Abstract
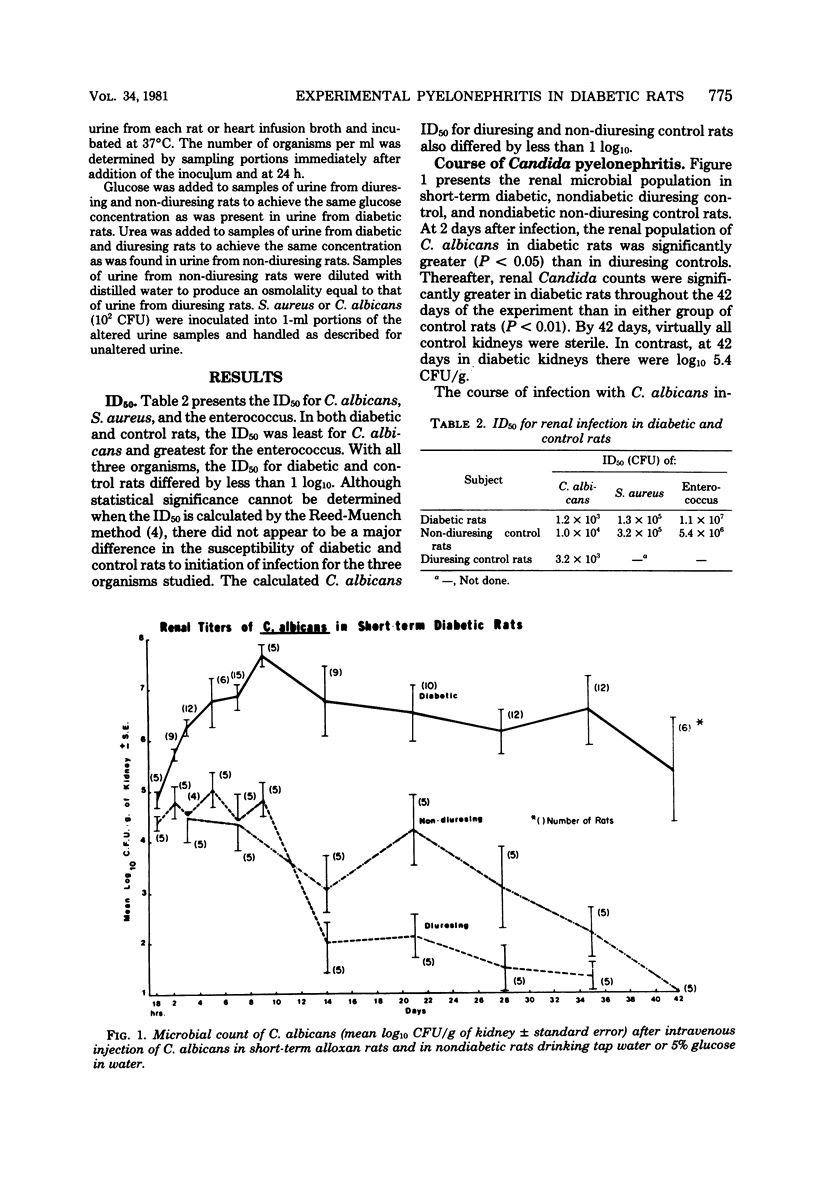
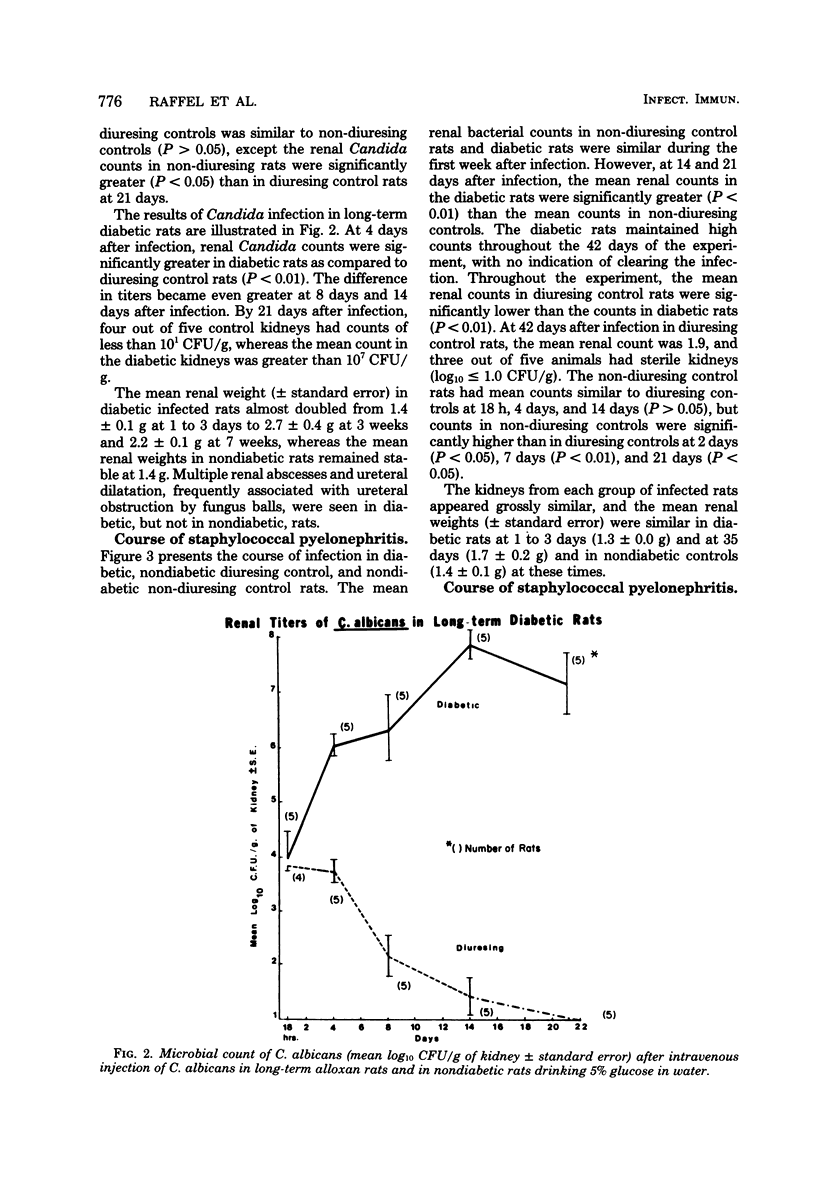
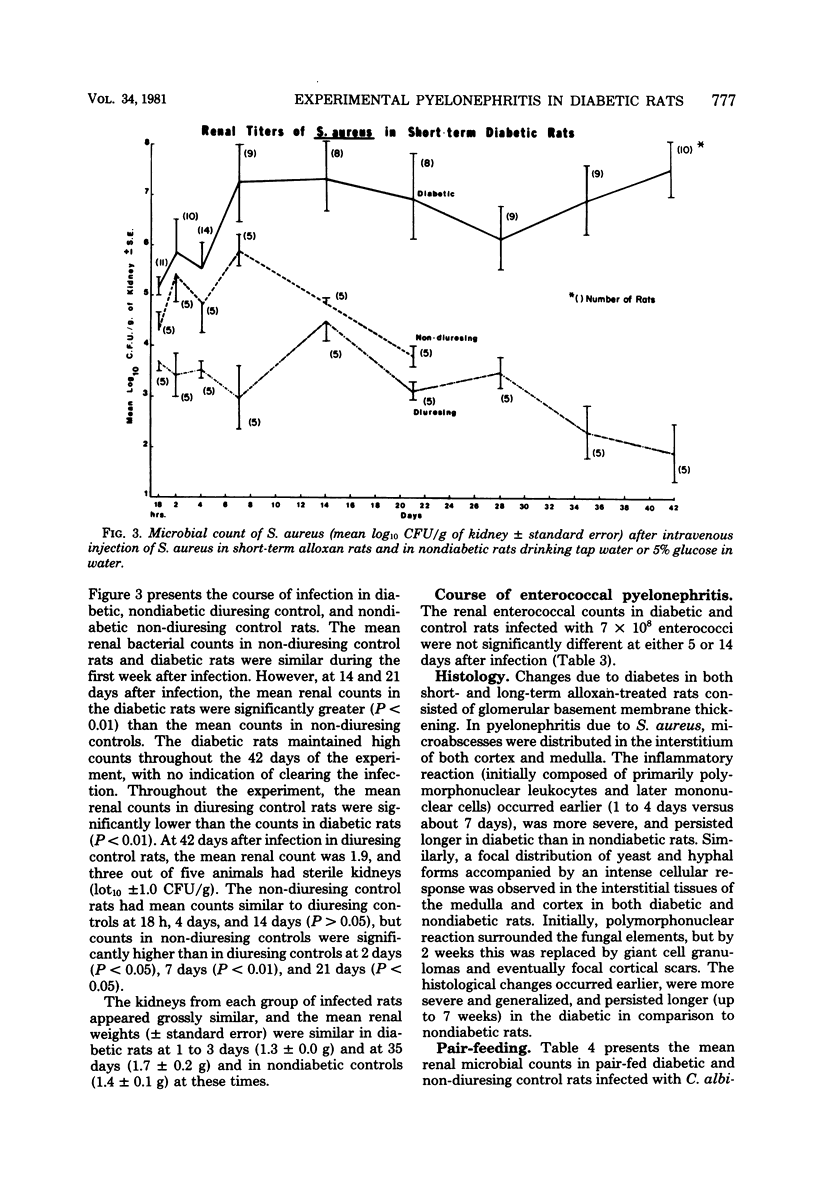
Pyelonephritis was studied after an intravenous injection of Candida albicans, Staphylococcus aureus, or enterococcus in alloxan-diabetic rats and in water-diuresing or non-diuresing nondiabetic rats. The renal microbial populations of C. albicans or S. aureus were found to be greater than 10(5) colony-forming units per g for up to 42 days in diabetic rats, whereas the kidneys tended to become sterile in nondiabetic rats. No significant difference was found in the course of enterococcal pyelonephritis in diabetic versus control rats. The difference in the 50% infective dose for each microorganism between diabetic and control rats was less than or equal to log10. Neither duration of diabetes nor weight loss contributed to the greater and more sustained renal populations of C. albicans and S. aureus in diabetic rats. The inflammatory reaction in kidneys infected with S. aureus or C. albicans was greater in diabetic rats. Fungus balls associated with ureteral obstruction and gross multiple renal abscesses occurred in diabetic, but not in nondiabetic, rats infected with Candida. Growth of C. albicans and S. aureus in vitro in urine from diabetic rats was significantly greater than it was in urine from control rats. Addition of water or glucose to the urine of non-diuresing, nondiabetic rats significantly increased in vitro growth of S. aureus and C. albicans. These studies demonstrate greater severity of infection in the diabetic kidney due to S. aureus and C. albicans, which can be partially explained by decreased inhibitory activity of urine for these organisms in diabetic rats.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN B. R., JACKSON G. G. Pyelitis, an important factor in the pathogenesis of retrograde pyelonephritis. J Exp Med. 1961 Sep 1;114:375–384. doi: 10.1084/jem.114.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANDRIOLE V. T., EPSTEIN F. H. PREVENTION OF PYELONEPHRITIS BY WATER DIURESIS: EVIDENCE FOR THE ROLE OF MEDULLARY HYPERTONICITY IN PROMOTING RENAL INFECTION. J Clin Invest. 1965 Jan;44:73–79. doi: 10.1172/JCI105128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANDRIOLE V. T., HASENCLEVER H. F. Factors influencing experimental candidiasis in mice. I. Alloxan diabetes. Yale J Biol Med. 1962 Aug;35:96–112. [PMC free article] [PubMed] [Google Scholar]
- CRUICKSHANK A. H. Resistance to infection in the alloxan-diabetic rabbit. J Pathol Bacteriol. 1954 Apr;67(2):323–334. doi: 10.1002/path.1700670205. [DOI] [PubMed] [Google Scholar]
- Coil J. A., Davis J. H. Altered host response to experimental pyelonephritis in alloxan diabetic rats. J Surg Res. 1967 Jan;7(1):26–34. doi: 10.1016/0022-4804(67)90006-6. [DOI] [PubMed] [Google Scholar]
- Hurley R. Experimental infection with Candida albicans in modified hosts. J Pathol Bacteriol. 1966 Jul;92(1):57–67. doi: 10.1002/path.1700920108. [DOI] [PubMed] [Google Scholar]
- Kaye D., Rocha H. Urinary concentrating ability in early experimental pyelonephritis. J Clin Invest. 1970 Jul;49(7):1427–1437. doi: 10.1172/JCI106360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levison S. P., Kaye D. Influence of water diuresis on antimicrobial treatment of enterococcal pyelonephritis. J Clin Invest. 1972 Sep;51(9):2408–2413. doi: 10.1172/JCI107053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud A. A., Cheever A. W., Warren K. S. Streptozotocin-induced diabetes mellitus and the host-parasite relation in murine schistosomiasis mansoni. J Infect Dis. 1975 Jun;131(6):634–642. doi: 10.1093/infdis/131.6.634. [DOI] [PubMed] [Google Scholar]
- Mahmoud A. A., Rodman H. M., Mandel M. A., Warren K. S. Induced and spontaneous diabetes mellitus and suppression of cell-mediated immunologic responses. Granuloma formation, delayed dermal reactivity and allograft rejection. J Clin Invest. 1976 Feb;57(2):362–367. doi: 10.1172/JCI108287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T., Scott L., Stewart E., North D. Modification by suppressor cells and serum factors of the cell-mediated immune response in experimental pyelonephritis. J Clin Invest. 1978 Apr;61(4):964–972. doi: 10.1172/JCI109021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherji A. K., Mallik K. C. Candida albicans infection in alloxan induced diabetic mice. Indian J Med Res. 1975 Apr;63(4):539–544. [PubMed] [Google Scholar]
- SCHOFIELD R. A., BAKER R. D. Experimental mucormycosis (Rhizopus infection) in mice; the failure of chronic alloxan diabetes to modify host susceptibility. AMA Arch Pathol. 1956 May;61(5):407–415. [PubMed] [Google Scholar]
- SHELDON W. H., BAUER H. The development of the acute inflammatory response to experimental cutaneous mucormycosis in normal and diabetic rabbits. J Exp Med. 1959 Dec 1;110:845–852. doi: 10.1084/jem.110.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood U., Agarwal D. S., Aurora A. L. A study of subcutaneous staphylococcal infection in alloxan diabetic mice. Indian J Med Res. 1975 Nov;63(11):1564–1576. [PubMed] [Google Scholar]
- WERTMAN K. F., HENNEY M. R. The effects of alloxan diabetes on phagocytosis and susceptibility to infection. J Immunol. 1962 Sep;89:314–317. [PubMed] [Google Scholar]
- Williams T. W., Friedlander A. M., Lyons J. M., Braude A. I. Cellular immunity in pyelonephritis: identification of suppressor cell activity of spleen cells in response to concanavalin A and inhibition of lymphocyte-mediated L cell cytotoxicity. J Immunol. 1976 Mar;116(3):778–781. [PubMed] [Google Scholar]


