Abstract
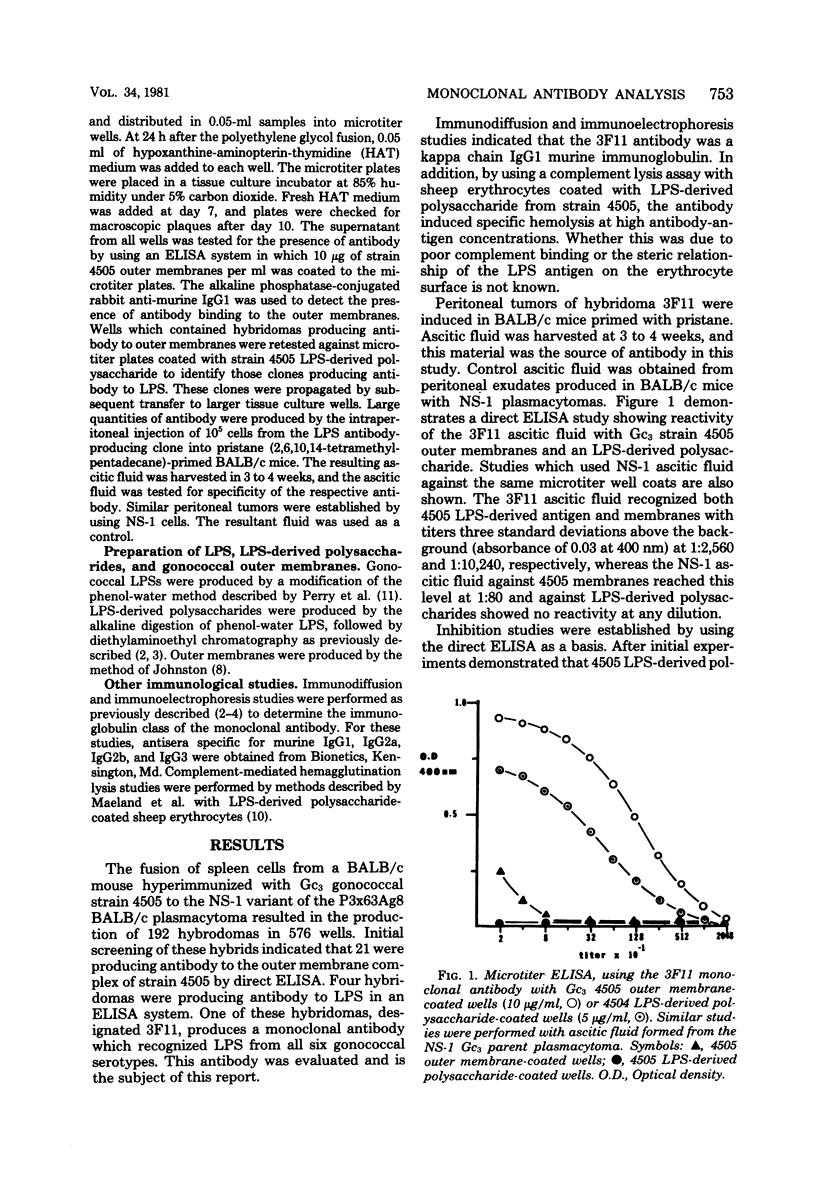
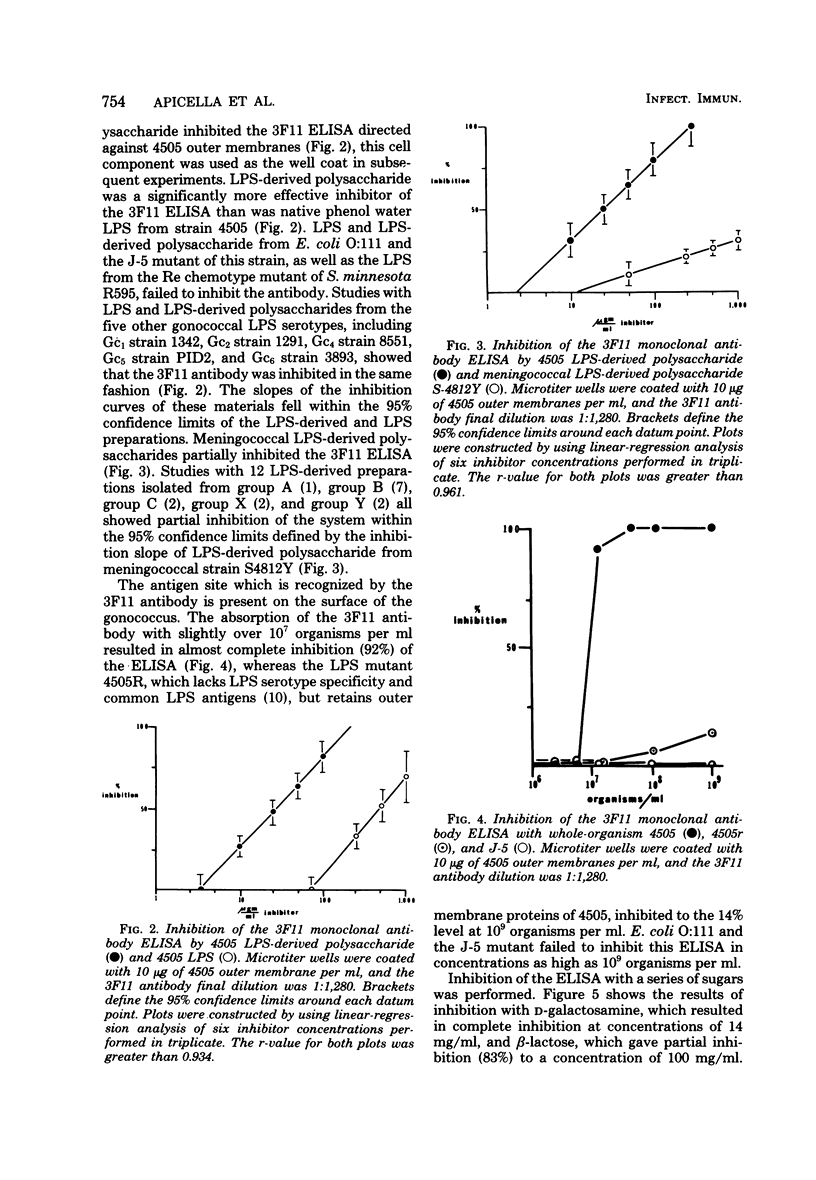
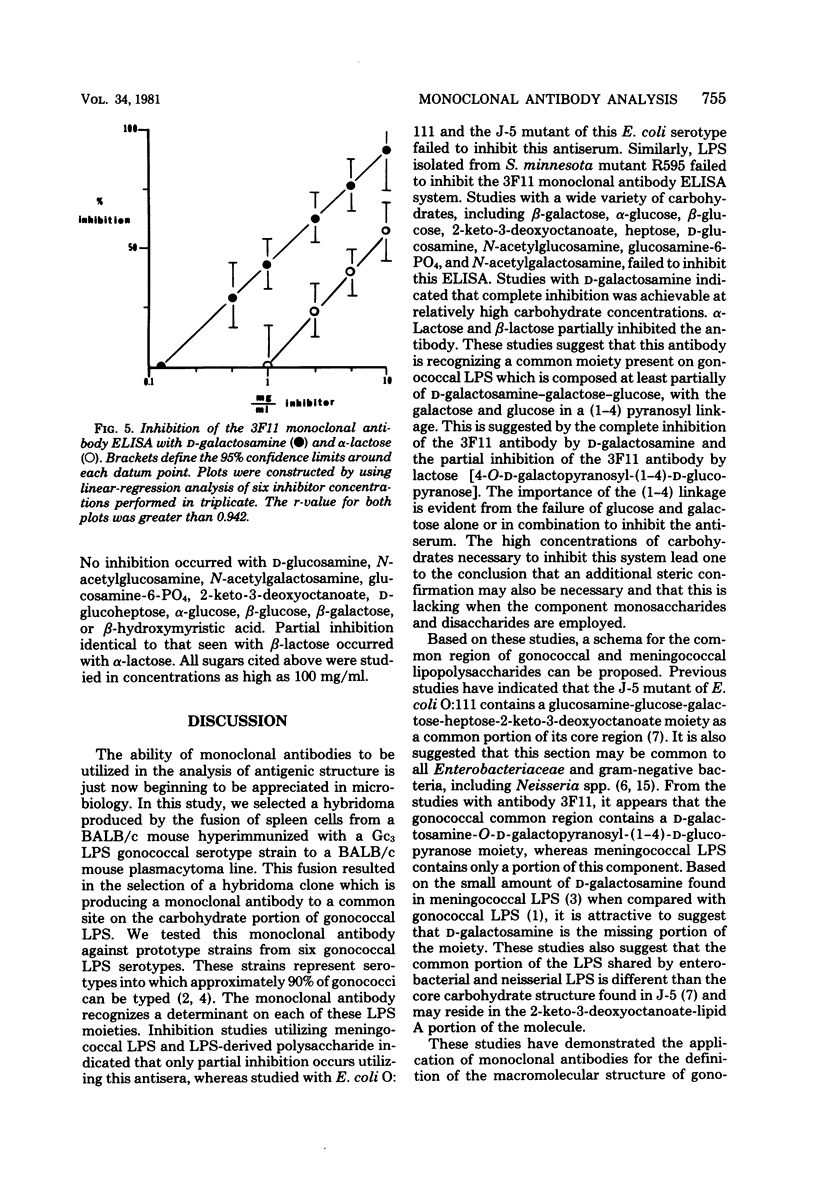
A hybridoma produced by the polyethylene glycol fusion of the NS-1 variant of the P3x63Ag8 BALB/c plasmacytoma to splenocytes harvested from a BALB/c mouse immunized with whole gonococci was found to be producing antibody to a common region on gonococcal lipopolysaccharide (LPS). Enzyme-linked immunosorbent assay inhibition systems were established by utilizing this antibody, designated 3F11, and 100% inhibition occurred with both LPS and the LPS-LPS and LPS-derived polysaccharides partially inhibited the enzyme-linked immunosorbent assay, whereas similar preparations isolated from Escherichia coli O:111, the J-5 mutant of this strain, and Salmonella minnesota Re595 failed to inhibit the assay. Studies utilizing whole gonococcal strains 4505 and the isogenic variant 4505r, which lacks both the LPS serotype and common determinants as inhibitors, demonstrated that the determinant recognized by the 3F11 antibody was present on the surface of 4505 and absent on 4505r. Inhibition studies were performed with beta-glucose, beta-galactose, D-glucosamine, D-galactosamine, heptose, 2-keto-3-deoxyoctanoate, N-acetylglucosamine, N-acetylgalactosamine, alpha-lactose, and beta-lactose. Complete inhibition of the enzyme-linked immunosorbent assay occurred with D-galactosamine, and partial inhibition was achieved with both alpha-lactose and beta-lactose. Based on these observations, the 3F11 antibody recognizes a site common to gonococcal LPS which is partially shared by meningococcal LPS. The chemical structure of the determinant appears to be a D-galactosamine-O-D-galactopyranosyl-(1-4)-D-glucopyranose. Additional specificity may be conferred by the steric relationship of the determinant on the intact LPS.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen P. Z., Connelly M. C., Apicella M. A. Interaction of lectins with Neisseria gonorrhoeae. Can J Microbiol. 1980 Apr;26(4):468–474. doi: 10.1139/m80-078. [DOI] [PubMed] [Google Scholar]
- Apicella M. A., Gagliardi N. C. Antigenic heterogeneity of the non-serogroup antigen structure of Neisseria gonorrhoeae lipopolysaccharides. Infect Immun. 1979 Dec;26(3):870–874. doi: 10.1128/iai.26.3.870-874.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apicella M. A. Lipopolysaccharide-derived serotype polysaccharides from Neisseria meningitidis group B. J Infect Dis. 1979 Jul;140(1):62–72. doi: 10.1093/infdis/140.1.62. [DOI] [PubMed] [Google Scholar]
- Apicella M. A. Serogrouping of Neisseria gonorrhoeae: identification of four immunologically distinct acidic polysaccharides. J Infect Dis. 1976 Oct;134(4):377–383. doi: 10.1093/infdis/134.4.377. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Davis C. E., Ziegler E. J., Arnold K. F. Neutralization of meningococcal endotoxin by antibody to core glycolipid. J Exp Med. 1978 Apr 1;147(4):1007–1017. doi: 10.1084/jem.147.4.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELBEIN A. D., HEATH E. C. THE BIOSYNTHESIS OF CELL WALL LIPOPOLYSACCHARIDE IN ESCHERICHIA COLI. I. THE BIOCHEMICAL PROPERTIES OF A URIDINE DIPHOSPHATE GALACTOSE 4-EPIMERASELESS MUTANT. J Biol Chem. 1965 May;240:1919–1925. [PubMed] [Google Scholar]
- Kennett R. H. Cell fusion. Methods Enzymol. 1979;58:345–359. doi: 10.1016/s0076-6879(79)58149-x. [DOI] [PubMed] [Google Scholar]
- Maeland J. A., Kristoffersen T., Hofstad T. Immunochemical investigations on neisseria gonorrhoeae endotoxin. 2. Serological multispecificity and other properties of phenol-water preparations. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(2):233–238. [PubMed] [Google Scholar]
- Perry M. B., Daoust V. The lipopolysaccharides of Neisseria gonorrhoeae colony types 1 and 4. Can J Biochem. 1975 May;53(5):623–629. doi: 10.1139/o75-084. [DOI] [PubMed] [Google Scholar]
- Voller A., Draper C., Bidwell D. E., Bartlett A. Microplate enzyme-linked immunosorbent assay for chagas' disease. Lancet. 1975 Feb 22;1(7904):426–428. doi: 10.1016/s0140-6736(75)91492-0. [DOI] [PubMed] [Google Scholar]
- Ziegler E. J., Douglas H., Sherman J. E., Davis C. E., Braude A. I. Treatment of E. coli and klebsiella bacteremia in agranulocytic animals with antiserum to a UDP-gal epimerase-deficient mutant. J Immunol. 1973 Aug;111(2):433–438. [PubMed] [Google Scholar]
- Zollinger W. D., Mandrell R. E. Outer-membrane protein and lipopolysaccharide serotyping of Neisseria meningitidis by inhibition of a solid-phase radioimmunoassay. Infect Immun. 1977 Nov;18(2):424–433. doi: 10.1128/iai.18.2.424-433.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


