Abstract
Poor executive functioning has been implicated in children’s concurrent and future behavioral difficulties, making work aimed at understanding processes related to the development of early executive function (EF) critical for models of developmental psychopathology. Deficits in EF have been associated with adverse prenatal experiences, genetic influences, and temperament characteristics. However, our ability to disentangle the predictive and independent effects of these influences has been limited by a dearth of genetically-informed research designs that also consider prenatal influences. The present study examined EF and language development in a sample of 361 toddlers who were adopted at birth and reared in non-relative adoptive families. Predictors included genetic influences (as inherited from birth mothers), prenatal risk, and growth in child negative emotionality. Structural equation modeling indicated that the effect of prenatal risk on toddler effortful attention at age 27 months became nonsignificant once genetic influences were considered in the model. In addition, genetic influences had unique effects on toddler effortful attention. Latent growth modeling indicated that increases in toddler negative emotionality from 9 to 27 months were associated with poorer delay of gratification and poorer language development. Similar results were obtained in models incorporating birth father data. Mechanisms of intergenerational transmission of EF deficits are discussed.
Keywords: executive function, toddler, adoption, genetic, prenatal, temperament
Deficits in executive function (EF), defined as poor skills in monitoring and controlling one’s thoughts, actions, and emotions (Carlson & Moses, 2001), have been associated with a host of deleterious outcomes in children, including aggression, inattention, behavior problems, and peer problems (Bridgett, Valentino, & Hayde, in press; Darwish, Esquivel, Houtz, & Alfonso, 2001; Fantuzzo, Weiss, Atkins, Meyers, & Nooze, 1998; Floyd & Kirby, 2001; Riggs, Blair, & Greenberg, 2003). Conversely, higher levels of EF are predictive of positive adjustment outcomes, including on-task behavior and perspective taking skills (Blair & Peters, 2003; Carlson, Mandell, & Williams, 2004). In addition, variation in EF has been associated with verbal intelligence, including language outcomes, suggesting the possibility of overlapping etiological pathways. For example, children with better EF evidence greater success in language-based subjects across the preschool and school-age years as compared to children with poorer EF, as indexed by measures of reading, spelling, and language (Blair & Razza, 2007; Deater-Deckard, Mullineaux, Petrill, & Thompson, 2009). Better EF has also been associated with better performance in mathematics (e.g., Bull, Espy, & Wiebe, 2008; Blair & Razza, 2007; Clark, Pritchard, & Woodward, 2010). Together, this body of work indicates the centrality of EF in affecting positive outcomes for children and suggests the importance of disentangling the factors that relate to its development.
A number of studies have indicated specific processes that contribute to children’s EF. Factors shown to be associated with variation in EF during early childhood include emotion regulation skills (Carlson & Wang, 2007; Eisenberg & Spinrad, 2004; Zelazo & Cunningham, 2007) and prenatal exposure to substance use (Connor, Sampson, Bookstein, Barr, & Streissguth, 2000). In addition, gender may also be associated with EF; a meta-analytic study indicated that girls have better EF than boys (Else-Quest, Hyde, Goldsmith, & Van Hulle, 2006), although some studies have not found a gender difference (Gunner, Tout, deHaan, & Pierce, 1997). Furthermore, there is evidence from twin studies that genetic influences explain a substantial portion of individual differences in EF performance (Friedman et al., 2008; Polderman et al., 2007). However, almost all prior developmental studies of EF have been conducted with children reared in biological families. When children are raised by biological parents, the influences of prenatal risk, genes, and the postnatal rearing environment cannot be disentangled because the child shares all three with the rearing parents. In addition to this limitation, few studies have examined the development of EF during very early childhood (0–2 years), and none have simultaneously considered genetic, negative emotion, and prenatal influences on EF during early development.
The present study integrates and extends prior work by using a prospective adoption design to disentangle the contributions of prenatal risk, genetic influences, and negative emotionality on children’s EF at 27 months of age, while simultaneously considering the association between EF and language. In the adoption design, children share genes and a prenatal environment with their birth parents but not with their rearing parents. As such, this design uniquely allows for an assessment of the independent contributions of genetic and prenatal influences on young children’s EF, separate from postnatal rearing environment factors.
Executive Function During Early Childhood
EF is a broad category of discrete inter-related cognitive processes, including attentional control, attention shifting, cognitive flexibility, self-monitoring, planning, inhibitory control of prepotent responses, and working memory, and has been associated with activation of the prefrontal cortex in neuroimaging studies (Anderson, 2002; Roth, Randolph, Koven, & Isquith, 2006; Wood & Smith, 2008). The prefrontal cortex undergoes a protracted period of postnatal development (e.g., increases in the density of synapses and gray matter) that begins during infancy and extends through adolescence and into adulthood. This development is linked with age-related improvements in tasks measuring various components of EF (Best, Miller, & Jones, 2009; Garon, Bryson, & Smith, 2008), suggesting the importance of studying factors associated with EF early in development. Several tasks reliably measure EF in 24-month old children, including a shape Stroop task and a gift delay task (Carlson et al., 2004). In addition, Kochanska, Murray, and Harlan (2000) found high reliability in EF at 22 months, as well as predictability from 22-month EF to preschool EF and emotion regulation. The present study used two validated EF tasks for young children: a shape Stroop task, conceptualized as drawing upon effortful attention skills, and a gift delay task, which requires that children employ behavioral inhibitory control skills for successful completion (Kochanska et al., 2000).
Prenatal Experiences and Executive Function Deficits
Extant research indicates associations between adverse prenatal experiences and later deficits in EF. Children whose mothers engage in substance use during pregnancy have an increased likelihood of EF deficits; preschoolers through adolescents with fetal alcohol syndrome/effects make more perseverative errors and show impairments in planning, response inhibition, abstract thinking, and cognitive flexibility (Kodituwakku, Handmaker, Cutler, Weathersby, & Handmaker, 1995; Kopera-Frye, Dehaene, & Streissguth, 1996; Mattson, Goodman, Caine, Delis, & Riley, 1999). These deficits remain even when IQ scores are controlled (Connor et al., 2000), suggesting that prenatal alcohol exposure might lead to selective effects on the prefrontal cortex. Prenatal exposure to tobacco (Huijbregts, Warren, Sonneville, & Swaab-Barneveld, 2008), marijuana (Fried, 2002; Fried & Smith, 2001), and cocaine (Bridgett & Mayes, 2011; Espy, Kaufmann, & Glisky, 1999) have also been linked to deficits in children’s EF. However, the vast majority of this work has been conducted with intact, biologically-related families, and it is unclear whether the effects of prenatal experiences on EF would remain once genetic and postnatal factors have been considered.
Genetic Influences on Executive Function
EF skills in young children are highly heritable, with 40–80% of the variance accounted for by genetic influences (Groot, de Sonneville, Stins, & Boomsma, 2004; Lemery-Chalfant, Doelger, & Goldsmith, 2008; Polderman et al., 2007). Two primary methodologies have been used to examine genetic influences on EF: twin studies (e.g., a comparison of similarities between monozygotic versus dizygotic twin pairs) and candidate gene association studies (e.g., molecular genetic studies that examine individual variation in a specific gene polymorphism in association with an observed outcome). Twin studies have shown that half or more of the variance in EF is due to genetic influences (Friedman et al., 2008; Polderman et al., 2007); candidate gene association studies have shown an association between specific polymorphisms such as COMT and PFC-based EFs (Goldberg & Weinberger, 2004).
The present study used a third type of genetically-informed methodology—a full adoption design. To the best of our knowledge, no studies have used this design to examine genetic influences on children’s EF, although studies using data from the Colorado Adoption Project have examined genetic influences on the Bayley Mental Scale and found evidence of heritability on general cognitive functioning and on subscales tapping memory function during infancy (Plomin & DeFries, 1985). In the full adoption design, the child is placed in a non-relative adoptive home at birth and the adopted child, adoptive parents, and birth parent(s) are assessed following placement. When post-placement contact between birth and adoptive families is statistically controlled, associations between birth parent characteristics and adopted child characteristics cannot result directly from postnatal factors, and are assumed to result from genetic influences or from prenatal influences (when there is no selective placement of children into adoptive families by matching to birth parent characteristics). Therefore, the adoption design provides a means of examining genetic and prenatal influences as transmitted intergenerationally from parent to child. A unique feature of this design is the ability to capture effects of the genome as expressed phenotypically through behavior; the inferential phenotype-based genetic approach of the adoption design has the potential to add significantly to the existing literature on genetic influences on EF.
Development of Negative Emotionality and Links with Executive Function
Factor analytic studies of the broad temperament characteristic of negative emotionality have consistently identified distress to limitations and anger as key subcomponents of negative emotionality during infancy and toddlerhood (Gartstein & Rothbart, 2003; Goldsmith, 1996; Putnam, Gartstein, & Rothbart, 2006). Negative emotionality tends to increase across the first year of life (e.g., Lipscomb et al., 2011; Sallquist et al., 2009), with substantial individual differences in development that have implications for later child outcomes (e.g., Gartstein et al., 2010). Most theoretical models typically emphasize that regulation of negative emotionality is driven by top-down EF processes (e.g., Ochsner & Gross, 2007). However, early in life, top-down cortical EF processes are less developed than subcortical structures involved in the generation of emotion (Calkins & Marcovitch, 2010). For example, although the executive attention system begins to come online toward the end of the first year of life, it is not until much later (i.e., late toddlerhood and early preschool ages) that this system undergoes a rapid period of maturation (Derryberry & Rothbart, 1997; Rueda, Posner, & Rothbart, 2005). Given the timing of both systems, negative emotionality may exert a powerful influence on the development of subsequent EF during the first two to three years of life before children are able to efficiently recruit top-down EF processes to assist in regulation. Consistent with this possibility, Stifter and Spinrad (2002) found that infants characterized as excessive criers (a marker of negative emotionality) displayed the lowest levels of subsequent self-regulation. Likewise, Bridgett et al. (2009) found that higher levels of negative emotionality at 4-months of age predicted lower trajectories of early orienting/regulation between 4- and 12-months of age.
Although studies using older age groups have identified negative associations between negative emotionality and EF (e.g., Carlson & Wang, 2007; Eisenberg et al., 1995), to our knowledge, no studies have examined the influence of early developing negative emotionality on subsequent EF. The current study addresses this gap by examining the effects of early and developing negative emotionality, from late infancy into the early toddler period, on measures of EF in toddlers. Relevant to the current study, negative emotionality has also been shown to be heritable during early childhood, with a heritability estimate of .64 (Goldsmith, Lemery, Buss, & Campos, 1999), suggesting that it might serve as a mediating mechanism between genetic influences on EF and the expression of EF deficits.
Associations Among Negative Emotionality, Language Development, and EF
Language development and EF are closely linked (see Cole, Armstrong, & Pemberton, 2010 for a discussion) and there is also evidence linking negative emotionality to language development (Cole et al., 2010). Early attention skills have been identified as important prerequisites to language acquisition. For example, Dixon and Smith (2000) found that 13-month old infants’ duration of orienting (i.e., focused attention) predicted language outcomes when children reached 20 months of age. Similarly, in a sample of toddlers, Salley and Dixon (2007) found that better attention focusing and attention shifting were concurrently related to better language skills. Inhibitory control, a component of EF, has also been implicated in the development of language. Infants need the ability to inhibit their attention from a distracting object or event to look at a speaker, infer the speaker’s intentions, and understand what the speaker is talking about (Baldwin & Moses, 2001). Consistent with the importance of inhibitory control for early language, Salley and Dixon (2007) found that toddlers’ inhibitory control was concurrently related to better language performance. Other studies have also documented strong concurrent and longitudinal associations between EF and language skills (e.g., Carlson et al., 2004, Pears & Fisher, 2005).
There is also evidence of associations between negative emotionality and early language, although most of this work has been done in families where parents and children are genetically related to one another. For example, children who are prone to displaying high negative affect often have difficulty allocating attention to language acquisition demands (Bloom, 1993); whereas children who can better maintain emotional neutrality have more attention resources to devote to language. Similarly, negative emotionality may inhibit children’s ability to process language at times when being able to do so would enhance language acquisition (Rieser-Danner, 2003). Because there is evidence of substantial genetic influences on both negative emotionality and language development in early childhood (Byrne et al., 2009; Goldsmith et al., 1999), such associations may be due to parents and children sharing genes rather than to the family socialization process. In the present study, we were able to examine possible genetic links between adopted children and their birth parents (who are not rearing them and thus provide no socialization with the family), thereby providing an index of genetic influences on the child that are independent of family socialization processes. We include measures of children’s language ability and birth parent verbal intelligence to examine these associations.
Summary of Hypotheses
Four primary hypotheses and one secondary hypothesis were examined. First, unique prenatal influences on toddler EF and language development at 27-months of age were expected. Second, it was hypothesized that unique genetic influences on EF and language development would manifest through associations between birth parent characteristics (birth parent EF and verbal intelligence) and toddler characteristics (child EF and language development). Third, we hypothesized that toddler negative emotionality and increases in negative emotionality from 9- to 27-months of age would be associated with poor child EF and language at age 27 months. Fourth, it was expected that negative emotionality would serve as a mediator of associations between birth parent characteristics and child EF and language development. Finally, in a secondary hypothesis consistent with meta-analytic work (e.g., Else-Quest et al., 2006), we expected that girls would have higher EF scores than boys.
Method
Participants & Procedures
Participants were 361 linked sets of adopted children, adoptive mothers and fathers, and birth mothers from Cohort I of the Early Growth and Development Study (EGDS). In addition, 121 linked birth fathers were included in secondary analyses. Participants were recruited between 2003 and 2006 through 33 adoption agencies located in 10 states spanning the Northwest, Mid-Atlantic, and Southwest regions of the United States. Participating agencies reflected the full range of adoption agencies in the United States: public, private, religious, secular, those favoring open adoptions, and those favoring closed adoptions.
Eligibility criteria for participation included the following: domestic adoption placement, placement occurring within 3 months postpartum, non-relative adoption placement, birth and adoptive parents able to understand English at the eighth-grade level, and no known major medical conditions such as extreme prematurity, extensive medical surgeries, or any condition that required the child to be in the NICU for more than one week. The children were 9-months old during the first assessment (M = 9.24, SD = 0.96), 18-months old during the second assessment (M = 18.00, SD = 1.32), and 27-months old during the third assessment (M = 27.36, SD = 1.56). Fifty-seven percent of the children were male. The median child age at adoption placement was 2 days (M = 7.11, SD = 13.28; range = 0–75 days).
Adoptive parents were typically college educated (median education level for adoptive mothers and fathers was a 4-year college degree), middle to upper class families (median income = $100,000–$125,000). At the first assessment, adoptive mother and adoptive father mean ages were 38 (SD = 5.5) and 39 (SD = 5.8) years, respectively. The ethnicity of adoptive mothers and fathers was: 91% and 90% Caucasian, 4% and 5% African American, 3% and 2% Hispanic or Latino, 1% and 1% multiracial, 1% and 1% Asian, <1% and 0% and American Indian or Alaskan Native, and 1% and 1% unknown or unreported, respectively.
The education level of birth parents was: a GED, high school degree, or lower (65% of sample); a trade school or two-year college degree (25% of sample); or a four-year college degree or higher (10% of sample). Birth parents typically had household incomes of less than $25,000. The birth mother and birth father mean ages were 24 (SD = 5.9) and 26 (SD = 7.5) years, respectively. Birth mother and birth father ethnicity was as follows: 71% and 73% Caucasian; 11% and 9% African-American; 7% and 9% Hispanic/Latino; 5% and 5% multiracial; 2% and 0% Asian, and 3% and 1% Native American, respectively; the remaining participants were not identified or of other ethnic status. For full demographic information refer to Leve, Neiderhiser, Scaramella, and Reiss (2010).
Birth parents were assessed in person at 3- to 6-months (T1) and 18-months (T2) postpartum. Adoptive parents were assessed in person when the child was 9-months (T1), 18-months (T2), and 27-months of age (T3). Birth parent self-report, adoptive parent report of child, and observational data of the child were used in the present study. Retention rates remained high throughout the course of the present study (94% for adoptive families, 92% for birth mothers, and 90% for birth fathers).
Measures
Birth parent predictors
Prenatal risk
Prenatal risk was assessed at the birth parent T1 assessment using birth mother report of her pregnancy complications (e.g., prenatal illness and fetal distress during pregnancy) and prenatal drug use (e.g., illicit substances/alcohol) via a pregnancy screener and a pregnancy calendar method to enhance recall (Caspi et al., 1996). The birth mother was first asked to identify key events (e.g., birthdays, anniversaries, holidays) that occurred during her pregnancy on a one-page pregnancy calendar. She then recalled the occurrence of a series of any obstetric complications during her pregnancy. Scoring was derived from the McNeil-Sjostrom Scale for Obstetric Complications (McNeil & Sjostrom, 1995). Responses to each item were assigned a score from 1 (not harmful or relevant) to 6 (very great harm to or deviation in offspring), indicating the level of risk indicated by each response. Consistent with McNeil-Sjostrom scoring protocols, a prenatal risk score that reflected severity of risk was created by summing items with a score of 3 or greater.
Verbal IQ
The Information subtest from the Wechsler Adult Intelligence Scale-III (Wechsler, 1997) was administered during the birth parents T2 assessment to obtain an estimate of verbal intelligence. This subtest, which loads onto the verbal comprehension index and is broadly considered to be a representative measure of g (g loading = .79), consists of 28 questions that measure knowledge of the world, basic facts, and information (Kaufman & Lichtenberger, 1999). Scaled scores were computed for each individual based on age.
Color Stroop Task
In this computerized Stroop task, interference is created between a word’s color and its meaning (MacLeod, 1991). The Stroop task was administered on a laptop computer. Letter strings composed of the color words “RED”, “BLUE”, “GREEN”, and “YELLOW” appeared in color on the screen. In the control trials, the word and color were congruent, with each word printed in the color identified by the word. In the interference trials, the word and color were non-congruent, with each word printed in a color different from that identified by the word. Reaction time was computed as the interval in milliseconds between a word's appearance on the screen and a key press that indicated the font color in which the word was printed. The difference score between control and interference trials was used in the present study, computed by subtracting the mean reaction time for the control trials from the mean reaction time for the interference trials (see Bryce, Szucs, Soltesz, & Whitebread, 2011; Tse, Balota, Yap, Duchek, & McCabe, 2010, for validity of reaction time in the Stroop and related EF tasks).
Mediating variable (9- to 27-months of age)
Negative Emotionality
Child negative emotionality was measured at T1 using adoptive mother report on the Distress to Limitations subscale of the Infant Behavior Questionnaire (Rothbart, 1981), and at T2 and T3 using mother report on the Anger Proneness subscale of the Toddler Behavior Assessment Questionnaire (Goldsmith, 1996). The Distress to Limitations subscale consists of 20 items rated from 1 (never) to 7 (always) reflecting fussing, crying, or showing distress while (a) in a confining place or position; (b) involved in caretaking activities; (c) unable to perform a desired action (α = .85). The Anger Proneness subscale is the analogous, developmentally-appropriate scale for toddlers, consisting of 28 items rated on the same scale as the Distress to Limitations subscale. Items reflect toddler crying, protesting, hitting, pouting, or other signs of anger in situations involving conflict with another child or the caregiver (α = .87 at T2 and .86 at T3). A mean score of scale items was computed separately at T1, T2, and T3, and was used in the subsequent modeling.
Toddler outcomes (27-months of age)
Effortful attention (Shape Stroop Task)
This task was modeled after Kochanska et al. (2000) and Carlson et al. (2004), who describe the task as an indicator of effortful attention that measures the child’s ability to sustain attentional focus on a subdominant rather than a dominant perceptual feature. The interviewer showed the child three large and three small pictures of the same fruits (apple, banana, orange). After reviewing the names and the meaning of each big-little dimension, the interviewer showed the child three pictures, each containing a small fruit embedded within a different large fruit (e.g., a small orange inside of a large apple). The interviewer then asked the child to point to each of the little fruits (e.g., “show me the little orange”). The prepotent response for young children is to point to the large fruit. After the fruit trials, the interviewer repeated the activity with a similar set of three trials with pictures of big and little animals (bunny, dog, teddy bear). Each trial was scored on a 1–3 scale, with values of 1 (ambiguous or incorrect response on both item and size of object), 2 (correct item but wrong size), or 3 (correct item and correct size).1 Items were averaged to compute the scale score (Cronbach’s α = .86). This scoring system is slightly modified from Kochanska et al. (2000) and Carlson et al. (2004), who included an additional scoring category for the child pointing to the incorrect item and then self-correcting by pointing to the correct item. In the present study, we did not witness such self-correcting behavior, perhaps due to the young age of the children. When children pointed to multiple items they were given a score of 1. A principal components analysis of the 6 task items obtained a one component solution (eigenvalue = 3.59) using parallel analyses and Velicer’s MAP tests, as recommended by O’Conner (2000).
Delay of gratification (Gift Delay Task)
This task was modeled after Kochanska et al. (2000) to measure children’s ability to inhibit behavior. The interviewer told the child that she had a present that she thought the child would really like, and told the child to sit with their hands over their eyes so that the interviewer could wrap the present. The interviewer instructed the child not to peek and then noisily wrapped the gift. After 1 minute, the interviewer gave the child the wrapped present but instructed the child not to touch the present until she returned with the bow. After 2 minutes, the interviewer returned with the bow and let the child open the present. Ratings of the child’s ability to inhibit impulses were coded by the interviewer after the session, referencing the video-recording, with the following three items: “How often did child peek?” (1 [continually] to 5 [never]); “How often did the child touch the gift when interviewer left the room?” (1 [yes, repeatedly] to 3 [no, not at all]); and “The child used distraction strategies” (1 [very true] to 4 [not true]). The 3 items were each rescaled 1 to 4 and then averaged to indicate greater ability to delay gratification (α = .54; r = .08, .32, and .46 among items). For 11% of the cases, a second staff member watched the gift delay task from the video-recording and completed the same set of items. The intraclass correlation between raters for this measure was .61.
Language development
Language development was assessed by adoptive mother and father report of the child’s language ability on the Language Development Survey of the Child Behavior Checklist (Achenbach & Rescorla, 2000). The Language Development Survey is a 310-item measure that lists a series of words (e.g., foods, body parts, actions, animals) and asks parents to indicate which words the child says spontaneously (not just imitating or understanding) (α = .99 mothers and 1.0 fathers). Total scores were converted to reflect the child’s percentile relative to same age peers, using norms provided by the authors of the instrument. Mother and father ratings were significantly correlated (r = .79, p <.001) and therefore aggregated into a single score reflecting the combined parent rating (α = .88). Scores on this measure correlate in the moderate range with scores on standardized vocabulary tests (r’s = .66–.87; Klee et al., 1998; Rescorla & Alley, 2001).
Covariates
Two covariates were included: toddler sex and openness in adoption (contact between birth and adoption parents). Sex of toddler was coded 0 for boys and 1 for girls. T1 openness in the adoption was included to control for similarities between birth parents and adopted children that might result from contact between the two parties. This was measured using a composite of birth mother, adoptive mother, and adoptive father ratings of perceived adoption openness (Ge et al., 2008), where each participant rated the degree and nature of contact and communication with their counterpart on a 7-point scale ranging from very closed (“you have no information about the adoptive parents”) to very open (“you have visits with the family at least once a month and communicate several times a month by phone, letters, or emails”). Interrater agreement was high (r range = .66–.81, p values all < .001).
Analytic Strategy
Study hypotheses were tested using structural equation modeling to examine the role of birth parent characteristics on toddler outcomes and whether their effects were mediated through toddler negative emotionality. Because toddler negative emotionality was measured over three waves, the models were specified as latent variable growth models in the Mplus 6.1 program (Muthén & Muthén, 2010). Latent growth models (LGM) provide advantages for modeling developmental changes over time. Repeated measure outcomes at Level 1 are nested within individuals at Level 2. Also known as random intercepts models, LGM estimates the individual differences or variation in the levels of child negative emotionality (random intercepts) and the increases or decreases in negative emotionality from 9 to 27 months for each individual at Level 1. The individual intercepts and slopes are then summarized as latent variable factor components at Level 2, representing the sample means and growth slopes. The present LGM analyses were modeled as two factors: initial status (9-month intercept) and linear growth. This was obtained by fixing the three random intercept factor loadings at 1, respectively, for 9, 18, and 27 months, and by specifying the chronometric time weights for the slope factor at 0, 1, and 2 (Biesanz, Deeb-Sossa, Papadakis, Bollen, & Curran, 2004).
Missing data and attrition analyses
We first evaluated missing data using missing values analyses and standard attrition analyses. Little’s test of missing data (Little, 1988) revealed the data were missing completely at random (MCAR) for the birth mother models [Little's MCAR χ2 (209) = 211.75, p = .43], indicating that missingness was not dependent on missing values of the observed or unobserved variables. Data for the birth father secondary analysis were also missing completely at random [MCAR χ2 (182) = 165.61, p = .80]. Following recommendations for data that are MAR or MCAR, models were estimated using full information maximum likelihood (FIML) which uses all available information from the observed data (see Table 1 for n’s for each variable). FIML estimates are computed by maximizing the likelihood of a missing value based on observed data (Jeličić, Phelps, & Lerner, 2009). Compared to mean-imputation, list-wise deletion, or pair-wise deletion, FIML provides more statistically reliable standard errors.
Table 1.
Means, Standard Deviations, Sample Size, and Bivariate Correlations Among Study Variables
| Variable | M | SD | n | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Birth Mother Predictors | |||||||||||||
| 1. Prenatal Risk | 9.18 | 6.49 | 361 | __ | |||||||||
| 2. Verbal IQ | 9.47 | 2.64 | 313 | −.04 | __ | ||||||||
| 3. Stroop Reaction Time | 123.12 | 103.02 | 308 | .06 | .08 | __ | |||||||
| Toddler Outcomes | |||||||||||||
| 4. Effortful Attention | 2.01 | 0.59 | 320 | −.10 | .20*** | −.04 | __ | ||||||
| 5. Delay of Gratification | 2.08 | 0.92 | 321 | −.05 | −.04 | −.03 | .03 | __ | |||||
| 6. Language Development | 54.76 | 21.41 | 314 | −.03 | .10 | −.00 | .33*** | .19** | __ | ||||
| Child Negative Emotionality | |||||||||||||
| 7. 9 Months | 3.13 | 0.76 | 350 | .00 | −.02 | −.06 | .10 | .02 | −.03 | __ | |||
| 8. 18 Months | 3.42 | 0.69 | 339 | −.05 | −.02 | .02 | −.01 | −.01 | −.00 .47*** | __ | |||
| 9. 27 Months | 3.63 | 0.70 | 316 | −.01 | −.06 | −.13* | −.02 | −.08 | −.17** | .44*** | .60*** | __ | |
| Covariates | |||||||||||||
| 10. Openness in Adoption | 4.73 | 1.18 | 361 | .02 | .20*** | −.01 | .17** | .02 | .24*** | .08 | .03 | −.03 | __ |
| 11. Child Sex (Girl) | .43 | .50 | 361 | .02 | −.07 | −.03 | .20*** | .16** | .06 | −.05 | .01 | .09 | −.08 |
Note.
p < .05;
p < .01;
<.001.
For standard attrition analyses we examined two comparisons, one that included 295 families with three complete waves of child negative emotionality and 66 families missing one or two waves of data, and one with 174 families with complete data on all 10 study variables and 187 families missing one or more variables. Both analyses revealed that among the six baseline measures (prenatal risk, birth mother verbal IQ, birth parent Stroop reaction time, toddler negative emotionality, openness in adoption, and sex of child), no differences were obtained except for openness in adoption. On both longitudinal attrition status and partial data status, comparisons revealed that families with incomplete data scored higher on openness in adoption ratings (M = 4.56, SD = 1.17 and M = 4.88, SD = 1.17, respectively, t = 2.64, p < .01 for attrition and M = 4.39, SD = 1.03 and M = 4.80, SD = 1.20, respectively, t = 1.99, p < .05 for partial data).
Results
The sample sizes, means, standard deviations, and pairwise bivariate correlations for study variables are presented in Table 1. Consistent with the study hypotheses, toddler effortful attention was positively associated with toddler language development, birth mother verbal IQ, and toddler sex (girl). Toddler delay of gratification was positively associated with toddler language development and toddler sex (girls), and toddler language development was negatively associated with 27-month toddler negative emotionality. In addition, birth mother Stroop reaction time was negatively associated with toddler negative emotionality at 27 months. Finally, the control variable of openness in adoption was positively associated with toddler effortful attention, toddler language development, and birth mother verbal IQ. The child negative emotionality variables were correlated with each other across all three time points.
Testing the Main Effects of Prenatal Risk and Genetic Influences
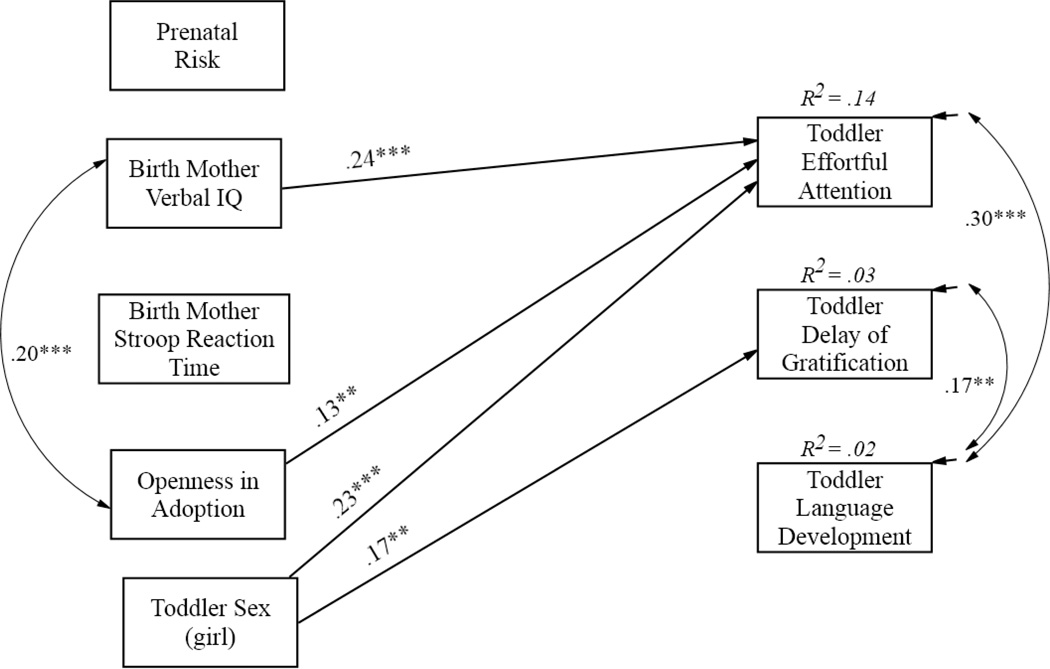
The first step of the analyses evaluated Hypotheses 1 and 2 regarding the unique prenatal and genetic influences on toddler EF. To isolate the unique contributions of genetic and prenatal influences, we estimated a set of three models entering genetic influences only, prenatal risk only, and both as predictors of the EF outcomes with openness in adoption and sex of child as covariates. In the genetic influences only model, birth mother verbal IQ significantly predicted children’s effortful attention (β = .24, p < .001) explaining 14% of the variance. No other associations were present. In the prenatal risk only model, prenatal risk also significantly predicted children’s effortful attention in the expected direction (β = −.11, p < .05). The final model tested unique effects in a multivariate path model, with both genetic and prenatal risk variables included. Results are shown in Figure 1 in the form of standardized beta paths. Although this was a saturated model with all possible paths from predictors to the three EF outcomes, only significant paths are shown for visual clarity. Hypotheses 1 and 2 were partially supported. Higher levels of birth mother verbal IQ were associated with greater effortful attention in toddlers (β = .24, p < .001). The effect of prenatal risk was nonsignificant in the presence of the genetic influence variables. Among the control variables, openness in adoption predicted higher effortful attention in toddlers (β = .13 p < .01), and girls exhibited higher effortful attention and delay of gratification relative to boys (β = .23, p <.001 and β = .17, p < .01, respectively). The model accounted for 14% of the variance in toddler effortful attention, 3% in delay of gratification, and 2% in language development.
Figure 1.
Test of main effects of prenatal influences and genetic influences from birth mother verbal IQ and birth mother Stroop reaction time on child 27-month outcomes. Paths are standardized beta coefficients and only significant paths are shown. Model is saturated with perfect fit. Significant covariances among predictors were freely estimated, else constrained.
***p < .001; **p < .01.
Testing the Effects of Toddler Growth in Negative Emotionality
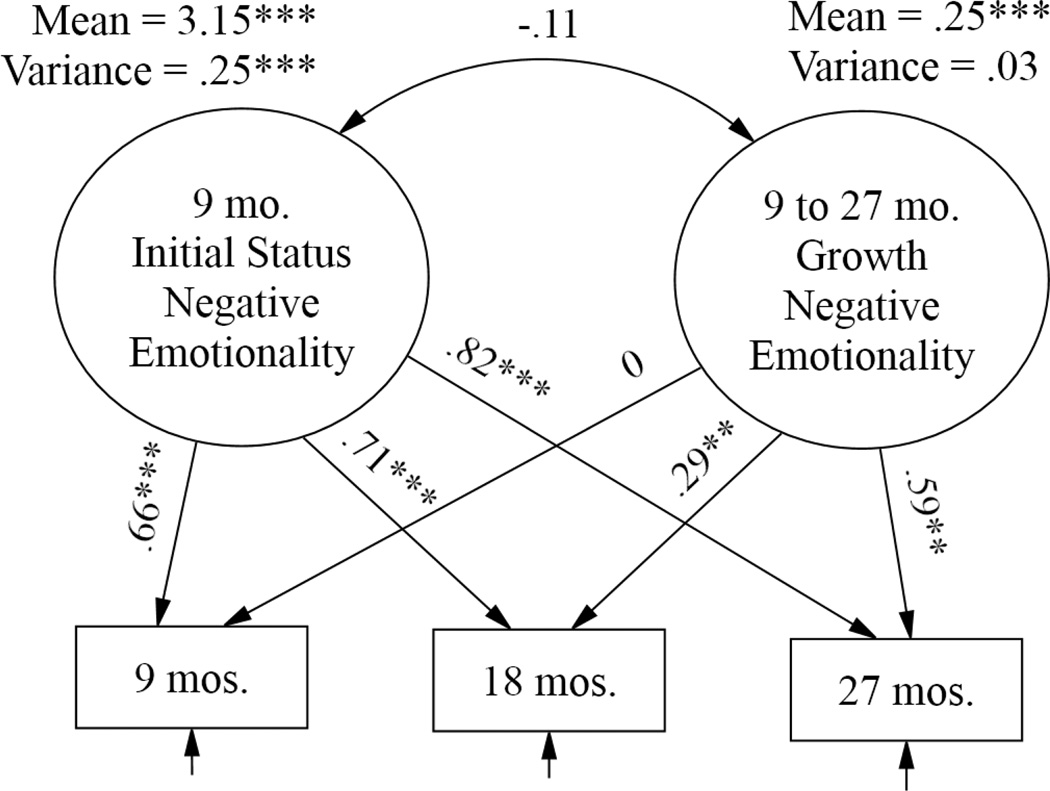
We next specified latent growth models (LGM) employing the repeated toddler negative emotionality measures to examine Hypotheses 3 and 4. The first step in the LGM analysis was to estimate the unconditional model describing the individual and summary patterns of change in toddler negative emotionality. The model specified an initial status factor and a linear growth slope factor. Results are shown in Figure 2 in the form of standardized beta paths. Specifying linear growth provided adequate fit to the observed data [χ2(1) = 2.87, p = .09; comparative fit index (CFI) = .99; root mean square error of approximation (RMSEA) = .07]. The estimated mean level for the sample at 9 months was significantly different from zero (M = 3.15, p < .001) and there were significant individual differences at 9 months (σ2 = .25, p < .001). The sample of children as a whole showed significant developmental increases in negative emotionality (M = .25, p < .001). However, no reliable individual differences in growth rates were obtained, indicating that on the whole, children’s negative emotionality grew at similar rates.
Figure 2.
Unconditional growth model of growth in child negative emotionality across the 18-month study period [χ2(1) = 2.87, p = .09; CFI = .99; RMSEA = .07]. Paths are standardized beta coefficients.
***p < .001; **p < .01.
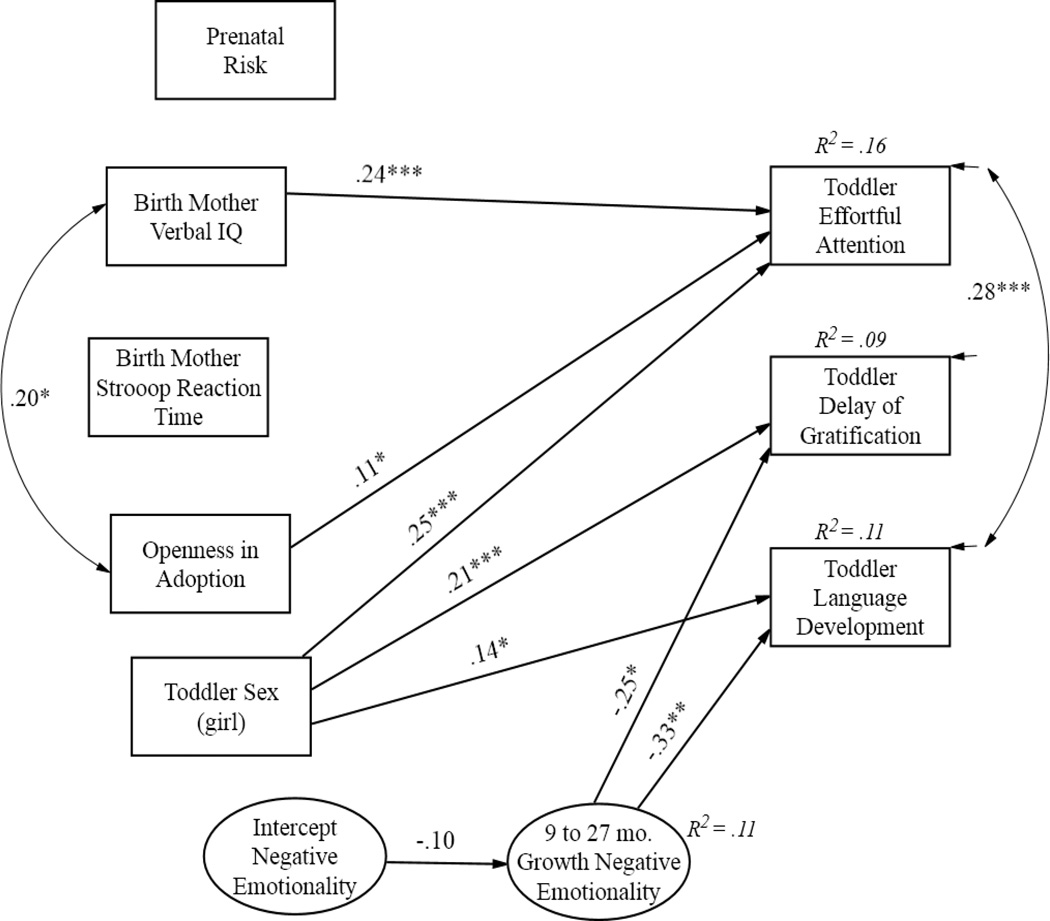
The next step of the analyses tested initial status and growth in negative emotionality as mechanisms linking birth parent characteristics and child EF outcomes. Growth rates also controlled for negative emotionality initial status. Results of the final model are displayed in Figure 3 [χ2(12) = 20.03, p = .07, CFI = .98, RMSEA = .04]. For visual clarity, only significant paths are displayed among the predictor paths. Results partially supported Hypotheses 3 and 4 (see Figure 3): negative emotionality initial status at 9 months did not predict 27-month outcomes; however, growth in negative emotionality was associated with lower levels of toddler delay of gratification (β = −.25 p < .05) and lower levels of language development (β = −.33 p < .01). The effect of birth mother verbal IQ, toddler sex (girl), and openness in adoption on toddler’s effortful attention remained significant in the final model. Consistent with our secondary hypothesis, girls performed better than boys on effortful attention, delay of gratification, and language development. Adding growth in negative emotionality explained an additional 6% of the variance in toddler’s delay of gratification and an additional 9% of the variance in language development. Eleven percent of the variance in growth rates of negative emotionality was explained. With nonsignificant effects between the genetic and prenatal risk variables and toddler negative emotionality as part of the mediational pathway, our mediation hypothesis was not supported and not further examined.
Figure 3.
Growth in toddler negative emotionality as a predictor of 27-month child outcomes [χ2(12) = 20.03, p = .07, CFI = .98, RMSEA = .04]. Paths are standardized beta coefficients and only significant paths are shown.
***p < .001; **p < .01; *p <.05.
Secondary Analyses With Birth Fathers
The final analyses examined full information models for the 121 participating birth fathers (models not shown). Due to these models serving as a replication of the birth mother models, and the smaller sample size, significance levels of p < .10 were considered. In the main effects multivariate model (Hypotheses 1 and 2), prenatal risk predicted children’s effortful attention (β = −.14, p < .01) and birth father verbal IQ marginally predicted children’s effortful attention (β = .11, p < .10). Birth father variables did not predict delay of gratification or language development. Upon entering negative emotionality (Hypotheses 3 and 4), birth fathers’ verbal IQ significantly predicted children’s effortful attention (β = .24, p < .01), indicating that growth in negative emotionality was partially suppressing the effect of birth fathers’ verbal IQ on children’s effortful attention. Holding negative emotionality constant, birth fathers’ verbal IQ showed the same pattern as birth mothers’ verbal IQ. Growth in negative emotionality showed a similar pattern of effects as was observed in the birth mother model in regards to delay of gratification (β = −.24, p < .05) and language development (β = −.35, p < .01). Overall, birth father predictors showed the same signed effects as the birth mother predictors to the respective specified developmental outcomes.
Discussion
The current study examined unique genetic, prenatal risk, and child temperament (i.e., negative emotionality) influences on toddler EF in the context of an adoption design, accounting for associations with language/verbal skills. The first two hypotheses tested whether there were unique influences of prenatal risk and genetic influences on toddler EF. The results provided partial support for our hypotheses. Significant associations were observed between prenatal risk and toddler effortful attention prior to inclusion of genetic influences; once genetic influences were included, prenatal risk was no longer significant. Although prior studies have also shown associations between prenatal risk and deficits in child EF (e.g., Connor et al., 2000), to our knowledge, its influence has never been examined in conjunction with genetic influences on EF. The current results suggest that prenatal risk may not have independent associations with EF once genetic factors are considered.
In comparison, when the effects of prenatal risk and genetic influence variables were considered simultaneously, genetic influences on toddler EF remained, with associations found between birth mother verbal IQ and toddler effortful attention. This genetic main effect was replicated in the models incorporating birth father verbal IQ data. This finding suggests a genetic etiology of toddler EF that is identifiable very early in development and may serve as an important mechanism of the intergenerational transmission of cognitive functioning. There were no significant associations between birth mother Stroop reaction time and toddler effortful attention. Thus, at least during toddlerhood, genetic influences on verbal IQ may be the predominant pathway to toddler effortful attention during the early stages of EF development. Consistent with this interpretation, our indicator of birth parent verbal IQ (the information subtest of the WAIS) not only assesses verbal dimensions of intelligence but also reflects long term memory and ability to accurately retrieve information (Kaufman & Lichtenberger, 1999), features central to the development of early effortful attention.
Contrary to our hypotheses, there were neither prenatal nor genetic effects on our second measure of toddler EF: delay of gratification. This set of null findings presents an interesting possibility. EF tasks that have inherent motivational properties, such as delay of gratification tasks (Hongwanishkul, Happaney, Lee, & Zelazo, 2005), have been conceptualized as drawing on “hot” EF. Conversely, more cognitive EF tasks that do not have a motivational aspect, such as the effortful attention task used in the current study, have been conceptualized as drawing upon “cool” EF functions (Zelazo & Cunningham, 2007; Zelazo & Muller, 2002). In the current study, only a measure of birth parent “cool” EF was available. Further, there is no inherent motivational aspect to completion of the birth parent measure of verbal IQ, which demonstrated connections with the child’s “cool” effortful attention task. This suggests that there may be either completely or partially distinct genetic pathways involved with “hot” and “cool” forms of EF during very early childhood.
Additional support for this possibility comes from at least two sources. First, previous work examining the underlying neural structures activated during “hot” and “cool” EF tasks has identified greater activation of the dorsolateral prefrontal cortex during performance of “cool” EF tasks. In contrast, greater activation of the orbital frontal cortex (OFC) during performance on measures of “hot” EF is often found (for an overview of findings, see Zelazo & Cunningham, 2007). Second, the current results indicate that increases in negative emotionality from 9 and 27 months were associated with poorer toddler delay of gratification. This links an aspect of emotion (negative emotionality) to performance during a “hot” EF task. Interestingly, this association was not observed with the more characteristically “cool” EF task used in the current investigation (shape Stroop). This finding is consistent with the iterative reprocessing model (Cunningham & Zelazo, 2007), which suggests that situations eliciting strong avoidance or approach motivation can impair EF in some circumstances (Zelazo, Qu, & Kesek, 2010). Future work using different methodologies is needed to further consider how emotion may differentially influence performance during tasks that measure “hot” and “cool” forms of EF.
Consistent with our third hypothesis, increases in negative emotionality across toddlerhood were negatively associated with poorer performance during the delay of gratification task. This finding is consistent with a large body of evidence indicating that negative emotionality is associated with lower EF skills, and specifically, with lower effortful control (e.g., Eisenberg et al., 1995). This finding is also consistent with prior work suggesting that high early negative emotionality may compromise later attentional and self-regulatory processes (e.g., Bridgett, et al., 2009; Stifter & Spinrad, 2002). Moreover, increases in negative emotionality were also associated with toddler language development. This is consistent with the theoretical perspective postulated by Riesner-Danner (2003) that having a reactive temperament may render some children less able to attend to and process relevant language cues at a time when being able to do so would facilitate language acquisition.
The final model examined whether increases in negative emotionality served as a mediator of the association between genetic and prenatal influences and toddler language development. Owing to the lack of association between genetic and prenatal variables with negative emotionality, this hypothesis was not supported. Future work may benefit from considering a broader range of genetic influences (e.g., birth parent psychopathology) that might be associated with toddler negative emotionality. Finally, support was provided for our secondary hypothesis that girls would outperform boys on effortful attention and delay of gratification, two measures of EF. Girls scored higher on effortful attention and delay of gratification than did boys. This is consistent with prior work in this area (e.g., Else-Quest et al., 2006) and also provides validation for the specific measures of EF used in the present study. In the final model, girls also had better language development than boys.
Strengths, Limitations, and Future Directions
One strength of the adoption design is the ability to isolate influences that result from prenatal and genetic influences. Because the child is adopted at birth and not reared by the birth parent(s), when there is the absence of selective placement (as in the current study, Leve et al., 2007) and contact/openness between birth and adoptive parents is controlled, associations between birth parents and the adopted child are inferred to result from genetic influences and/or prenatal experiences rather than postnatal rearing conditions. Nevertheless, although examination of the postnatal rearing environment was beyond the scope of the present study, research indicates the intricate interplay between genetic, prenatal, and caregiving influences. For example, the influences of one factor are likely associated with or conditioned by the influences of another factor through gene-environment (GxE) interaction and/or gene-environment correlation (Johnson, 2007; Rutter, 2006). GxE interactions have been identified for outcomes related to language and EF development (e.g., Taylor, Roehrig, Hensler, Connor, & Schatschneider, 2010). Consequently, it is plausible that exposure to specific caregiving conditions (e.g., interparental hostility) during infancy may disrupt the development of effortful control through inducing higher levels of negative emotionality. Such emotions could overwhelm children’s fledgling abilities to voluntarily enact attentional and behavioral regulation. This could play out by increasing children’s proneness for negative emotionality, amplifying the pathway found in current study. Likewise, recent work suggests that parental self-regulation, including aspects of EF, is important for parenting behavior (e.g., Deater-Deckard, Sewell, Petrill, & Thompson, 2010), and other work has identified connections between parental effortful control, parenting, and subsequent toddler effortful control (e.g., Bridgett et al., 2011; Spinrad et al., 2007). EF in adoptive parents was not measured during the present study, but the effects of the postnatal family environment on EF are avenues for future exploration within the context of a genetically-sensitive design.
A second area where additional research would be beneficial is in examining the contribution of birth father characteristics. Although the present sample of 121 birth fathers is the largest sample assessed in an adoption design, and their inclusion permitted a supplementary examination of the expressed effects of the genome as transmitted from birth father (and generally replicated the effects from the birth mother models), the birth father sample size was underpowered to detect genetic effects. We are currently assessing a second cohort of families (n = 200) using the same assessment protocols as the present study (Leve et al., 2010). This will ultimately provide greater power to examine potential sex-linked patterns of association.
Another strength of the present study was the ability of the adoption design to facilitate an examination of the behavioral expression of genetic influences through associations between birth parent characteristics and child characteristics. The adoption design examines gene expression using an anonymous but full genome approach by testing behavioral similarities between a child and his/her birth parent. This approach therefore serves as a broad inquiry into the extent to which specific phenotypes have a genetic etiology, and whether the expressed effects of genes can be moderated by specific environmental contexts to reduce genetic risk and enhance genetic strengths. In addition, this is the first study of which we are aware to examine prenatal risk and genetic influences on EF simultaneously. The results suggest that studies which measure prenatal risk but not genetic influences may inadvertently overestimate the role of prenatal risk and underestimate the role of genetic influences. Future work in this area could explore implications for the development of psychopathology by examining whether the identified associations between genetic influences, prenatal risk, and negative emotionality with toddler EF are predictive of subsequent child behavior problems. Specifically, associations between early increases in negative emotionality and later behavioral difficulties might be mediated by EF processes, and these pathways might be mediated or moderated by genetic, prenatal, or family environmental factors.
Despite the strengths of the present study, a cautionary note is warranted. First, although the sample was representative of the domestic infant adoption population at large (Leve et al., 2007), adoptive parents had relatively high levels of education and income and were primarily of Caucasian ethnicity. As such, explorations of caregiving influences on toddler EF would need to take into consideration how these characteristics might enhance or reduce the effects of prenatal and genetic effects. In addition, our measurement of child language development was based on adoptive parent report; observational studies of child language may yield more reliable associations with child EF. Also, factors such as openness in the adoption need to be considered because they might affect the extent to which associations between children and birth parents may be influenced by contact between birth parents and the adoptive family. In the current study, the effects of openness in the adoption on associations between birth parent and child characteristics were statistically controlled through the inclusion of openness in the multivariate models. Nonetheless, openness in adoption remained a significant predictor of toddler effortful attention, and was positively associated with birth parent verbal IQ. Although these associations were not anticipated, they may reflect an aspect of cognitive flexibility, which is an underlying component of EF (e.g., Rougier, Noelle, Braver, Cohen, & O’Reilly, 2005). Birth and adoptive parents who choose more open adoptions might have more flexible thinking processes to adapt to the complex notion of shared adult contact with the child as compared to individuals who chose more closed adoptions. Alternatively, they may reflect other aspects of birth parent personality or temperament. Additional research is needed to further investigate these possibilities, which might open new avenues of exploration regarding caregiver cognitive flexibility or specific personality traits as predictors of child EF. These study limitations notwithstanding, the current study adds to the field by providing new knowledge about the underlying and unique influences of prenatal risk, genetic influences, and negative emotionality on the development of EF during very early childhood.
Acknowledgments
This project was supported by grant R01 HD042608, NICHD, NIDA, and OBSSR, NIH, U.S. PHS (PI Years 1–5: David Reiss, MD; PI Years 6–10: Leslie Leve, PhD). The writing of this manuscript was partially supported by R01 DA020585 (PI: Jenae Neiderhiser) and P30 DA023920 (PI: Patricia Chamberlain), NIDA, NIH, U.S. PHS. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health & Human Development or the National Institutes of Health. The contributions of the late John Reid and Xiaojia Ge to this work and the ongoing study remain invaluable. We thank the adoptive and birth family participants, and Rand Conger, Jody Ganiban, and Laura Scaramella for their contributions to the full study.
Footnotes
Note. Scaling was also computed on a 0–1 scale for each item (0 = incorrect, 1 = correct) and similar results were obtained. Because accuracy on one dimension conveyed greater cognitive skill that accuracy on zero dimensions, we retained the three-level scoring.
Contributor Information
Leslie D. Leve, Oregon Social Learning Center
David S. DeGarmo, Oregon Social Learning Center
David J. Bridgett, Northern Illinois University
Jenae M. Neiderhiser, The Pennsylvania State University
Daniel S. Shaw, University of Pittsburgh
Gordon T. Harold, University of Leicester
Misaki N. Natsuaki, University of California, Riverside
David Reiss, Yale Child Study Center.
References
- Achenbach TM, Rescorla LA. Manual for the ASEBA Preschool Forms and Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families; 2000. [Google Scholar]
- Anderson P. Assessment and development of executive function (EF) during childhood. Child Neuropsychology. 2002;8:71–82. doi: 10.1076/chin.8.2.71.8724. [DOI] [PubMed] [Google Scholar]
- Baldwin DA, Moses LJ. Links between social understanding and early word learning: Challenges to current accounts. Social Development. 2001;10:309–329. [Google Scholar]
- Best JR, Miller PH, Jones LL. Executive functions after age 5: Changes and correlates. Developmental Review. 2009;29:180–200. doi: 10.1016/j.dr.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biesanz J, Deeb-Sossa N, Papadakis A, Bollen KA, Curran P. The role of coding time in estimating and interpreting growth curve models. Psychological Methods. 2004;9:30–52. doi: 10.1037/1082-989X.9.1.30. [DOI] [PubMed] [Google Scholar]
- Blair C, Peters R. Physiological and neurocognitive correlates of adaptive behavior in preschool among children in Head Start. Developmental Neuropsychology. 2003;24:479–497. doi: 10.1207/S15326942DN2401_04. [DOI] [PubMed] [Google Scholar]
- Blair C, Razza RP. Relating effortful control, executive function, and false belief understanding to emerging math and literacy ability in kindergarten. Child Development. 2007;78:647–663. doi: 10.1111/j.1467-8624.2007.01019.x. [DOI] [PubMed] [Google Scholar]
- Bloom L. The transition from infancy to language: Acquiring the power of expression. Cambridge, England: Cambridge University Press; 1993. [Google Scholar]
- Bridgett DJ, Gartstein MA, Putnam SP, Lance KB, Iddins E, Waits R, Lee L. Emerging effortful control in toddlerhood: The role of infant orienting/regulation, maternal effortful control, and maternal time in caregiving activities. Infant Behavior and Development. 2011;34:189–199. doi: 10.1016/j.infbeh.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Bridgett DJ, Gartstein MA, Putnam S, McKay T, Iddins E, Robertson C, Rittmueller A. Maternal and contextual influences and the effect of temperament development during infancy on parenting in toddlerhood. Infant Behavior and Development. 2009;32:103–116. doi: 10.1016/j.infbeh.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Bridgett DJ, Mayes LC. Development of inhibitory control among prenatally cocaine exposed and non-cocaine exposed youths from late childhood to early adolescence: The effects of gender and risk and subsequent aggressive behavior. Neurotoxicology and Teratology. 2011;33:47–60. doi: 10.1016/j.ntt.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgett DJ, Valentino K, Hayden LC. The contribution of children’s temperamental fear and effortful control to restraint and seclusion during inpatient treatment in a psychiatric hospital. Child Psychiatry and Human Development. doi: 10.1007/s10578-012-0298-x. (in press) [DOI] [PubMed] [Google Scholar]
- Bryce D, Szucs D, Soltesz F, Whitebread D. The development of inhibitory control: An averaged and single-trial Lateralized Readiness Potential study. NeuroImage. 2011;57:671–685. doi: 10.1016/j.neuroimage.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Bryne B, Coventry WL, Olson RK, Samuelsson S, Corley R, Willcutt EG, DeFries JC. Genetic and environmental influences on aspects of literacy and language in early childhood: Continuity and change from preschool to grade 2. Journal of Neurolinguistics. 2009;22:219–236. doi: 10.1016/j.jneuroling.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull R, Espy KA, Wiebe SA. Short-term memory, working memory, and executive functions in preschoolers: Longitudinal predictors of mathematical achievement at age 7 years. Developmental Neuropsychology. 2008;33:205–228. doi: 10.1080/87565640801982312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins SD, Marcovitch S. Emotion regulation and executive functioning in early development: Integrated mechanisms of control supporting adaptive functioning. In: Calkins SD, Bell MA, editors. Child development at the intersection of emotion and cognitive. Washington, DC: American Psychological Association; 2010. pp. 37–57. [Google Scholar]
- Carlson SM, Mandell DJ, Williams L. Executive functioning and theory of mind: Stability and prediction from ages 2 to 3. Developmental Psychology. 2004;40:1105–1122. doi: 10.1037/0012-1649.40.6.1105. [DOI] [PubMed] [Google Scholar]
- Carlson SM, Moses LJ. Individual differences in inhibitory control and children’s theory of mind. Child Development. 2001;72:1032–1053. doi: 10.1111/1467-8624.00333. [DOI] [PubMed] [Google Scholar]
- Carlson SM, Wang TS. Inhibitory control and emotion regulation in preschool children. Cognitive Development. 2007;22:489–510. [Google Scholar]
- Caspi A, Moffitt T, Thornton A, Freedman D, Amell JW, Harrington H, Silva PA. The life history calendar: A research and clinical assessment method for collecting retrospective event-history data. International Journal of Methods in Psychiatric Research. 1996;6:101–114. [Google Scholar]
- Clark CAC, Pritchard VW, Woodward LJ. Preschool executive functioning abilities predict early mathematics achievement. Developmental Psychology. 2010;46:1176–1191. doi: 10.1037/a0019672. [DOI] [PubMed] [Google Scholar]
- Cole PM, Armstrong LM, Pemberton CK. The role of language in the development of emotion regulation. In: Calkins SD, Bell MA, editors. Child development at the intersection of emotion and cognitive. Washington, DC: American Psychological Association; 2010. pp. 59–77. [Google Scholar]
- Connor PD, Sampson PD, Bookstein FL, Barr HM, Streissguth AP. Direct and indirect effects of prenatal alcohol damage on executive function. Developmental Neuropsychology. 2000;18:331–354. doi: 10.1207/S1532694204Connor. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Zelzao PD. Attitudes and evaluation: A social cognitive neuroscience perspective. Trends in Cognitive Sciences. 2007;11:97–104. doi: 10.1016/j.tics.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Darwish D, Esquivel GB, Houtz JC, Alfonso VC. Play and social skills in maltreated and non-maltreated preschoolers during peer interactions. Child Abuse & Neglect. 2001;25:13–31. doi: 10.1016/s0145-2134(00)00228-3. [DOI] [PubMed] [Google Scholar]
- Deater-Deckard K, Mullineaux PY, Petrill SA, Thompson LA. Effortful control, surgency, and reading skills in middle childhood. Reading and Writing. 2009;22:107–116. doi: 10.1007/s11145-007-9111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deater-Deckard K, Sewell MD, Petrill SA, Thompson LA. Maternal working memory and reactive negativity in parenting. Psychological Science. 2010;21:75–79. doi: 10.1177/0956797609354073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derryberry D, Rothbart MK. Reactive and effortful processes in the organization of temperament. Development and Psychopathology. 1997;9:633–652. doi: 10.1017/s0954579497001375. [DOI] [PubMed] [Google Scholar]
- Dixon W, Smith PH. Links between early temperament and language acquisition. Merrill-Palmer Quarterly. 2000;46:417–440. [Google Scholar]
- Eisenberg N, Fabes RA, Murphy B, Maszk P, Smith M, Karbon M. The role of emotionality and regulation in children's social functioning: A longitudinal study. Child Development. 1995;66:1360–1384. [PubMed] [Google Scholar]
- Eisenberg N, Spinrad TL. Emotion-related regulation: Sharpening the definition. Child Development. 2004;75:334–339. doi: 10.1111/j.1467-8624.2004.00674.x. [DOI] [PubMed] [Google Scholar]
- Else-Quest NM, Hyde JS, Goldsmith HH, Van Hulle CA. Gender differences in temperament: A meta-analysis. Psychological Bulletin. 2006;132:33–72. doi: 10.1037/0033-2909.132.1.33. [DOI] [PubMed] [Google Scholar]
- Espy KA, Kaufmann PM, Glisky ML. Neuropsychologic function in toddlers exposed to cocaine in utero: A preliminary study. Developmental Neuropsychology. 1999;15:447–460. [Google Scholar]
- Fantuzzo JW, Weiss AD, Atkins M, Meyers R, Nooze M. A contextually relevant assessment of the impact of child maltreatment on the social competencies of low-income urban children. Journal of the American Academy of Child & Adolescent Psychiatry. 1998;37:1201–1208. [PubMed] [Google Scholar]
- Floyd RG, Kirby EA. Psychometric properties of measures of behavioral inhibition with preschool-age children: Implications for assessment of children at risk for ADHD. Journal of Attention Disorders. 2001;5:79–91. [Google Scholar]
- Fried PA. Conceptual issues in behavioral teratology and their application in determining long-term sequelae of prenatal marihuana exposure. Journal of Child Psychology & Psychiatry. 2002;43:81–102. doi: 10.1111/1469-7610.00005. [DOI] [PubMed] [Google Scholar]
- Fried PA, Smith AM. A literature review of the consequences of prenatal marihuana exposure: An emerging theme of a deficiency in aspects of executive function. Neurotoxicology & Teratology. 2001;23:1–11. doi: 10.1016/s0892-0362(00)00119-7. [DOI] [PubMed] [Google Scholar]
- Friedman NP, Miyaki A, Young SE, DeFries JC, Corley RP, Hewitt JK. Individual differences in executive functions are almost entirely genetic in origin. Journal of Experimental Psychology General. 2008;137:201–225. doi: 10.1037/0096-3445.137.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garon N, Bryson SE, Smith IM. Executive function in preschoolers: A review using an integrative framework. Psychological Bulletin. 2008;134:31–60. doi: 10.1037/0033-2909.134.1.31. [DOI] [PubMed] [Google Scholar]
- Gartstein MA, Bridgett DJ, Rothbart MK, Robertson C, Iddins E, Ramsay K, Schlect S. A latent growth examination of fear development in infancy: Contributions of maternal depression and the risk for toddler anxiety. Developmental Psychology. 2010;46:651–668. doi: 10.1037/a0018898. [DOI] [PubMed] [Google Scholar]
- Gartstein MA, Rothbart MK. Studying infant temperament via the Revised Infant Behavior Questionnaire. Infant Behavior and Development. 2003;166:1–23. [Google Scholar]
- Ge X, Natsuaki MN, Martin D, Leve LD, Neiderhiser JM, Shaw DS, Reiss D. Bridging the divide: Openness in adoption and post-adoption psychosocial adjustment among birth and adoptive parents. Journal of Family Psychology. 2008;22:529–540. doi: 10.1037/a0012817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg TE, Weinberger DR. Genes and the parsing of cognitive processes. Trends in Cognitive Sciences. 2004;8:325–335. doi: 10.1016/j.tics.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Goldsmith HH. Studying temperament via construction of the Toddler Behavior Assessment Questionnaire. Child Development. 1996;67:218–235. [PubMed] [Google Scholar]
- Goldsmith HH, Lemery KS, Buss KA, Campos J. Genetic analyses of focal aspects of infant temperament. Developmental Psychology. 1999;35:972–985. [PubMed] [Google Scholar]
- Groot AS, de Sonneville LMJ, Stins JF, Boomsma DI. Familial influences on sustained attention and inhibition in preschoolers. Journal of Child Psychology and Psychiatry. 2004;45:306–314. doi: 10.1111/j.1469-7610.2004.00222.x. [DOI] [PubMed] [Google Scholar]
- Gunnar M, Tout K, deHaan M, Pierce S. Temperament, social competence, and adrenocortical activity in preschoolers. Developmental Psychobiology. 1997;31:65–85. doi: 10.1002/(sici)1098-2302(199707)31:1<65::aid-dev6>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Hongwanishkul D, Happaney KR, Lee W, Zelazo PD. Hot and cool executive function: Age-related changes and individual differences. Developmental Neuropsychology. 2005;28:617–644. doi: 10.1207/s15326942dn2802_4. [DOI] [PubMed] [Google Scholar]
- Huijbregts SC, Warren AJ, de Sonneville LM, Swaab-Barneveld H. Hot and cool forms of inhibitory control and externalizing behavior in children of mothers who smoked during pregnancy: An exploratory study. Journal of Abnormal Child Psychology. 2008;36:323–333. doi: 10.1007/s10802-007-9180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeličić H, Phelps E, Lerner RM. Use of missing data methods in longitudinal studies: The persistence of bad practices in developmental psychology. Developmental Psychology. 2009;45:1195–1199. doi: 10.1037/a0015665. [DOI] [PubMed] [Google Scholar]
- Johnson W. Genetic and environmental influences on behavior: Capturing all the interplay. Psychological Review. 2007;114:423–440. doi: 10.1037/0033-295X.114.2.423. [DOI] [PubMed] [Google Scholar]
- Kaufman AS, Lichtenberger EO. Essentials of WAIS-III assessment. New York: John Wiley & Sons; 1999. [Google Scholar]
- Klee T, Carson DK, Gavin WJ, Hall L, Kent A, Reece S. Concurrent and predictive validity of an early language screening program. Journal of Speech, Language, and Hearing Research. 1998;41:627–641. doi: 10.1044/jslhr.4103.627. [DOI] [PubMed] [Google Scholar]
- Kochanska G, Murray KT, Harlan ET. Effortful control in early childhood: Continuity and change, antecedents, and implications for social development. Developmental Psychology. 2000;36:220–232. [PubMed] [Google Scholar]
- Kodituwakku PW, Handmaker NS, Cutler SK, Weathersby EK, Handmaker SD. Specific impairments in self-regulation in children exposed to alcohol prenatally. Alcoholism: Clinical and Experimental Research. 1995;19:1558–1564. doi: 10.1111/j.1530-0277.1995.tb01024.x. [DOI] [PubMed] [Google Scholar]
- Kopera-Frye K, Dehaene S, Streissguth AP. Impairments of number processing induced by prenatal alcohol exposure. Neuropsychologia. 1996;34:1187–1196. doi: 10.1016/0028-3932(96)00043-7. [DOI] [PubMed] [Google Scholar]
- Lemery-Chalfant K, Doelger L, Goldsmith HH. Genetic relations between effortful and attentional control and symptoms of psychopathology in middle childhood. Infant and Child Development. 2008;17:365–385. doi: 10.1002/icd.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leve LD, Neiderhiser JM, Ge X, Scaramella LV, Conger RD, Reid JB, Reiss D. The Early Growth and Development Study: A prospective adoption design. Twin Research and Human Genetics. 2007;10:84–95. doi: 10.1375/twin.10.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leve LD, Neiderhiser JM, Scaramella LV, Reiss D. The Early Growth and Development Study: Using the prospective adoption design to examine genotype-environment interplay. [Special section] Behavior Genetics. 2010;40:306–314. doi: 10.1007/s10519-010-9353-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipscomb ST, Leve LD, Harold GT, Neiderhiser JM, Shaw DS, Ge X, Reiss D. Trajectories of parenting and child negative emotionality during infancy and toddlerhood: A longitudinal analysis. Child Development. 2011;82:1661–1675. doi: 10.1111/j.1467-8624.2011.01639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little RJA. A test of missing completely at random for multivariate data with missing values. Journal of the American Statistical Association. 1988;83:1198–1202. [Google Scholar]
- MacLeod CM. Half a century of research on the Stroop effect: An integrative review. Psychological Bulletin. 1991;109:163–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Goodman AM, Caine C, Delis DC, Riley EP. Executive functioning in children with heavy prenatal alcohol exposure. Alcoholism: Clinical & Experimental Research. 1999;23:1808–1815. [PubMed] [Google Scholar]
- McNeil TF, Sjostrom K. The McNeil-Sjostrom Scale for Obstetric Complications. Malmo, Sweden: Department of Psychiatry, University Hospital, Lund University; 1995. [Google Scholar]
- Muthén LK, Muthén BO. Mplus: Statistical Analysis with Latent Variables User's Guide. Sixth ed. Los Angeles, CA: StatModel; 2010. [Google Scholar]
- O'Connor BP. SPSS and SAS programs for determining the number of components using parallel analysis and Velicer's MAP test. Behavior Research Methods, Instrumentation, and Components. 2000;32:396–402. doi: 10.3758/bf03200807. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The neural architecture of emotion regulation. In: Gross JJ, editor. Handbook of emotion regulation. New York, NY: Guilford Press; 2007. pp. 87–109. [Google Scholar]
- Pears KC, Fisher P. Developmental, cognitive, and neuropsychological functioning in preschool-aged foster children: Associations with prior maltreatment and placement history. Journal of Developmental and Behavioral Pediatrics. 2005;26:112–122. doi: 10.1097/00004703-200504000-00006. [DOI] [PubMed] [Google Scholar]
- Plomin R, DeFries JC. A parent-offspring adoption study of cognitive abilities in early childhood. Intelligence. 1985;9:341–356. [Google Scholar]
- Polderman TJC, Posthuma D, De Sonneville LMJ, Stins JF, Verhulst FC, Boomsma DI. Genetic analyses of the stability of executive functioning during childhood. Biological Psychology. 2007;76:11–20. doi: 10.1016/j.biopsycho.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Putnam SP, Gartstein MA, Rothbart MK. Measurement of fine-grained aspects of toddler temperament: The Early Childhood Behavior Questionnaire. Infant Behavior & Development. 2006;29:386–401. doi: 10.1016/j.infbeh.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla L, Alley A. Validation of the Language Development Survey (LDS): A parent report tool for identifying language delay in toddlers. Journal of Speech, Language, and Hearing Research. 2001;44:434–445. doi: 10.1044/1092-4388(2001/035). [DOI] [PubMed] [Google Scholar]
- Rieser-Danner L. Individual differences in infant fearfulness and cognitive performance: A testing, performance, or competence effect? Genetic, Social, and General Psychology Monographs. 2003;129:41–71. [PubMed] [Google Scholar]
- Riggs NR, Blair CB, Greenberg MT. Concurrent and 2-year longitudinal relations between executive function and the behavior of 1st and 2nd grade children. Child Neuropsychology. 2003;9:267–276. doi: 10.1076/chin.9.4.267.23513. [DOI] [PubMed] [Google Scholar]
- Rothbart MK. Measurement of temperament in infancy. Child Development. 1981;52:569–578. [Google Scholar]
- Roth RM, Randolph JJ, Koven NS, Isquith PK. Neural substrates of executive functions: Insights from functional neuroimaging. In: Dupri JR, editor. Focus on neuropsychology research. Hauppauge, NY: Nova Science Publishers; 2006. pp. 1–36. [Google Scholar]
- Rougier NP, Noelle DC, Braver TS, Cohen JD, O’Reilly RC. Prefrontal cortex and flexible cognitive control: Rules without symbols. PNAS Proceedings of the National Academy of Sciences of the United States of America. 2005;102:7338–7343. doi: 10.1073/pnas.0502455102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda MR, Posner MI, Rothbart MK. The developmental of executive attention: Contributions to the emergence of self-regulation. Developmental Neuropsychology. 2005;28:573–594. doi: 10.1207/s15326942dn2802_2. [DOI] [PubMed] [Google Scholar]
- Rutter M. Genes and behavior: Nature-nurture interplay explained. Malden, MA: Blackwell; 2006. [Google Scholar]
- Salley BJ, Dixon WE. Temperamental joint attention predictors of language development. Merrill-Palmer Quarterly. 2007;53:131–154. doi: 10.1353/mpq.2007.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallquist JV, Eisenberg N, Spinrad TL, Reiser M, Hofer C, Zhou Q, Eggum N. Positive and negative emotionality: Trajectories across six years and relations with social competence. Emotion. 2009;9:15–28. doi: 10.1037/a0013970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinrad TL, Eisenberg N, Gaertner B, Popp T, Smith CL, Kupfer A, Hofer C. Relations of maternal socialization and toddlers’ effortful control to children’s adjustment and social competence. Developmental Psychology. 2007;43:1170–1186. doi: 10.1037/0012-1649.43.5.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stifter CA, Spinrad TL. The effect of excessive crying on the development of emotion regulation. Infancy. 2002;3:133–152. doi: 10.1207/S15327078IN0302_2. [DOI] [PubMed] [Google Scholar]
- Taylor J, Roehrig AD, Hensler BS, Connor CM, Schatschneider C. Teacher quality moderates the genetic effects on early reading. Science. 2010;328:512–514. doi: 10.1126/science.1186149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse C, Balota DA, Yap MJ, Duchek JM, McCabe D. Effects of healthy aging and early stage dementia of the Alzheimer's type on components of response time distributions in three attention tasks. Neuropsychology. 2010;24:300–315. doi: 10.1037/a0018274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. WAIS-III administration and scoring manual. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Wood AG, Smith E. Pediatric neuroimaging studies: A window to neurocognitive development of the frontal lobes. In: Anderson V, Jacobs R, Anderson PJ, editors. Executive functions and the frontal lobes: A lifespan perspective. Philadelphia, PA: Taylor & Francis; 2008. pp. 203–216. [Google Scholar]
- Zelazo PD, Cunningham WA. Executive Function: Mechanisms Underlying Emotion Regulation. In: Gross JJ, editor. Handbook of emotion regulation. New York, NY, US: Guilford Press; 2007. pp. 135–158. [Google Scholar]
- Zelazo PD, Muller U. Executive function in typical and atypical development. In: Goswami U, editor. Handbook of childhood cognitive development. Oxford, England: Blackwell; 2002. pp. 445–469. [Google Scholar]
- Zelazo PD, Qu L, Kesek AC. Hot executive function: Emotion and the development of cognitive control. In: Calkins SD, Bell MA, editors. Child development at the intersection of emotion and cognition. Washington, DC: American Psychological Association; 2010. pp. 97–111. [Google Scholar]