Abstract
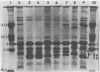
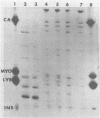
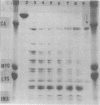
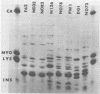
The major outer membrane protein (MOMP), or protein 1, of Neisseria gonorrhoeae is one of the immunodominant proteins of the gonococcal cell surface. It is at least partially responsible for imparting serotyping specificity. This study attempted to compare the primary structure of MOMP molecules isolated from several gonococcal strains by preparative polyacrylamide gel electrophoresis. These isolated proteins were then subjected to enzymatic digestion with staphylococcal V8 protease and alpha-chymotrypsin. The generated peptides were separated by polyacrylamide gradient gel electrophoresis in the presence of sodium dodecyl sulfate. Of the eight strains analyzed, six exhibited distinct peptide maps after either staphylococcal V8 protease or alpha-chymotrypsin digestion. These six strains have also been shown to be immunologically distinct. The data suggest that the gene pool coding for the MOMP is diverse and reflect the complexity of the evolution of N. gonorrhoeae.
Full text
PDF

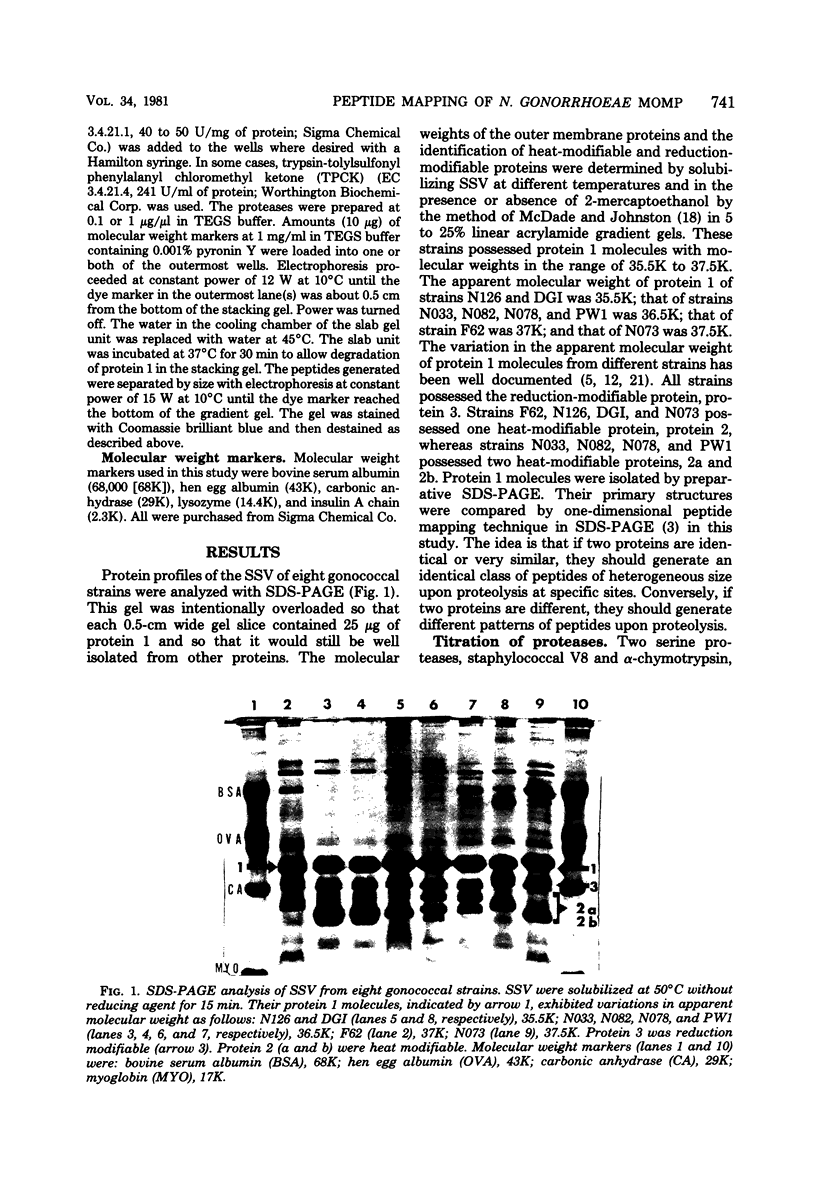
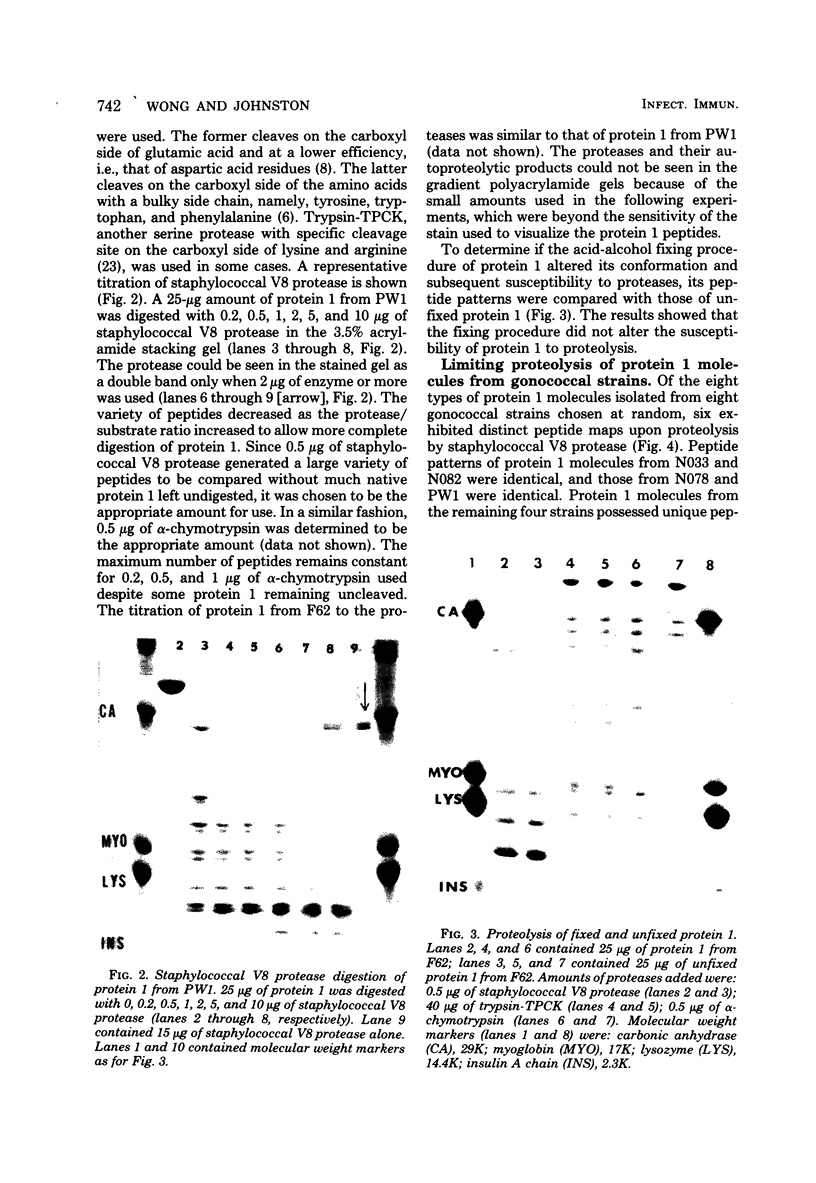
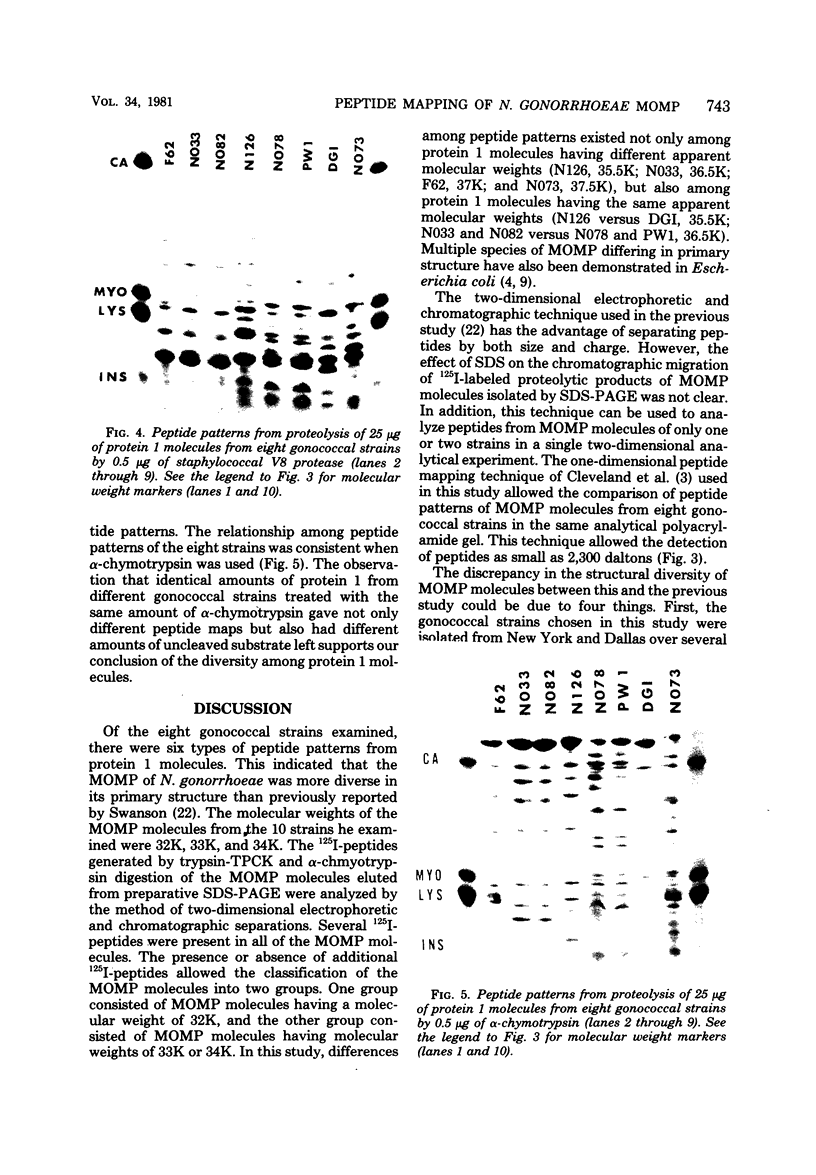


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apicella M. A. Serogrouping of Neisseria gonorrhoeae: identification of four immunologically distinct acidic polysaccharides. J Infect Dis. 1976 Oct;134(4):377–383. doi: 10.1093/infdis/134.4.377. [DOI] [PubMed] [Google Scholar]
- Buchanan T. M. Antigenic heterogeneity of gonococcal pili. J Exp Med. 1975 Jun 1;141(6):1470–1475. doi: 10.1084/jem.141.6.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Gamon K., Chen R., Henning U. Major proteins of the outer cell envelope membrane of Escherichia coli K12: multiple species of protein I differ in primary structure. Mol Gen Genet. 1978 Oct 30;166(2):187–192. doi: 10.1007/BF00285921. [DOI] [PubMed] [Google Scholar]
- Heckels J. E. The surface properties of Neisseria gonorrhoeae: topographical distribution of the outer membrane protein antigens. J Gen Microbiol. 1978 Oct;108(2):213–219. doi: 10.1099/00221287-108-2-213. [DOI] [PubMed] [Google Scholar]
- Hildebrandt J. F., Mayer L. W., Wang S. P., Buchanan T. M. Neisseria gonorrhoeae acquire a new principal outer-membrane protein when transformed to resistance to serum bactericidal activity. Infect Immun. 1978 Apr;20(1):267–272. doi: 10.1128/iai.20.1.267-272.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houmard J., Drapeau G. R. Staphylococcal protease: a proteolytic enzyme specific for glutamoyl bonds. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3506–3509. doi: 10.1073/pnas.69.12.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihara S., Mizushima S. Characterization of major outer membrane proteins O-8 and O-9 of Escherichia coli K-12. Evidence that structural genes for the two proteins are different. J Biochem. 1978 Apr;83(4):1095–1100. doi: 10.1093/oxfordjournals.jbchem.a131998. [DOI] [PubMed] [Google Scholar]
- Johnston K. H., Gotschlich E. C. Isolation and characterization of the outer membrane of Neisseria gonorrhoeae. J Bacteriol. 1974 Jul;119(1):250–257. doi: 10.1128/jb.119.1.250-257.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston K. H., Holmes K. K., Gotschlich E. C. The serological classification of Neisseria gonorrhoeae. I. Isolation of the outer membrane complex responsible for serotypic specificity. J Exp Med. 1976 Apr 1;143(4):741–758. doi: 10.1084/jem.143.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G. J., Swanson J. Studies on gonococcus infection. XV. Identification of surface proteins of Neisseria gonorrhoeae correlated with leukocyte association. Infect Immun. 1978 Aug;21(2):575–584. doi: 10.1128/iai.21.2.575-584.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krohn K. A., Knight L. C., Harwig J. F., Welch M. J. Differences in the sites of iodination of proteins following four methods of radioiodination. Biochim Biophys Acta. 1977 Feb 22;490(2):497–505. doi: 10.1016/0005-2795(77)90026-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambden P. R., Heckels J. E., James L. T., Watt P. J. Variations in surface protein composition associated with virulence properties in opacity types of Neisseria gonorrhoeae. J Gen Microbiol. 1979 Oct;114(2):305–312. doi: 10.1099/00221287-114-2-305. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- McDade R. L., Jr, Johnston K. H. Characterization of serologically dominant outer membrane proteins of Neisseria gonorrhoeae. J Bacteriol. 1980 Mar;141(3):1183–1191. doi: 10.1128/jb.141.3.1183-1191.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockley R. K., Coffee E. E., Johnston K. H. SJ-GC, a modified complete medium for growth of Neisseria gonorrhoeae. J Clin Microbiol. 1980 Jul;12(1):35–38. doi: 10.1128/jcm.12.1.35-38.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XIV. Cell wall protein differences among color/opacity colony variants of Neisseria gonorrhoeae. Infect Immun. 1978 Jul;21(1):292–302. doi: 10.1128/iai.21.1.292-302.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XVIII. 125I-labeled peptide mapping of the major protein of the gonococcal cell wall outer membrane. Infect Immun. 1979 Mar;23(3):799–810. doi: 10.1128/iai.23.3.799-810.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong T. P., Shockley R. K., Johnston K. H. WSJM, a simple chemically defined medium for growth of Neisseria gonorrhoeae. J Clin Microbiol. 1980 Apr;11(4):363–369. doi: 10.1128/jcm.11.4.363-369.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]