AMH has been reported to fall to very low levels as early as 5 years prior to the final menstrual period 13. Although AMH’s main role is believed to be the regulation of primordial follicle recruitment 14–17, recent reports in mice indicate that AMH may have neuroprotective effects in the brain 18. It is not known currently whether AMH has direct effects on other non-ovarian tissues.
The purpose of the study presented here was to determine retrospectively whether premenopausal ovarian reserve, as indicated by plasma AMH concentration, is associated with subsequent atherosclerosis progression. The data thus represent an opportunistic observation from a larger study designed to determine the effect of ovarian reserve on risk for chronic disease 19.
Methods
Study Design and Diet
Sixty six cynomologus females (Macaca fascicularis, imported from the Institut Pertanian Bogor, Indonesia) were used for this study. These monkeys are part of an ongoing study designed to investigate the effects of ovarian reserve on cardiovascular and metabolic disease risk. The study design has been described previously 19. Briefly, adult status was confirmed by dentition and evidence of epiphyseal closure, and the mean age and body weight of the monkeys was 15 years (range 7–21yrs) and 3.1 kg. All monkeys were housed in social groups of 2 to 4 individuals and were fed a semi-purified diet formulated to be similar to diets consumed by women in North America. The diet was prepared in the Wake Forest Primate Center diet laboratory and contained 0.20–0.28 mg cholesterol per calorie and derived 19% of its energy from protein (casein and lactalbumin), ~35% from fat and ~47% from carbohydrates. The monkeys were fed 120 Cal of diet/kg body weight daily.
For the first 5 months of the study, monkeys were exposed to a moderate amount of cholesterol (0.20mg/Cal). Cholesterol content of the diet was then increased to 0.28mg chol/Cal for the remainder of the study. After consuming diet for ~ 7 months (baseline period), monkeys were randomized to one of three conditions: 1) bilateral ovariectomy – OVX, n = 17, 2) premenopausal, PRE, n = 20 or 3) premenopausal with reduced ovarian reserve, ROR, n= 29. Following ovariectomy, plasma concentrations of estradiol were monitored to document completeness of the ovariectomy (estradiol concentrations below the level of assay detection). Three monkeys were excluded from the original 20 monkeys in the OVX group, due to resumption of intermittent menstrual bleeding and measureable estradiol concentrations, indicative of the presence of ectopic ovarian tissue 20. Reduced ovarian reserve was achieved by the surgical placement of a biodegradable peri-ovarian implant containing a compound that specifically targets primordial follicles (4-vinlycyclohexenediepoxide, VCD), thereby decreasing ovarian reserve and mimicking the early stages of the menopausal transition 21. This procedure coincided with the ovariectomy surgeries. Treatment with VCD reduced AMH ~ 56% (14.1 ± 1.7 to 6.7 ± 0.5 ng/ml) within four months 19 and remained above the level of detection for the duration of the experiment.
Following ovariectomy and induction ROR, monkeys consumed the Western diet consumption for 26 months. The iliac artery was then biopsied to allow the measurement of atherosclerosis extent (plaque area mm2) without sacrificing the animal. The iliac artery was used because it provides an estimate of coronary artery atherosclerosis extent due to its high correlation with coronary artery atherosclerosis, r > 0.71 22.
All animal manipulations were approved by the Wake Forest University Animal Care and Use Committee and were conducted in accordance with state and federal laws and following the guidelines of the US Department of Health and HumanServices.
Plasma Measures
Antimullerian hormone (AMH) and plasma lipid determinations were done following 5 months consumption of the diet (baseline) and within two months prior to artery collection. Serum AMH was measured using ELISA (DSL, Texas, Intra assay CV: 4.53% at13.62 ng/ml; Inter assay CV: 19.75% at 0.23 ng/ml, 11.42% at 9.61 ng/ml, and 11.27% at1.89 ng/ml). Total plasma cholesterol (TPC) concentrations, high-density lipoprotein cholesterol (HDL-C) concentrations, and plasma triglycerides (TGs) were determined in the Comparative Medicine Clinical Chemistry and Endocrinology Laboratory using reagents (ACE cholesterol, ACE HDL-C, and ACE triglycerides) and instrumentation (ACE ALERA autoanalyzer) from Alfa Wasserman Diagnostic Technologies (West Caldwell, NJ). TPC and HDL-C were standardized to calibrated controls from the Centers for Disease Control and Prevention-National Heart, Lung, and Blood Lipid Standardization Program. Intra- and inter-assay coefficients of variation were less than 5% for all analytes. Non-HDL-C, which approximates the sum of low-density lipoprotein (LDL) cholesterol and very-low-density lipoprotein (VLDL) cholesterol, was calculated by subtracting HDL-C from TPC. Plasma glucose concentrations were determined by the glucose oxidase method using a Glucose Analyzer 2 (Beckman Instruments, Brea CA, USA).Estradiol was measured during the follicular phase across two baseline menstrual cycles, approximately 4 and 5 months after arrival from Indonesia. E2 was measured from serum by a modification 23 of a commercially available radioimmunoassay (RIA) from Diagnostic Products Corporation (Los Angeles, CA). Intra-assay and inter-assay CVs were <4% and 10%, respectively.
Measurement of Atherosclerosis
The common iliac artery was collected from all monkeys after consuming the diet for a total of ~ 33 months (7 months baseline plus 26 months post-OVX). Once collected, the left common iliac (LCI) was opened longitudinally, laid flat, fixed in 4% paraformaldehyde for 24 hrs and then transferred to 70% ethanol. After fixation, the artery was divided into the 3 equal segments, embedded in paraffin, and sections (4 μm) from each block were stained with Verhoeffvan Gieson’s stain. Images of arteries were digitally captured and the extent of atherosclerosis (mm2) determined using methods published previously 24.
Statistical Analysis
For this investigation, the treatment groups (OVX, ROR and PRE) were balanced prior to intervention at baseline for body weight, AMH and TPC: HDLC ratio. To meet normality and equality of variance assumptions, atherosclerosis plaque area (mm2) and AMH were square root (SQRT) transformed and the data were then back transformed for presentation. There were no significant differences in the square root of plaque extent among the 3 arterial blocks measured per LCI (block-1 = 0.7 ± 0.04, block-2 = 0.67 ± 0.05, block-3 = 0.68 ± 0.05, p = 0.22) and therefore the mean of plaque area (mm2) for all three blocks was used as the outcome variable for all further analysis of atherosclerosis extent. AMH for all monkeys measured at baseline was divided into tertiles with the following ranges: AMH-Low = 0.96–7.6, AMH-Mid = 7.71–14.4 and AMH-High =14.6–50.6 ng/ml. Atherosclerosis extent was also analysed according to baseline median AMH within each treatment group. Statistical analysis was done using SAS 9.2 and the SAS-based software -JMP® V-9. General linear models were used to examine the differences in plaque area, plasma lipids, estradiol (E2) and body weight among tertiles of AMH as well as among treatment groups. A multivariate regression model was used to determine correlations among variables. The significance was set at p<.05.
Results
Atherosclerosis extent is not affected by treatment but is predicted by baseline AMH
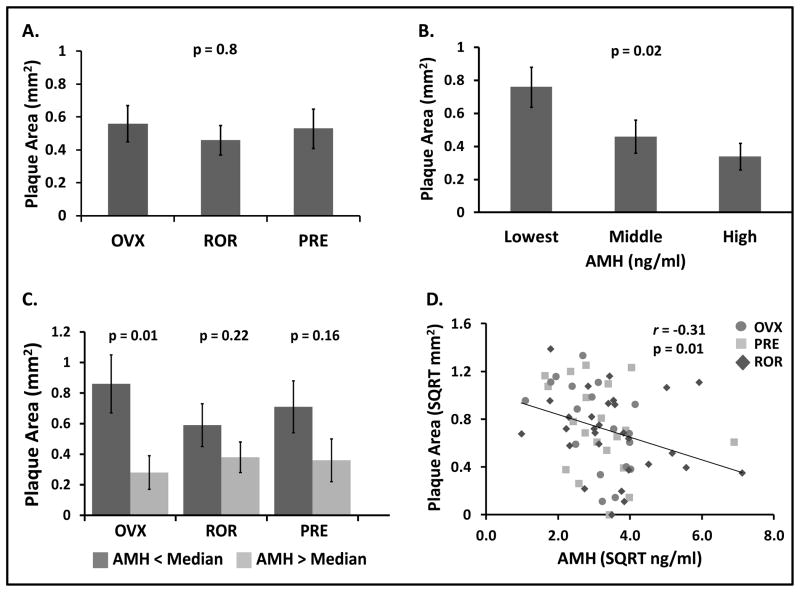
The common iliac artery was collected from all monkeys after consuming the diet for a total of ~ 33 months (baseline plus post-treatment period). Mean plaque area (mm2) did not differ among the treatment groups (OVX = 0.75 ± 0.08, ROR-0.68± 0.07PRE = 0.73 ± 0.08, p = 0.8 overall, non-transformed means) (Figure 1A). Because the treatment groups were balanced prior to treatment according to baseline AMH, a procedure not done in previous studies, data were then analyzed to determine whether baseline AMH for all monkeys was associated with subsequent atherosclerosis extent. Atherosclerosis extent according to tertile of baseline AMH is depicted in Figure 1B. Monkeys with the lowest plasma AMH at baseline developed the most atherosclerosis 26 months later. To investigate the relationship between baseline AMH and subsequent atherosclerosis in each individual treatment group, monkeys were divided into two groups using the median of baseline AMH. Figure 1C shows that in all groups, baseline AMH appears to be related to atherosclerosis outcomes, although only the OVX group reached statistical significance. Finally, the negative correlation between baseline AMH and plaque area was also significant (r = − 0.31, p <0.01) (Figure 1D). Neither AMH measured at the time of artery collection, nor percent change in AMH from baseline to post-treatment, were significantly associated with plaque extent (p = 0.6 for both comparisons).
FIG. 1.
A: Iliac artery atherosclerosis plaque area (mm2) among OVX (n = 17), ROR (n = 29), and PRE (n = 20) cynomolgus monkeys after ~2 years of diet consumption. B: Plaque area depicted according to tertiles of baseline plasma antimullerian hormone (AMH), all treatment groups combined. C: Plaque area depicted according to median baseline AMH for each individual treatment group. D: Correlation between baseline AMH and atherosclerosis, all treatment groups combined: OVX (○), PREMENOP (□), and ROR subset (diamond shape). Data are presented as mean ± SE plaque area (back transformed from SQRT) (A–C) and as the association between the SQRT of plaque size ± SE and SQRT of AMH (r = −0.31, P < 0.01) (D). OVX, ovariectomized; ROR, reduced ovarian reserve; PRE, premenopausal control; AMH, antimüllerian hormone; PREMENOP, premenopausal; SQRT, square root.
Plasma Lipids Correlate Significantly with Atherosclerosis
Plasma lipids measured at baseline (seven months after beginning to consume a cholesterol containing diet) correlated significantly with plaque area (TPC, r = 0.42, p <0.001; LDL+VLDLC, r = 0.46, p <0.001; HDLC r = −0.38, p=0.002; and TPC:HDLC, r=0.44, p <0.001). The correlations between lipids and atherosclerosis remained significant after 33 months of consuming the experimental diet (TPC, r = 0.70, p < 0.001; LDL+VLDLC r = 0.72, p < 0.001; HDLC r = − 0.39, p=0.001; and TPC: HDLC r = 0.61, p < 0.001).
Baseline and Post-Ovariectomy AMH, BW, E2 and Plasma Lipids
AMH, body weight, and plasma lipids were measured after consuming the diet for 7 months (baseline) and 26 months after OVX. Follicular phase estradiol was measured at baseline only. Please note: PRE and ROR groups were combined into a single PRE group (n=49) for analysis because no baseline or post-treatment differences were observed between the two with respect to the aforementioned variables. There were no significant differences at baseline between monkeys assigned to OVX (n=17) or PRE (n= 49) conditions: (AMH, 10.6 ± 2 vs. 13 ± 1.3 ng/mL, p = 0.3; estradiol, 21.8 ± 6 vs. 25.5 ± 4 pg/mL, p = 0.6; BW, 2.99 ± 0.1 vs. 3.15 ± 0.1 kg, p = 0.3; TPC, 241.7 ± 13 vs. 238.1 ± 7.9 mg/dL, p = 0.8; HDLC, 45.3 ± 3.5 vs. 43.9 ± 2.1 mg/dL, p = 0.7; LDL+VLDLC, 196.4 ± 14.5 vs. 193.5 ± 9 mg/dL, p = 0.9 or TPC:HDLC, 6.0 ± 0.7 vs. 6.1 ± 0.4, p=0.9). Four months post-ovariectomy AMH was below the level of detection. After 26 months, no significant differences were observed in between OVX and PRE monkeys respectively in BW (3.14 ± 0.2 vs. 3.46 ± 0.12 kg, p = 0.2), TPC (318.2 ± 86 vs. 300.3 ± 71 mg/dL, p = 0.4), HDLC (46.7± 17 vs. 45.9 ± 19 mg/dL, p= 0.9) or LDL+VLDLC (271.6 ± 19.5 vs. 254.5 ± 12 mg/dL, p = 0.5), TPC:HDLC (7.8 ± 3 vs. 7.6 ± 3, p=0.9). Similarly, baseline plasma lipids, BW, E2, nor post-treatment plasma lipids and BW differed according to baseline AMH tertile (Table 1).
Table 1.
Atherosclerosis and CHD Risk Variables According to Tertile of Baseline Antimullerian Hormone
| AMH Low (n = 22) | AMH Mid (n = 22) | AMH High (n = 22) | p | |
|---|---|---|---|---|
| Iliac Artery Plaque Area (mm2) | ||||
| Post-Treatment | 0.76 ± 0.12 | 0.46 ± 0.1 | 0.34 ± 0.08 | 0.02 |
| AMH (ng/ml) | ||||
| Baseline Tertile Mean | 4.94 ± 1.4 | 10.59 ± 1.3 | 21.3 ± 1.3 | 0.001 |
| Tertile Range | 0.96–7.6 | 7.71 – 14.4 | 14.6 – 50.6 | NA |
| Estradiol (pg/ml) | ||||
| Baseline | 20.1 ± 5.3 | 21.6 ± 5.5 | 31.6 ± 5.3 | 0.3 |
| Body weight (kg) | ||||
| Baseline | 3.2 ± 0.1 | 3.1 ± 0.1 | 3.1 ± 0.1 | 0.86 |
| Post-Treatment | 3.36 ± 0.2 | 3.31 ± 0.2 | 3.36 ± 0.2 | 0.97 |
| TPC (mg/dl) | ||||
| Baseline | 245.1 ± 11 | 237.2 ± 13 | 235.2 ± 11 | 0.82 |
| Post-Treatment | 327.9 ± 16 | 290.9 ± 15 | 297.5 ± 17 | 0.23 |
| HDLC (mg/dl) | ||||
| Baseline | 44.2 ± 3 | 45.8 ± 3 | 42.8 ± 3 | 0.8 |
| Post-Treatment | 44.7 ± 4 | 46.9 ± 4 | 43.3 ± 4 | 0.86 |
| LDLC + VLDLC (mg/dl) | ||||
| Baseline | 199.5 ± 14 | 191.4 ± 13 | 192.5 ± 13 | 0.9 |
| Post-Treatment | 283.2 ± 18 | 244 ± 17 | 254.2 ± 19 | 0.3 |
| TPC: HDLC | ||||
| Baseline | 6.2 ± 0.6 | 6.1 ± 0.6 | 6.0 ± 0.6 | 0.5 |
| Post-Treatment | 8.4 ± 0.7 | 7.4 ± 0.8 | 7.6 ± 0.8 | 0.56 |
| LDLC + VLDLC: HDCL | ||||
| Baseline | 5.2 ± 0.6 | 5.1 ± 0.6 | 5.0 ± 0.6 | 1 |
| Post-Treatment | 7.4 ± 0.7 | 6.4 ± 0.7 | 6.6 ± 0.8 | 0.6 |
Baseline and 26 months post-diet measures of atherosclerosis, hormone and risk variable data are presented according to baseline (pre-treatment group assignment) tertiles of plasma AMH for all monkeys. Data are Mean ±SE.
Discussion
In the present study there was no effect of ovariectomy or reduced ovarian reserve on atherosclerosis extent when compared to premenopausal control monkeys. This finding was unexpected given previous reports of increased CHD risk following oophorectomy in both monkeys and women 25, 26. However, the current study differs from previous monkeys studies because each treatment condition was balanced for plasma AMH (a previously validated marker of ovarian reserve or reproductive age) prior to the intervention. Consequently, we investigated retrospectively, the potential relationship between AMH and subsequent atherosclerosis development. As a result, we found that AMH, measured prior to long-term (26 months) consumption of an atherogenic diet, was negatively correlated with atherosclerosis extent and there was a significant, monotonic increase in atherosclerosis extent across tertiles of baseline AMH. Furthermore, within each individual treatment group, monkeys that fell below the median AMH at baseline developed more atherosclerosis than those above the median. Interestingly, it was only in the OVX group that this finding reached statistical significance. There were no differences in plasma lipids or lipoproteins among the tertiles of AMH, either at baseline or at the time of atherosclerosis measurement, indicating that the observed relationships between AMH and subsequent atherosclerosis were independent of plasma lipids. Taken together, these data suggest that AMH may be a marker of arterial susceptibility to atherosclerosis, a finding that may have been worsened by ovariectomy. Consistent with some studies of women however, the data also indicate that the trajectory of atherosclerosis progression was not influenced by estrogen depletion (ovariectomy) compared to premenopausal subjects.
The observed relationship between low AMH and subsequent atherosclerosis development in premenopausal monkeys may relate to arterial estrogen exposure prior to initiation of the atherogenic diet. That is, AMH may be a marker of arterial susceptibility to atherosclerosis rather than having a direct relationship with atherosclerosis progression. It is well accepted that estrogen is atheroprotective through increased vascular responsiveness, attenuation of inflammatory mediators and cytokines, and decreased oxidative stress 27. In addition, estrogen inhibits smooth muscle cell proliferation and reduces cholesterol and macrophage influx into the artery wall 28. AMH is produced by the granulosa cells of small growing ovarian follicles (primary, secondary and small pre-antral) and is highly correlated with larger antral follicle number 29, 30. Waves of growing and antral follicles are responsible for the majority of estradiol production by the ovary during the menstrual cycle. Consequently, lower AMH may indicate lower overall estradiol-production. In support of this hypothesis are the results of a study of premenopausal women > 40 years of age in which peak estradiol was observed to be lowest in women who were also in the lowest tertile of AMH 31. In addition, it has been reported that premenopausal women with diminished ovarian reserve (and likely low AMH) had impaired urinary excretions of estrone conjugate 32. Taken together this data indicate that it is possible that the monkeys in our study that were in the lowest tertile of AMH prior to dietary challenge may have had a history of chronically lower estrogen levels, resulting in increased susceptibility to initiation and progression of atherosclerosis. In addition, if AMH is a marker of estradiol exposure and subsequent artery susceptibility to the initiation of atherosclerosis, then it is not surprising that experimental reduction of AMH via chemical or surgical means was not associated with atherosclerosis extent.
We were not able to confirm a relationship between baseline E2 and atherosclerosis in this study. This finding might be explained in relation to the E2 sampling time period. Monkeys were sampled within ~ 5 months of arrival at our center from Indonesia and follicular phase samples were collected from two menstrual cycles only (1 sample each cycle). Cynomologus monkeys are known to experience hypothalamic suppression of menstrual cyclicity and cyclic hormones (estradiol, progesterone) following extreme stressors, such as travel and introduction to new social groups 6–33. Therefore, our sampling method may not adequately represent premenopausal E2 exposure, and thus degree of pre-existing atheroprotection. More frequent measures of E2 across numerous menstrual cycles, over a longer period (12–18 months) may be required for accurate determinations of pre-existing E2 status.
Another potential explanation for our finding may relate to arterial aging. AMH is a marker of reproductive aging and is becoming accepted as a tool for predicting time to menopause. A recent long-term follow up study of normo-ovulatory women reported that women with low AMH for their age became menopausal earlier than those in the higher percentiles of AMH 34. It is possible that ovarian aging and somatic aging occur in parallel, and that AMH is also a marker for generalized aging. Studies in rodents indicate that estrogen’s vasoprotective and anti-inflammatory effects are diminished in aging arteries of ovariectomized older rats compared to young OVX rats 35. It is also possible that circulating pro-inflammatory mediators increase with age, irrespective of estradiol production, resulting in increased arterial susceptibility to atherosclerosis 36–38.
It is notable that atherosclerosis extent was not increased in ovariectomized monkeys compared to premenopausal control subjects. It is unclear how this observation may or may not be in contrast to some previous studies dealing with the effect of ovariectomy on atherosclerosis progression. Monkeys in the study presented here consumed an atherogenic diet for 7 months prior to ovariectomy and from historical studies we expect them to have developed small fibro-fatty plaques by this time 39. The amount of cholesterol in the atherogenic diet and the length of time the monkeys consume the diet prior to ovariectomy may modulate the effect of estrogen depletion on atherosclerosis progression. That is, it is possible that the trajectory for atherosclerosis progression was set and was not further exacerbated by estrogen depletion, except in monkeys that had low AMH prior to OVX. Never the less, this finding would appear to be in contrast to a previous cynomologus monkey study that observed increased coronary artery atherosclerosis in ovariectomized monkeys compared to premenopausal monkeys 25. Interestingly, OVX monkeys in that study did not differ in atherosclerosis extent from premenopausal females classified as socially stressed (subordinate) and anovulatory (progesterone values below expected for ovulatory cycles). Thus, the main driver of atherosclerosis protection in that study was dominant social status; a finding reported previously to be associated with normal ovarian function 6. There was no effect of social status on atherosclerosis in the present study. In contrast to the aforementioned study, findings from the present study are supported by a nonhuman primate experiment that included both premenopausal and OVX groups fed an amount of cholesterol in the diet (0. 28mg chol/Cal) similar to our study, for a similar amount of time (30 months)40. In that study, no significant differences in coronary artery atherosclerosis were observed between OVX and premenopausal monkeys (0.20 ± 0.04 vs. 0.11± 0.04, p>0.05).
There are limitations to this study that deserve mention. First, our hypothesis was not formulated apriori. As such, these findings should be viewed as retrospective and therefore preliminary until tested prospectively. Second, we know from historical studies 41 that cynomologus macaques do not develop significant coronary artery atherosclerosis while consuming low cholesterol diets (i.e. in the wild or at the Indonesian Primate Center) and therefore it is assumed that the monkeys in this study did not have significant iliac artery atherosclerosis at the time of diet initiation. However, arterial function can be compromised in the absence of atherosclerosis (i.e. arterial stiffness), and thus other baseline measures of arterial function (i.e. pulse wave velocity, in vitro vascular reactivity of arteries taken via biopsy, arterial gene expression) might have been informative and should be studied prospectively. Finally, AMH is not perfectly correlated with follicle numbers and the coefficient of variation can be large. However, AMH was measured 3 times at baseline and approximately every 6 months post-treatment (data not shown) and the inter-individual variation across time in premenopausal monkeys was less than 10% overall when the samples were assayed together in a single batch, thus giving us confidence that baseline AMH was representative of ovarian reserve in our monkeys.
Conclusion
In conclusion, our primary findings indicate that a relationship exists between a marker of ovarian reserve (AMH) and atherosclerosis progression in monkeys. This relationship is independent of the strong association between plasma lipid concentrations and atherosclerosis. This finding may have implications for CHD risk determination and early prevention in women who are late reproductive age or transitioning to menopause.
Acknowledgments
Financial support: None
Funding Support: This work was supported by the National Institute of Aging (R01AG027847-SEA).
The author would like to acknowledge Patricia B. Hoyer, University of Arizona, for her intellectual contributions and guidance related to the non human primate reduced ovarian reserve model. In addition, we wish to thank Nanette Santoro and Rogerio Lobo for their advice and support and A. Dewayne Cairnes, Margaret May, Maryanne Post, Debbie Golden, Edison Floyd for their technical support.
Abbreviations
- AMH
antimüllerian hormone
- E2
estradiol
Footnotes
Conflict of Interest: None
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, et al. Writing Group Members, On behalf of the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2012;125(1):188–197. doi: 10.1161/CIR.0b013e3182456d46. [DOI] [PubMed] [Google Scholar]
- 2.Deo R, Vittinghoff E, Lin F, Tseng ZH, Hulley SB, Shlipak MG. Risk factor and prediction modeling for sudden cardiac death in women with coronary artery disease. Arch Intern Med. 2011;171:1703–9. doi: 10.1001/archinternmed.2011.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Michos ED, Nasir K, Braunstein JB, Rumberger JA, Budoff MJ, Post WS, Blumenthal RS. Framingham risk equation underestimates subclinical atherosclerosis risk in asymptomatic women. Atherosclerosis. 2006;184:201–6. doi: 10.1016/j.atherosclerosis.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Hodis HN, Mack WJ. A “window of opportunity:” the reduction of coronary heart disease and total mortality with menopausal therapies is age- and time-dependent. Brain Res. 2011;1379:244–52. doi: 10.1016/j.brainres.2010.10.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atsma F, Bartelink MEL, Grobbee DE, van der Schow YT. Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: a meta-analysis. Menopause. 2006:265–279. doi: 10.1097/01.gme.0000218683.97338.ea. [DOI] [PubMed] [Google Scholar]
- 6.Kaplan JR, Chen H, Appt SE, Lees CJ, Franke AA, Berga SL, Wilson ME, Manuck SB, Clarkson TB. Impairment of ovarian function and associated health-related abnormalities are attributable to low social status in premenopausal monkeys and not mitigated by a high-isoflavone soy diet. Hum Reprod. 2010;25:3083–94. doi: 10.1093/humrep/deq288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Donnell E, Goodman JM, Harvey PJ. Cardiovascular Consequences of Ovarian Disruption: A Focus on Functional Hypothalamic Amenorrhea in Physically Active Women. J Clin Endocrinol Metab. 2011;96:3638–48. doi: 10.1210/jc.2011-1223. [DOI] [PubMed] [Google Scholar]
- 8.Hansen KR, Craig LB, Zavy MT, Klein NA, Soules MR. Ovarian primordial and non growing follicle counts according to the Stages of Reproductive Aging Workshop (STRAW) staging system. Menopause. 2012;19:164–71. doi: 10.1097/gme.0b013e31823b0b2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hansen KR, Hodnett GM, Knowlton N, Craig LB. Correlation of ovarian reserve tests with histologically determined primordial follicle number. Fertility and Sterility. 2011;95:171–175. doi: 10.1016/j.fertnstert.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 10.GÜlekli B, Bulbul Y, Onvural A, Yorukoglu K, Posaci C, Demir N, Erten O. Accuracy of ovarian reserve tests. Hum Reprod. 1999;14:2822–6. doi: 10.1093/humrep/14.11.2822. [DOI] [PubMed] [Google Scholar]
- 11.Appt SE, Clarkson TB, Chen H, Adams MR, Christian PJ, Hoyer PB, Wilson ME, Kaplan JR. Serum antimüllerian hormone predicts ovarian reserve in a monkey model. Menopause. 2009;16:597–601. doi: 10.1097/gme.0b013e3181906fb6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tehrani FR, Solaymani-Dodaran M, Azizi F. A single test of antimullerian hormone in late reproductive-aged women is a good predictor of menopause. Menopause. 2009 Jul;16:797–802. doi: 10.1097/gme.0b013e318193e95d. [DOI] [PubMed] [Google Scholar]
- 13.Sowers MR, Eyvazzadeh AD, McConnell D, Yosef M, Jannausch ML, Zhang D, Harlow S, Randolph JF., Jr Anti-mullerian hormone and inhibin B in the definition of ovarian aging and the menopause transition. J Clin Endocrinol Metab. 2008;93:3478–83. doi: 10.1210/jc.2008-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Modi D, Bhartiya D, Puri C. Developmental expression and cellular distribution of Mullerian inhibiting substance in the primate ovary. Reproduction. 2006;132:443–53. doi: 10.1530/rep.1.01178. [DOI] [PubMed] [Google Scholar]
- 15.Visser JA, Themmen AP. Anti-Müllerian hormone and folliculogenesis. Mol Cell Endocrinol. 2005;234:81–6. doi: 10.1016/j.mce.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Downs JL, Urbanski HF. Neuroendocrine changes in the aging reproductive axis of female rhesus macaques (Macaca mulatta) Biol Reprod. 2006;75:539–46. doi: 10.1095/biolreprod.106.051839. [DOI] [PubMed] [Google Scholar]
- 17.Sahambi SK, Visser JA, Themmen AP, Mayer LP, Devine PJ. Correlation of serum anti Müllerian hormone with accelerated follicle loss following 4-vinylcyclohexene diepoxide-induced follicle loss in mice. Reprod Toxicol. 2008;26:116–22. doi: 10.1016/j.reprotox.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Lebeurrier N, Launay S, Macrez R, Maubert E, Legros H, Leclerc A, Jamin SP, Picard JY, Marret S, Laudenbach V, Berger P, Sonderegger P, Ali C, di Clemente N, Vivien D. Anti-Mullerian-hormone-dependent regulation of the brain serine-protease inhibitor neuroserpin. J Cell Sci. 2008;121:3357–65. doi: 10.1242/jcs.031872. [DOI] [PubMed] [Google Scholar]
- 19.Appt SE, Clarkson TB, Hoyer PB, Kock ND, Goode AK, May MC, Persyn JT, Vail NK, Ethun KF, Chen H, Sen N, Kaplan JR. Experimental induction of reduced ovarian reserve in a nonhuman primate model (Macaca fascicularis) Comp Med. 2010;60:380–8. [PMC free article] [PubMed] [Google Scholar]
- 20.Ethun KF, Cline JM, Appt SE. Endocrine profile of an ovariectomized cynomologus monkey (Macaca fascicularis) with a supernumerary ovary. Comp Med. 2011 Oct;61(5):462–6. [PMC free article] [PubMed] [Google Scholar]
- 21.Hoyer PB, Sipes IG. Development of an animal model for ovotoxicity using 4-vinylcyclohexene: A case study. Birth Defects Research Part B: Developmental and Reproductive Toxicology. 2007;80:113–125. doi: 10.1002/bdrb.20103. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan JR, Adams MR, Anthony MS, Morgan TM, Manuck SB, Clarkson TB. Dominant social status and contraceptive hormone treatment inhibit atherogenesis in premenopausal monkeys. Arterioscler Thromb Vasc Biol. 1995;15:2094–100. doi: 10.1161/01.atv.15.12.2094. [DOI] [PubMed] [Google Scholar]
- 23.Pazol K, Wilson ME, Wallen K. Medroxyprogesterone acetate antagonizes the effects of estrogen treatment on social and sexual behavior in female macaques. J Clin Endocrinol Metab. 2004;89:2998–3006. doi: 10.1210/jc.2003-032086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clarkson TB, Anthony MS, Morgan TM. Inhibition of postmenopausal atherosclerosis progression: a comparison of the effects of conjugated equine estrogens and soy phytoestrogens. J Clin Endocrinol Metab. 2001;86:41–47. doi: 10.1210/jcem.86.1.7151. [DOI] [PubMed] [Google Scholar]
- 25.Adams MR, Kaplan JR, Clarkson TB, Koritnik DR. Ovariectomy, social status, and atherosclerosis in cynomolgus monkeys. Arteriosclerosis. 1985 Mar-Apr;5(2):192–200. doi: 10.1161/01.atv.5.2.192. [DOI] [PubMed] [Google Scholar]
- 26.Parker WH, Broder MS, Chang E, Feskanich D, Farquhar C, Liu Z, Shoupe D, Berek JS, Hankinson S, Manson JE. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the nurses’ health study. Obstet Gynecol. 2009 May;113(5):1027–37. doi: 10.1097/AOG.0b013e3181a11c64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xing D, Nozell S, Chen Y, Hage F, Oparil S. Estrogen and mechanisms of vascular protection. Arertioscler Thromb Vasc Biol. 2009;29:289–295. doi: 10.1161/ATVBAHA.108.182279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adams MR, Washburn SA, Wagner JA, Williams JK, Clarkson TB. Arterial Changes: Estrogen deficiency and effects of hormone replacement. In: Lobo RA, editor. Treatment of the postmenopausal woman; basic and clinical aspects. 2. Lippincott Williams & Wilkins; Philadelphia, PA: 1999. [Google Scholar]
- 29.de Vet A, Laven JS, de Jong FH, Themmen AP, Fauser BC. Antimüllerian hormone serum levels: a putative marker for ovarian aging. Fertil Steril. 2002;77:357–62. doi: 10.1016/s0015-0282(01)02993-4. [DOI] [PubMed] [Google Scholar]
- 30.Kunt C, Ozaksit G, Keskin Kurt R, Cakir Gungor AN, Kanat-Pektas M, Kilic S, Dede A. AntiMullerian hormone is a better marker than inhibin B, follicle stimulating hormone, estradiol or antral follicle count in predicting the outcome of in vitro fertilization. Arch Gynecol Obstet. 2011;283:1415–21. doi: 10.1007/s00404-011-1889-7. [DOI] [PubMed] [Google Scholar]
- 31.Lee RK, Wu FS, Lin MH, Lin SY, Hwu YM. The predictability of serum anti-Müllerian level in IVF/ICSI outcomes for patients of advanced reproductive age. Reprod Biol Endocrinol. 2011;9:115. doi: 10.1186/1477-7827-9-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pal L, Zhang K, Zeitlian G, Santoro N. Characterizing the reproductive hormone milieu in infertile women with diminished ovarian reserve. Fertil Steril. 2010;93:1074–9. doi: 10.1016/j.fertnstert.2008.10.069. [DOI] [PubMed] [Google Scholar]
- 33.Kaplan JR, Adams MR, Clarkson TB, Koritnik DR. Psychosocial influences on female ‘protection’ among cynomolgus macaques. Atherosclerosis. 1984;53:283–95. doi: 10.1016/0021-9150(84)90129-1. [DOI] [PubMed] [Google Scholar]
- 34.Broer SL, Eijkemans MJ, Scheffer GJ, van Rooij IA, de Vet A, Themmen AP, Laven JS, de Jong FH, Te Velde ER, Fauser BC, Broekmans FJ. Anti-mullerian hormone predicts menopause: a long-term follow-up study in normoovulatory women. J Clin Endocrinol Metab. 2011;96:2532–9. doi: 10.1210/jc.2010-2776. [DOI] [PubMed] [Google Scholar]
- 35.Miller AP, Xing D, Feng W, Fintel M, Chen YF, Oparil S. Aged rats lose vasoprotective and antiinflammatory actions of estrogen in injured arteries. Menopause. 2007;14:251–60. doi: 10.1097/01.gme.0000235366.39726.f6. [DOI] [PubMed] [Google Scholar]
- 36.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–46. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 37.Najjar SS, Scuteri A, Lakatta EG. Arterial aging: is it an immutable cardiovascular risk factor? Hypertension. 2005;46:454–62. doi: 10.1161/01.HYP.0000177474.06749.98. [DOI] [PubMed] [Google Scholar]
- 38.Wang M, Takagi G, Asai K, Resuello RG, Natividad FF, Vatner DE, Vatner SF, Lakatta EG. Aging increases aortic MMP-2 activity and angiotensin II in nonhuman primates. Hypertension. 2003;41:1308–16. doi: 10.1161/01.HYP.0000073843.56046.45. [DOI] [PubMed] [Google Scholar]
- 39.Clarkson TB, Mehaffey MH. Coronary heart disease of females: lessons learned from nonhuman primates. Am J Primatol. 2009;71:785–93. doi: 10.1002/ajp.20693. [DOI] [PubMed] [Google Scholar]
- 40.Obasanjo LO, Clarkson TB, Weaver DS. Effects of anabolic steroid nandrolone decanoate on plasma lipids and coronary arteries of female cynomologus macaques. Metabolism. 1996;45:463–468. doi: 10.1016/s0026-0495(96)90220-6. [DOI] [PubMed] [Google Scholar]
- 41.Shelton KA, Clarkson TB, Kaplan JR. Nonhuman primate models of atherosclerosis. In: Abee CR, Mansfield K, Tardif SD, Morris T, editors. Nonhuman Primates in Biomedical Research. 2. Elsevier; in press. [Google Scholar]