Abstract
Aims
To evaluate reciprocal enhancement (combining treatments to offset their relative weaknesses) as a strategy to improve cannabis treatment outcomes. Contingency management (CM) with reinforcement for homework completion and session attendance was used as a strategy to enhance cognitive–behavioral therapy (CBT) via greater exposure to skills training; CBT was used as a strategy to enhance durability of CM with rewards for abstinence.
Setting
Community-based out-patient treatment program in New Haven, Connecticut, USA.
Design
Twelve-week randomized clinical trial of four treatment conditions: CM for abstinence alone or combined with CBT, CBT alone or combined with CM with rewards for CBT session attendance and homework completion.
Participants
A total of 127 treatment-seeking young adults (84.3% male, 81.1% minority, 93.7% referred by criminal justice system, average age 25.7 years).
Measurements
Weekly urine specimens testing positive for cannabis, days of cannabis use via the time-line follow-back method.
Findings
Within treatment, reinforcing homework and attendance did not significantly improve CBT outcomes, and the addition of CBT worsened outcomes when added to CM for abstinence (75.5 versus 57.1% cannabis-free urine specimens, F = 2.25, P = 0.02). The CM for abstinence condition had the lowest percentage of cannabis-negative urine specimens and the highest mean number of consecutive cannabis-free urine specimens (3.3, F = 2.33, P = 0.02). Attrition was higher in the CBT alone condition, but random effect regression analyses indicated this condition was associated with the greatest rate of change overall. Cannabis use during the 1-year follow-up increased most rapidly for the two enhanced groups.
Conclusions
Combining contingency management and cognitive–behavioural therapy does not appear to improve success rates of treatment for cannabis dependence in clients involved with the criminal justice system.
Keywords: Cannabis dependence, cognitive behavioral therapy, contingency management, criminal justice system, randomized clinical trial
INTRODUCTION
Randomized trials have not yet yielded an effective pharmacological strategy for cannabis dependence [1,2]. The most promising behavioral therapies involve combinations of motivational, cognitive–behavioral therapy (CBT) and contingency management (CM) approaches [3–5]. Even with these approaches, however, abstinence rates remain modest [4,6]. The need to develop more powerful and durable approaches suggests that new strategies should be evaluated to understand more clearly whether outcomes can be improved further. One such strategy may be combining existing treatments to enhance their distinctive strengths and offset their relative weaknesses [7].
CBT approaches have demonstrated effectiveness across a range of substance use disorders [8–12]. A distinctive strength of CBT is that its benefits appear to be particularly durable [13–15], and may relate to its emphasis on skills training and homework assignments that provide opportunities to practice and generalize new coping skills [16–18]. Although CBT appears to retain substance users comparatively well [10], a notable limitation is that a significant proportion drop out before completing treatment and hence are insufficiently exposed to a full course of skills training. Because retention in CBT is associated with both enhanced skill acquisition and better drug use outcomes [19,20], strategies that effectively retain patients may strengthen CBT’s effects.
CM has also gained strong empirical support from rigorous clinical trials [21,22], demonstrating consistent robust effects in terms of retention and fostering abstinence in diverse samples [23]. One important strength of CM is the precision with which it can be targeted to specific behaviors [24–27]. CM could thus be used to facilitate exposure to specific targets or active ingredients of behavioral therapies. Thus, CM could be used as a strategy to stabilize patients and increase the likelihood of sufficient exposure to putative active ingredients of other therapies, such as CBT.
A weakness of CM is that its effects tend to drop off once the target behavior is no longer reinforced [28–30]. Thus, the longer-term effectiveness of CM may be enhanced by integrating it with therapies such as CBT, the durability of which may diminish rebound when the target behavior is no longer reinforced. CBT might be an ideal candidate for maximizing the effects of CM, as it emphasizes implementation and generalization of behavior change strategies. Thus, teaching patients practical strategies to initiate and sustain abstinence and become exposed to alternative reinforcers may strengthen the durability of CM’s effects. CBT strategies could be targeted specifically in this context to: (i) encourage patients to attribute their decisions to not use drugs to internal forces rather than entirely to the external contingencies; (ii) recognize, develop and integrate the strategies they are using to avoid drug use; (iii) facilitate greater patient exposure to CM reinforcement through goal-setting and problem-solving strategies; and (iv) build self-reward strategies to offset dependence on external rewards.
In a previous dismantling study among 240 outpatient cannabis users, Kadden and colleagues [6] compared a combination of CBT and motivational enhancement therapy (MET) [31], CBT + MET plus voucher-based CM, CM alone and supportive case management. CM had the highest rate of abstinence at the end of the 9-week treatment, and the CBT + MET + CM condition had the best outcomes at the 1-year follow-up. However, to our knowledge, no previous study has made explicit efforts to use specific cognitive–behavioral strategies to diminish rebound effects of CM, nor to deploy CM to strengthen the putative active ingredients of CBT by reinforcing attendance or homework completion.
In this study, we evaluated the benefits of adding specific enhancements of either CBT or CM to address their respective weaknesses. We hypothesized that adding reinforcement via CM for attendance and homework completion to standard CBT would enhance its efficacy in reducing cannabis use (contrast 1). Secondly, we hypothesized that adding targeted skills training via CBT would improve outcomes for CM for abstinence; however, this effect might be stronger during follow-up (contrast 2). Thirdly, we hypothesized that, during treatment, interventions involving any form of CM would have better outcomes than CBT alone (contrast 3). Finally, we hypothesized that during the course of a 1-year follow-up, the combination of CM for CBT attendance and homework would outperform CM for abstinence due to the tendency of CM effects to weaken over time.
METHODS
Participants
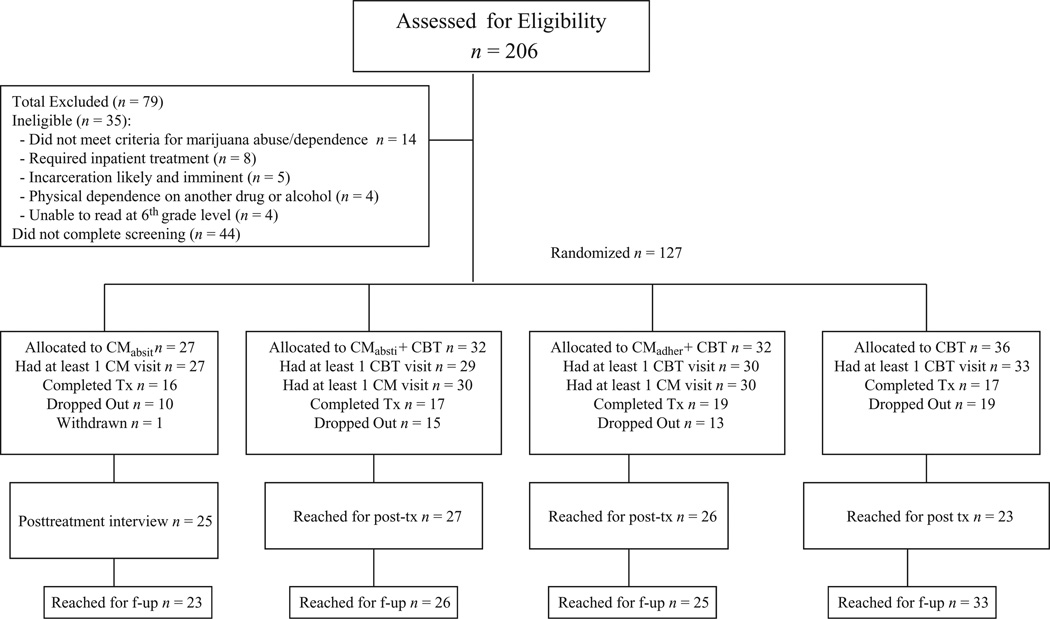
Participants were individuals aged 18 years and above, who were either self-referred or referred for treatment by the Office of Adult Probation to the Substance Abuse Treatment Unit in New Haven, Connecticut, and who met criteria for current cannabis dependence. Of 206 individuals screened, 44 did not complete the screening/eligibility process and 35 did not meet inclusion/exclusion criteria (Fig. 1). Thus, 127 individuals provided written informed consent and were randomized via an urn randomization program [32,33], which enhanced probabilities of balance across groups on gender, ethnicity, severity of cannabis use and referral source.
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram: flow of participants
Treatments
Cognitive–behavioral therapy (CBTalone)
CBTalone was delivered in 50-minute individual weekly sessions by 15 clinicians (11 female, four male: seven held doctorates and eight were master’s level) who had completed a 2-day didactic seminar and at least one closely supervised pilot case, and demonstrated competence in CBT by meeting pre-specified criteria for competence on the basis of ratings of their training cases using a validated treatment process rating system [34,35]. As described in the manual [36], the goal of CBT is abstinence from cannabis via functional analysis of high-risk situations, development of effective coping strategies and altering maladaptive cognitions associated with the maintenance of cannabis use. Material discussed during each session is supplemented with extra-session homework tasks intended to foster implementation and mastery of skills.
CBT + CM for adherence (CBT + CMadher)
In addition to CBT as above, participants were offered chances to draw prizes from a bowl contingent upon session attendance and homework completion. Following procedures developed by Petry [37,38], participants earned two draws each time they attended a CBT session. The number of draws earned escalated by one draw per consecutive day of scheduled attendance. If a participant failed to attend a scheduled session, the number of draws earned reset to one for the next session attended.
To promote extra-session skill practice, participants could earn bonus draws contingent upon bringing completed homework assignments to their CBT sessions. Reinforcement for homework completion also occurred on an escalating schedule, with the number of bonus draws escalating by one draw per consecutive times homework was completed, to a maximum of 13 bonus draws per session. Participants who were fully compliant with attendance and homework assignments could earn a maximum of 178 draws.
The same prize bowl was used for all three CM conditions where, on average, participants had an expected maximum earning of $250 in prizes. The bowl contained 650 cards, of which 375 were winning cards. Of these, 269 were small prizes (participant’s choice of $1 fast-food coupons, bus tokens), 75 were medium prizes, worth up to $5 in value (t-shirts, gloves, hats or five small prizes), 30 were large prizes, worth up to $20 in value (movie tickets, CDs, telephone cards) and one jumbo prize worth up to $100 (small television, or five medium prizes).
CM for abstinence (CMabst)
In this condition, participants had the opportunity to draw from a bowl and earn prizes each time they provided urine samples that were negative for cannabis at the 12-weekly assessment sessions. At the first assessment session, where the participant provided a urine sample that tested negative for cannabis, they earned four draws. To promote continuous abstinence, the number of draws participants earned increased by two for each successive negative sample submitted, up to a maximum of 26 draws per day. Participants could earn up to 180 drawings.
Participants assigned to this condition were not offered individual CBT or other treatment. As few studies have evaluated the feasibility, safety or efficacy of CM delivered without regular clinician contact [6,39], participants met weekly with a research assistant. Meetings lasted no more than 5 minutes and were limited to collection of urine samples, calculation and redemption of prizes, as well as minimal monitoring via the time-line follow-back method (TLFB). Standard criteria were used for clinical deterioration [40]; only one of the 27 individuals randomized to this condition was withdrawn due to unimproved cannabis use after several weeks.
CM for abstinence plus CBT (CMabst + CBT)
Participants randomized to this condition received prize CM for submitting urine specimens negative for cannabis and weekly individual CBT, as above. CBT was adapted for this condition in order to facilitate more durable CM effects. An addendum to the CBT manual encouraged therapists to address the following issues to: (i) identify what behaviors or skills were implemented when the participant submitted cannabis-negative urine specimens; (ii) focus on the participant’s cognitions regarding his decision to use or not use cannabis, encouraging recognition of these decisions as choices so as to foster internal attribution of change; (iii) practice specific skills and strategies the participant could use to earn draws in the future; and (iv) encourage self-rewards to offset dependence on external reinforcers.
Assessment of treatment fidelity
To evaluate fidelity and clinicians’ skill level, all sessions were videotaped for supervision, and 246 session videotapes were rated by seven master’s-level independent evaluators trained to criterion in previous trials [41–43]. Raters were unaware of the participants’ treatment assignment. These tapes covered 42% of all sessions recorded and were selected to include tapes from each clinician and all three groups assigned to CBT (CBT + CMabst, 39%: CBT + CMadher, 34% and CBTalone, 27%). The Yale Adherence and Competence Scale (YACS) [34], which includes several scales evaluating interventions characterizing specific therapies (e.g. CBT, CM) and general counseling (e.g. assessment, general support), was used for process ratings. The YACS has been demonstrated to have excellent reliability, concurrent and factorial validity and to discriminate treatments [34,35,42,43]. For this study, four items were added to monitor the extent to which the clinicians focused on CM in the CBT sessions as specified in the adapted manual.
Estimates of inter-rater reliability were based on nine tapes rated by all seven raters (e.g. a complete block design). A comparatively small reliability sample was used because this group of raters had been well trained in the YACS and had achieved high inter-rater reliability measures in several recent studies [41,42,44]. The mean intraclass correlation coefficient (ICC) estimate from the random effects model [45] for the CBT scale were 0.84 for the adherence scale and 0.84 for the competence scale. For the adapted CM scale, ICCs were 0.82 and 0.90, respectively; and for the general counseling scale, ICCs were 0.86 and 0.87, respectively.
Analysis of variance (ANOVA) analyses suggested that the study treatments were implemented as intended, as CM adherence scores were significantly higher in the two groups assigned to CM (F = 3.54, P = 0.03, d.f. = 2, 243). Moreover, there were no significant differences across conditions in mean CBT adherence (F = 1.04, P = 0.35, d.f. = 2, 243) or competence (F = 0.84, P = 0.43, d.f. = 2, 239) scores, or in mean general counseling adherence (F = 1.48, P = 0.23, d.f. = 2, 243) or competence scores (F = 0.65, P = 0.52, d.f. = 2, 229), suggesting that these approaches were implemented consistently across conditions.
Assessments
Participants were assessed at baseline, weekly during treatment at the 12-week post-treatment assessment and at 3-month intervals during the 1-year follow-up. Weekly assessments included urinalysis with temperature and adulterant checks, as well as self-reports of substance use using the TLFB method, a reliable and valid method for assessing substance use [46–49]. Psychiatric diagnoses were assessed using the Structured Clinical Interview for DSM-IV (SCID) [50].
In cases where a randomized participant did not initiate or dropped out of treatment, he or she was interviewed at post-treatment. Of the 58 participants who did not complete the study, 84% (49 of 58) were tracked successfully and interviewed at the 12-week point. Thus, complete self-report data for the 12-week study period were available for 111 of 127 (87%) of the randomized sample. Follow-up interviews included collection of urine/breath samples and the TLFB. Of the 127 individuals randomized, 84% were reached for at least one follow-up and 69% were reached for the 12-month follow-up, with no significant differences by treatment condition.
Self-reports of cannabis were verified through urine toxicology screens obtained weekly during treatment and each follow-up. Of 681 urine specimens collected from participants who had contributed at least 7 days of data from which to calculate agreement between self-reports and toxicology results, 80% were consistent with self-report; 16% were positive for cannabis when the participant denied use in the past 7 days and 4% were negative, although the participant reported cannabis use within 7 days. This level of discrepancy is comparable with rates reported previously [51,52].
Data analyses
For all within-treatment data, statistical models tested the a priori hypotheses using the following contrasts: contrast 1: CBT + CMadher versus CBTalone; contrast 2: CBT + CMabst versus CMabst; and contrast 3: all three groups receiving CM (CBT + CMadher, CBT + CMabst, and CMabst) versus CBTalone. ANOVA models were used to analyze stationary variables that summarized outcomes across treatment (e.g. longest period of continuous abstinence during treatment, percentage of cannabis-free urine specimens). Follow-up and longitudinal data were analyzed using random-effect regression models [53].
RESULTS
Participant characteristics, treatment exposure and integrity
Demographic, substance use and psychosocial functioning variables at baseline for the 127 randomized individuals are presented in Table 1. With the exception of rates of antisocial personality disorder (ASP), there were no statistically significant differences by treatment condition on any of these variables.
Table 1.
Baseline demographic, psychiatric and marijuana use data; randomized participants by treatment condition.
| CBTalone | CBT + CMadher | CBT + CMabst | CMabst | Total | |||
|---|---|---|---|---|---|---|---|
| Variable | n = 36 | n = 32 | n = 32 | n = 27 | n = 127 | F or X2 | P |
| % female (number, %) | 6 (16.7) | 5 (15.6) | 3 (9.4) | 6 (22.2) | 20 (15.7) | 1.86 | 0.60 |
| Race | |||||||
| Caucasian | 3 (8.3) | 8 (25) | 6 (18.8) | 7 (25.9) | 24 (18.9) | 10.52 | 0.57 |
| African American | 24 (66.7) | 20 (62.5) | 19 (59.4) | 18 (66.7) | 81 (63.8) | ||
| Hispanic | 6 (16.7) | 4 (12.5) | 5 (15.6) | 1 (3.7) | 16 (12.6) | ||
| Asian | 1 (2.8) | 0 | 0 | 0 | 1 (0.8) | ||
| Biracial | 2 (5.6) | 0 | 2 (6.3) | 1 (3.7) | 5 (3.9) | ||
| Completed high school | 23 (63.9) | 21 (65.6) | 19 (59.4) | 16 (59.3) | 79 (62.2) | 0.41 | 0.94 |
| Never married/living alone | 34 (94.4) | 29 (90.6) | 29 (90.6) | 23 (85.2) | 115 (90.6) | 1.55 | 0.67 |
| Unemployed | 15 (41.7) | 11 (34.4) | 10 (31.3) | 16 (59.3) | 52 (40.9) | 5.57 | 0.13 |
| Referred by criminal justice system | 35 (97.2) | 29 (90.6) | 29 (90.6) | 26 (96.3) | 119 (93.7) | 2.09 | 0.55 |
| Life-time alcohol use disordera | 6 (16.7) | 9 (28.1) | 9 (28.1) | 6 (22.2) | 30 (23.6) | 1.71 | 0.63 |
| Life-time major depressive disorder | 1 (2.8) | 2 (6.3) | 1 (3.1) | 2 (7.4) | 6 (4.7) | 1.08 | 0.78 |
| Life-time anxiety disorder | 5 (13.9) | 3 (9.4) | 5 (15.6) | 3 (11.1) | 16 (12.6) | 0.68 | 0.88 |
| Antisocial personality disorder | 10 (27.8) | 3 (9.4) | 14 (43.8) | 5 (18.5) | 32 (25.2) | 10.9 | 0.013 |
| Age (mean, SD) | 24.3 (4.3) | 25.4 (7.9) | 26.2 (5.4) | 27.6 (10.2) | 25.7 (7.1) | 1.23 | 0.30 |
| Days of marijuana use past 28 | 15.6 (9.8) | 17.6 (8.6) | 17.9 (9.6) | 14.1 (10.6) | 16.4 (9.7) | 1.03 | 0.38 |
| Days of cigarette use, past 28 | 18.7 (12.9) | 16.9 (13.2) | 16.9 (13.2) | 19.3 (12.9) | 17.9 (12.9) | 0.26 | 0.85 |
| Days alcohol use, past 28 | 2.3 (2.7) | 1.5 (2.4) | 2.7 (3.7) | 1.8 (4.0) | 2.1 (3.2) | 0.89 | 0.45 |
| Years of regular marijuana use | 9.5 (4.6) | 9.9 (7.4) | 10.6 (6.1) | 12.6 (10.7) | 10.5 (7.3) | 1.07 | 0.36 |
| ASI medical compositeb | 0.05 + 0.18 | 0.10 + 0.24 | 0.10 + 0.22 | 0.06 + 0.19 | 0.08 + 0.21 | 0.53 | 0.66 |
| ASI employment composite | 0.62 + 0.29 | 0.58 + 0.28 | 0.58 + 0.28 | 0.73 + 0.29 | 0.62 + 0.29 | 1.85 | 0.14 |
| ASI alcohol composite | 0.03 + 0.03 | 0.03 + 0.05 | 0.04 + 0.05 | 0.02 + 0.04 | 0.03 + 0.04 | 1.09 | 0.37 |
| ASI marijuana composite | 0.33 + 0.26 | 0.34 + 0.27 | 0.32 + 0.27 | 0.25 + 0.23 | 0.31 + 0.26 | 0.81 | 0.49 |
| ASI other drug composite | 0.00 + 0.01 | 0.01 + 0.03 | 0.01 + 0.02 | 0.01 + 0.02 | 0.01 + 0.02 | 0.51 | 0.68 |
| ASI legal composite | 0.13 + 0.16 | 0.16 + 0.16 | 0.14 + 0.16 | 0.09 + 0.12 | 0.13 + 0.15 | 1.12 | 0.35 |
| ASI family composite | 0.06 + 0.09 | 0.07 + 0.10 | 0.07 + 0.10 | 0.06 + 0.09 | 0.06 + 0.10 | 0.12 | 0.94 |
| ASI psychological composite | 0.05 + 0.10 | 0.05 + 0.09 | 0.07 + 0.12 | 0.04 + 0.08 | 0.05 + 0.10 | 0.61 | 0.61 |
| Life-time number of arrests | 4.6 (5.0) | 3.7 (3.4) | 6.1 (5.5) | 4.3 (4.6) | 4.7 (4.7) | 1.48 | 0.22 |
| Estimated IQ from Shipleyc | 90.0 (8.9) | 87.9 (14.6) | 92.3 (9.8) | 85.7 (13.6) | 89.1 (12.0) | 1.50 | 0.22 |
CBT: cognitive–behavioral therapy; CM: contingency management.
Indicates life-time DSM-IV psychiatric diagnosis from Structured Clinical Interview for DSM-IV (SCID) interviews.
Indicates Addiction Severity Index (ASI) composite scores, range 0–1, with higher scores indicating more severe problems.
Indicates age-corrected IQ estimate from Shipley Institute of Living scale. SD: standard deviation.
Overall, participants remained in the study an average of 61 days [standard deviation (SD) = 27.9]. There was a significant effect for the CBT + CMadher versus CBTalone contrast, indicating that those assigned to CBT + CMadher had better retention in the study (67.8 versus 53.0 days, contrast t = 2.2, P = 0.03; d.f. = 1123, d = 0.52). There were no differences in number of CBT sessions across the three conditions that involved CBT (mean = 5.9, SD = 3.8, F = 1.0, P = 0.36, d.f. = 2, 97, eta2 = 0.02) or by contrast. Although levels of CBT homework completion were low, there were significantly more homework assignments submitted by participants assigned to the condition where homework submission was reinforced (mean number completed: CBTalone = 1.25, CBT + CMadher = 3.1, CBT + CMabst = 1.4, omnibus F(97) = 7.8, P < 0.001, partial eta2 = 0.50, contrast 1: t = 3.6, P < 0.001). There were no significant differences in terms of total number of urine specimens submitted by treatment condition. Rates of exposure to reinforcement (percentage of participants earning at least one drawing) were significantly higher in the condition that provided drawings for attending sessions than in the two conditions where drawings were earned for cannabis-free urines (CBT + CMadher 94%, CMabst = 59%, CBT + CMabst = 34%, χ2(2) = 24.3, P < 0.001).
Cannabis use outcomes
Within-treatment cannabis use outcomes by treatment condition and contrast are summarized in Table 2. Contrasts 1 (CBT + CMadher versus CBTalone) and 3 (CMabst, CBT + CMabst, CBT + CMadher versus CBTalone) were not statistically significant for the stationary cannabis outcomes. There was a significant effect for contrast 2, opposite to the hypothesized direction: there was a higher proportion of positive urine specimens in the group assigned to CBT + CMabst versus those assigned to CMabst alone. Contrast 2 was also statistically significant for maximum consecutive negative urine specimens submitted, again favoring those assigned to CMabst alone versus CBT + CMabst. The longitudinal model, evaluating frequency of cannabis by week during the study indicated a main effect for time (F = 23.51, d.f. = 1, 948, P < 0.001) and an interaction of contrast 3 by time (t = 2.07, d.f. = 1152, P = 0.04, illustrated in Fig. 2). This suggests an overall reduction in marijuana use frequency for all participants, with a greater reduction for CBTalone compared with the other three conditions.
Table 2.
Within-treatment marijuana use outcomes by treatment condition and a priori contrasts.
| CBTalone | CBT + CMadher |
CBT + CM abst |
CMabst | Total | Contrast 1 CBT + CMadher versus CBTalone |
Contrast 2 CBT + CMabst versus CM abst |
Contrast 3 CBTalone versus CBT + CMadher, CBT + CMabst, CMabst |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | n = 36 | n = 32 | n = 32 | n = 27 | n = 127 | F | P | F | P | F | P | |
| Percentage of marijuana-positive urine specimens |
Mean | 73.1 | 75.6 | 75.5 | 57.1 | 70.9 | 0.38 | 0.74 | 2.25 | 0.02 | 0.67 | 0.55 |
| SD | 31.2 | 23.2 | 29.9 | 38.4 | 31.3 | |||||||
| Max. consecutive marijuana-negative urine specimens |
Mean | 2.14 | 1.59 | 1.41 | 3.3 | 2.06 | 0.73 | 0.47 | 2.33 | 0.02 | 0.08 | 0.94 |
| SD | 3.57 | 1.92 | 2.65 | 3.91 | 3.13 | |||||||
| Max. consecutive days abstinent (84 days max.) |
Mean | 35.8 | 33.9 | 28.8 | 33.8 | 33.2 | 1.5 | 0.79 | 0.65 | 0.52 | 0.62 | 0.54 |
| SD | 29.8 | 23.7 | 30.5 | 30.5 | 28.5 | |||||||
| Percentage cannabis-using days using by self-report |
Mean | 35.6 | 34.4 | 49.3 | 31.9 | 38.1 | 0.1 | 0.89 | 1.7 | 0.07 | 0.43 | 0.69 |
| SD | 36.2 | 31.1 | 37.2 | 38.0 | 35.9 | |||||||
CBT: cognitive–behavioral therapy; CM: contingency management; SD: standard deviation.
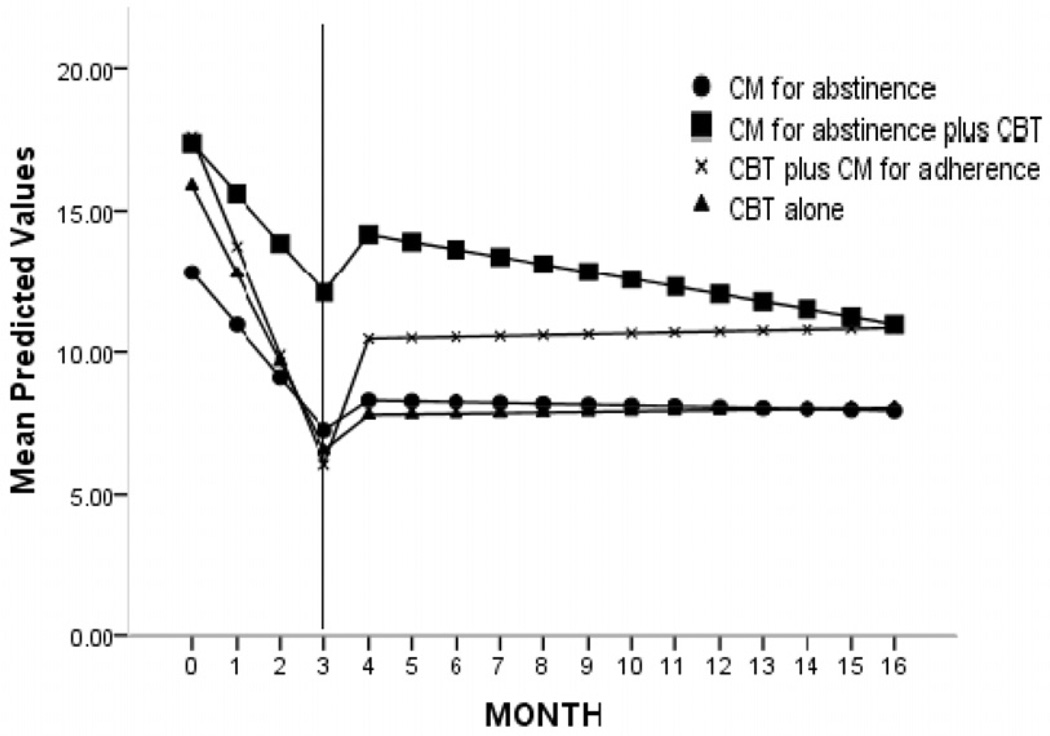
Figure 2.
Frequency of marijuana use by treatment condition (days of marijuana use by month), estimates from random regression models using piecewise model; within the study (months 0–3) versus 1-year follow-up (months 4–15). CBT: cognitive–behavioral therapy; CM: contingency management.
Given the unbalanced rate of antisocial personality disorder (ASPD) across conditions, exploratory analyses were conducted for the primary contrasts, with the diagnosis of ASPD included in the model as a covariate. Individuals diagnosed with ASPD had poorer outcomes than those who were not, as indicated by main effects of ASPD on retention (F = 4.5, P = 0.04, d.f. = 1119, partial eta2 = 0.04) and percentage of days of abstinence (F = 5.3, P = 0.02, d.f. = 1104, partial eta2 = 0.05). While samples sizes were insufficient for firm conclusions, there was some indication of improved outcomes for individuals with ASPD when assigned to any type of CM and poorer outcomes for those who received CBTalone.
Follow-up outcomes
Estimates of frequency of cannabis use by month for the full duration of the protocol (months 0–15) are presented in Fig. 2. These analyses represent a piecewise random regression model, which allows modeling of two discrete time-periods (study months 0–3 versus follow-up months 4–15), as the slopes during these two periods are expected to be different [54,55]. There was a main effect of slope, suggesting a significant reduction in cannabis use from randomization to end of treatment across condition (F = 65.5, d.f. = 1,1557, P = 0.00). There was also a main effect of period, suggesting that rate of change of cannabis use during the study was higher than during the follow-up period (F = 37.44, d.f. = 1,1566, P = 0.00). Finally, there was a significant three-way interaction indicating that the slopes of the treatment conditions differed by period (F = 15.4, d.f. = 4,1557, P = 0.00). As shown, CMabst, CBT + CMadher and CBTalone demonstrated the greatest reduction during the study. During follow-up, CMabst and CBTalone had the smallest slopes, suggesting that the reductions seen during the study were better maintained in these groups.
DISCUSSION
Results from this randomized clinical trial, evaluating the value of adding adaptations of CM for abstinence and CBT to enhance outcomes of each treatment, suggested that none of our hypotheses were supported in this trial. Neither of the planned enhancements of CBT or CM was associated with significant improvements in outcome. Rather, for those contrasts where there was a significant difference, adding CBT to CMabst was associated with poorer cannabis outcomes relative to CMabst alone. Overall, in terms of use throughout the 1-year follow-up, CBTalone and CMabst were associated with smaller rebounds and less frequent cannabis use relative to the enhanced conditions.
What might account for these unanticipated findings? One possibility is that the hypothesized enhancements were not implemented appropriately. However, available implementation checks and process analyses support the internal validity of the trial: independent analysis of session audiotapes indicated greater emphasis on CM concepts in the CBT + CMabst and CBT + CMadher conditions, as expected. Levels of CBT adherence and competence did not vary across the three conditions which included CBT. Similarly, reinforcement of session attendance and homework completion (CBT + CMadher) was associated with significantly more homework completion and better retention compared to CBTalone. However, the latter effect was very modest, suggesting limited uptake of coping skills training in this sample and possibly the need for higher levels of reward.
Regarding implementation of abstinence-based CM, the need for rapid, non-quantitative urinalyses methods and the long half-life of cannabis make it difficult to detect and reinforce changes in cannabis use quickly [6,56]. It is possible that larger, more powerful rewards may have produced better outcomes. However, given that CMabst and CBTalone tended to be more effective when delivered alone than when enhanced, this suggests that, at least in the case of adding CBT to CMabst, in this sample, reinforcement may have ‘backfired’ to some extent.
Another possibility is that the hypothesized enhancements were not well suited to this population. This sample, composed primarily of young adult male cannabis users, sought treatment largely because they were pressed to do so by the criminal justice system. It is conceivable that providing rewards for attendance, homework or abstinence produced some form of reactance in this sample (e.g. devaluing behavior because one is extrinsically, rather than intrinsically motivated) [57]. Less demanding approaches may be comparatively attractive, or effective, in samples where individuals do not seek treatment of their own volition.
The comparatively positive outcomes associated with the CMabst condition are notable, as relatively few trials have implemented CM without concurrent behavioral therapy of some type [39]. As in the Kadden et al. study [6], CM for abstinence was associated with better short-term cannabis outcomes. Thus, in this trial CM alone appeared safe, and largely sufficient, with this sample of young, adult, urban males at high risk of substance-related problems.
This study had several limitations: comparatively high rates of dropout, as seen here, are common among cannabis-using samples [1,3]; however, our efforts to interview dropouts, as well as the use of random regression models reduced, to some extent, issues associated with missing data. Secondly, as ASPD was associated with poorer outcome, and rates of ASPD were higher in the CBT + CMabst group, this may have depressed outcomes in disproportionately this condition. The trend towards better outcomes for those with ASPD when assigned to CM is consistent with previous findings [58], but the sample size was too small to resolve this issue directly.
Although the proposed enhancements did not improve outcomes as hypothesized, it is also notable that the level of reduction of cannabis use across treatment conditions, considered in terms of current conventions for ‘clinically significant’ outcomes (two or more consecutive weeks of documented abstinence in the last 2 weeks of treatment) [59,60], compare favorably with other recent randomized clinical trials with adult cannabis-dependent individuals [1,6,56] and support the efficacy of CBT and CM as implemented without the enhancements used here.
Acknowledgements
Support for this study was provided by National Institute on Drug Abuse grants P50-DA09241, R37-DA 015969, U10 DA13038 and T32-DA007238. We gratefully acknowledge the assistance of Karen Hunkele for data management and preparation, Joanne Corvino for training and quality assurance, and Sally Vitollo, Haley Ford and Monica Canning-Ball in carrying out the study. We express special thanks to Drs Sam Ball, Brian Kiluk and Steve Martino for their helpful comments on earlier drafts of the manuscript; and Bruce.
Footnotes
Declarations of interest
None.
References
- 1.Levin FR, Mariani JJ, Brooks DJ, Pavlicova M, Cheng W, Nunes EV. Dronabinol for the treatment of cannabis dependence: a randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 2011;116:142–150. doi: 10.1016/j.drugalcdep.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vandrey R, Haney M. Pharmacotherapy for cannabis dependence: how close are we? CNS Drugs. 2009;23:543–553. doi: 10.2165/00023210-200923070-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Budney AJ, Roffman R, Stephens RS, Walker D. Marijuana dependence and its treatment. Addict Sci Clin Pract. 2007;4:4–16. doi: 10.1151/ascp07414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denis C, Lavie E, Fatseas M, Auriacombe M. Psychotherapeutic interventions for cannabis abuse and/or dependence in outpatient settings. Cochrane Database Syst Rev. 2006;3 doi: 10.1002/14651858.CD005336.pub2. CD005336. [DOI] [PubMed] [Google Scholar]
- 5.Carroll KM, Easton CJ, Nich C, Hunkele KA, Neavins TM, Sinha R, et al. The use of contingency management and motivational/skills-building therapy to treat young adults with marijuana dependence. J Consult Clin Psychol. 2006;74:955–966. doi: 10.1037/0022-006X.74.5.955. PMCID: PMC2148500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kadden RM, Litt MD, Kabela-Cormier E, Petry NM. Abstinence rates following behavioral treatments for marijuana dependence. Addict Behav. 2007;32:1220–1236. doi: 10.1016/j.addbeh.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carroll KM. Combined treatments for substance dependence. In: Sammons MT, Schmidt NB, editors. Combined Treatments for Mental Disorders: Pharmacological and Psychotherapeutic Strategies for Intervention. Washington, DC: American Psychological Assosciation Press; 2001. pp. 215–238. [Google Scholar]
- 8.DeRubeis RJ, Crits-Christoph P. Empirically supported individual and group psychological treatments for adult mental disorders. J Consult Clin Psychol. 1998;66:37–52. doi: 10.1037//0022-006x.66.1.37. [DOI] [PubMed] [Google Scholar]
- 9.Carroll KM. Relapse prevention as a psychosocial treatment approach: a review of controlled clinical trials. Exp Clin Psychopharmacol. 1996;4:46–54. [Google Scholar]
- 10.Irvin JE, Bowers CA, Dunn ME, Wong MC. Efficacy of relapse prevention: a meta-analytic review. J Consult Clin Psychol. 1999;67:563–570. doi: 10.1037//0022-006x.67.4.563. [DOI] [PubMed] [Google Scholar]
- 11.National Institutes on Drug Abuse (NIDA) Principles of Drug Addiction Treatment: A Research-Based Guide. Bethesda, MD: NIDA; 2000. [Google Scholar]
- 12.Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW. A meta-analytic review of psychosocial interventions for substance use disorders. Am J Psychiatry. 2008;165:179–187. doi: 10.1176/appi.ajp.2007.06111851. [DOI] [PubMed] [Google Scholar]
- 13.Carroll KM, Rounsaville BJ, Nich C, Gordon LT, Wirtz PW, Gawin FH. One year follow-up of psychotherapy and pharmacotherapy for cocaine dependence: delayed emergence of psychotherapy effects. Arch Gen Psychiatry. 1994;51:989–997. doi: 10.1001/archpsyc.1994.03950120061010. [DOI] [PubMed] [Google Scholar]
- 14.Carroll KM, Nich C, Ball SA, McCance-Katz EF, Frankforter TF, Rounsaville BJ. One year follow-up of disulfiram and psychotherapy for cocaine-alcohol abusers: sustained effects of treatment. Addiction. 2000;95:1335–1349. doi: 10.1046/j.1360-0443.2000.95913355.x. [DOI] [PubMed] [Google Scholar]
- 15.O’Malley SS, Jaffe AJ, Chang G, Rode S, Schottenfeld R, Meyer RE, et al. Six month follow-up of naltrexone and psychotherapy for alcohol dependence. Arch Gen Psychiatry. 1996;53:217–224. doi: 10.1001/archpsyc.1996.01830030039007. [DOI] [PubMed] [Google Scholar]
- 16.Blagys MD, Hilsenroth MJ. Distinctive activities of cognitive behavioral therapy: a review of the comparative psychotherapy process literature. Clin Psychol Rev. 2002;22:671–706. doi: 10.1016/s0272-7358(01)00117-9. [DOI] [PubMed] [Google Scholar]
- 17.Burns DD, Spangler DL. Does psychotherapy homework lead to improvements in depression in cognitive–behavioral therapy or does improvement lead to increased homework compliance? J Consult Clin Psychol. 2000;68:46–56. doi: 10.1037//0022-006x.68.1.46. [DOI] [PubMed] [Google Scholar]
- 18.Hollon SD. Does cognitive therapy have an enduring effect? Cogn Ther Res. 2003;27:71–75. [Google Scholar]
- 19.Carroll KM, Nich C, Frankforter TL, Bisighini RM. Do patients change in the way we intend? Treatment-specific skill acquisition in cocaine-dependent patients using the Cocaine Risk Response Test. Psychol Assess. 1999;11:77–85. [Google Scholar]
- 20.Brown TG, Seraganian P, Tremblay J, Annis HM. Process and outcome changes with relapse prevention versus 12-Step aftercare programs for substance abusers. Addiction. 2002;97:677–689. doi: 10.1046/j.1360-0443.2002.00101.x. [DOI] [PubMed] [Google Scholar]
- 21.Griffith JD, Rowan-Szal GA, Roark RR, Simpson DD. Contingency management in outpatient methadone treatment: a meta-analysis. Drug Alcohol Depend. 2000;58:55–66. doi: 10.1016/s0376-8716(99)00068-x. [DOI] [PubMed] [Google Scholar]
- 22.Petry NM. A comprehensive guide to the application of contigency management procedures in clinical settings. Drug Alcohol Depend. 2000;58:9–25. doi: 10.1016/s0376-8716(99)00071-x. [DOI] [PubMed] [Google Scholar]
- 23.Higgins ST, Silverman K. Motivating Behavior Change Among Illicit-Drug Abusers. Washington, DC: American Psychological Association; 1999. [Google Scholar]
- 24.Petry NM, Petrakis I, Trevisan L, Wiredu L, Boutros N, Martin B, et al. Contingency management interventions: from research to practice. Am J Psychiatry. 2001;20:33–44. doi: 10.1176/appi.ajp.158.5.694. [DOI] [PubMed] [Google Scholar]
- 25.Carroll KM, Ball SA, Nich C, O’Connor PG, Eagan D, Frankforter TL, et al. Targeting behavioral therapies to enhance naltrexone treatment of opioid dependence: efficacy of contingency management and significant other involvement. Arch Gen Psychiatry. 2001;58:755–761. doi: 10.1001/archpsyc.58.8.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carroll KM, Sinha R, Nich C, Babuscio T, Rounsaville BJ. Contingency management to enhance naltrexone treatment of opioid dependence: a randomized clinical trial of reinforcement magnitude. Exp Clin Psychopharmacol. 2002;10:54–63. doi: 10.1037//1064-1297.10.1.54. [DOI] [PubMed] [Google Scholar]
- 27.Preston KL, Silverman K, Umbricht A, DeJesus A, Montoya ID, Schuster CR. Improvement in naltrexone treatment compliance with contingency management. Drug Alcohol Depend. 1999;54:127–135. doi: 10.1016/s0376-8716(98)00152-5. [DOI] [PubMed] [Google Scholar]
- 28.Crowley TJ. Research on contingency management treatment of drug dependence: clinical implications and future directions. In: Higgins ST, Silverman K, editors. Motivating Behavior Change Among Illicit Drug Abusers. Washington, DC: American Psychological Association; 1999. pp. 345–370. [Google Scholar]
- 29.Silverman K, Higgins ST, Brooner RK, Montoya ID, Cone EJ, Schuster CR, et al. Sustained cocaine abstinence in methadone maintenance patients through voucher-based reinforcement therapy. Arch Gen Psychiatry. 1996;53:409–415. doi: 10.1001/archpsyc.1996.01830050045007. [DOI] [PubMed] [Google Scholar]
- 30.Marlatt GA. Integrating contingency management with relapse prevention skills training: comment on Silverman et al. (2001) Exp Clin Psychopharmacol. 2001;9:33–34. doi: 10.1037/1064-1297.9.1.33. [DOI] [PubMed] [Google Scholar]
- 31.MTP Research Group. Brief treatments for cannabis dependence: findings from a randomized multisite trial. J Consult Clin Psychol. 2004;72:455–466. doi: 10.1037/0022-006X.72.3.455. [DOI] [PubMed] [Google Scholar]
- 32.Wei LJ, Lachin JM. Properties of the urn randomization in clinical trials. Control Clin Trials. 1988;9:345–364. doi: 10.1016/0197-2456(88)90048-7. [DOI] [PubMed] [Google Scholar]
- 33.Stout RL, Wirtz PW, Carbonari JP, DelBoca FK. Ensuring balanced distribution of prognostic factors in treatment outcome research. J Stud Alcohol Suppl. 1994;12:70–75. doi: 10.15288/jsas.1994.s12.70. [DOI] [PubMed] [Google Scholar]
- 34.Carroll KM, Nich C, Sifry R, Frankforter TL, Nuro KF, Ball SA, et al. A general system for evaluating therapist adherence and competence in psychotherapy research in the addictions. Drug Alcohol Depend. 2000;57:225–238. doi: 10.1016/s0376-8716(99)00049-6. [DOI] [PubMed] [Google Scholar]
- 35.Carroll KM, Connors GJ, Cooney NL, DiClemente CC, Donovan DM, Longabaugh RL, et al. Internal validity of Project MATCH treatments: discriminability and integrity. J Consult Clin Psychol. 1998;66:290–303. doi: 10.1037//0022-006x.66.2.290. [DOI] [PubMed] [Google Scholar]
- 36.Steinberg K, Roffman RA, Carroll KM, McRee B, Babor TF, Miller M, et al. Brief Counseling for Marijuana Dependence: A Manual for Treating Adults. Rockville, MD: Center for Substance Abuse Treatment, Substance Abuse and Mental Health Services Administration; 2005. [Google Scholar]
- 37.Petry NM, Martin B, Cooney JL, Kranzler HR. Give them prizes and they will come: contingency management treatment of alcohol dependence. J Consult Clin Psychol. 2000;68:250–257. doi: 10.1037//0022-006x.68.2.250. [DOI] [PubMed] [Google Scholar]
- 38.Petry NM, Alessi SM, Marx J, Austin M, Tardiff M. Vouchers versus prizes: contingency management of treatment of substance abusers in community settings. J Consult Clin Psychol. 2005;73:1005–1014. doi: 10.1037/0022-006X.73.6.1005. [DOI] [PubMed] [Google Scholar]
- 39.Higgins ST, Sigmon SC, Wong CJ, Heil SH, Badger GL, Donham R, et al. Community reinforcement therapy for cocaine-dependent outpatients. Arch Gen Psychiatry. 2003;60:1043–1052. doi: 10.1001/archpsyc.60.9.1043. [DOI] [PubMed] [Google Scholar]
- 40.Petry NM, Roll JM, Rounsaville BJ, Ball SA, Stitzer M, Peirce JM, et al. Serious adverse events in randomized psychosocial treatment studies: safety or arbitrary edicts? J Consult Clin Psychol. 2008;76:1076–1082. doi: 10.1037/a0013679. PMCID: PMC2756150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gibbons CJ, Nich C, Steinberg K, Roffman RA, Corvino J, Babor TF, et al. Treatment process, alliance and outcome in brief versus extended treatments for marijuana dependence. Addiction. 2010;105:1799–1808. doi: 10.1111/j.1360-0443.2010.03047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martino S, Ball SA, Nich C, Frankforter TL, Carroll KM. Community program therapist adherence and competence in motivational enhancement therapy. Drug Alcohol Depend. 2008;97:37–48. doi: 10.1016/j.drugalcdep.2008.01.020. PMCID: PMC2692429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martino S, Ball SA, Nich C, Frankforter TL, Carroll KM. Informal discussions in substance abuse treatment sessions. J Subst Abuse Treat. 2009;36:366–375. doi: 10.1016/j.jsat.2008.08.003. PMCID: PMC2705985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martino S, Ball SA, Nich C, Canning-Ball M, Rounsaville BJ, Carroll KM. Teaching community program clinicians motivational interviewing using expert and train-the-trainer strategies. Addiction. 2011;106:428–441. doi: 10.1111/j.1360-0443.2010.03135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86:420–429. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 46.Miller WR, DelBoca FK. Measurement of drinking behavior using the Form 90 family of instruments. J Stud Alcohol Suppl. 1994;12:112–117. doi: 10.15288/jsas.1994.s12.112. [DOI] [PubMed] [Google Scholar]
- 47.Sobell LC, Sobell MB. Timeline followback: a technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen J, editors. Measuring Alcohol Consumption: Psychosocial and Biological Methods. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- 48.Fals-Stewart W, O’Farrell TJ, Freitas TT, McFarlin SK, Rutigliano P. The timeline followback reports of psychoactive substance use by drug-abusing patients: psychometric properties. J Consult Clin Psychol. 2000;68:134–144. doi: 10.1037//0022-006x.68.1.134. [DOI] [PubMed] [Google Scholar]
- 49.Babor TF, Steinberg K, Anton RF, Del Boca FK. Talk is cheap: measuring drinking outcomes in clinical trials. J Stud Alcohol. 2000;61:55–63. doi: 10.15288/jsa.2000.61.55. [DOI] [PubMed] [Google Scholar]
- 50.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV, Patient Edition. Washington, DC: American Psychiatric Press; 1995. [Google Scholar]
- 51.Carroll KM, Ball SA, Nich C, Martino S, Frankforter TL, Crits-Christoph P, et al. Motivational interviewing to improve treatment engagement and outcome in individuals seeking treatment for substance abuse: a multisite effectiveness study. Drug Alcohol Depend. 2006;81:301–312. doi: 10.1016/j.drugalcdep.2005.08.002. PMCID: PMC2386852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Budney AJ, Higgins ST, Radonovich KJ, Novy PL. Adding voucher-based incentives to coping skills and motivational enhancement improves outcomes during treatment for marijuana dependence. J Consult Clin Psychol. 2000;68:1051–1061. doi: 10.1037//0022-006x.68.6.1051. [DOI] [PubMed] [Google Scholar]
- 53.Gibbons RD, Hedeker D, Elkin I, Waternaux C, Kraemer HC, Greenhouse JB, et al. Some conceptual and statistical issues in analyses of longitudinal psychiatric data. Arch Gen Psychiatry. 1993;50:739–750. doi: 10.1001/archpsyc.1993.01820210073009. [DOI] [PubMed] [Google Scholar]
- 54.Singer JD, Willett JB. Applying Longitudinal Data Analysis: Modeling Change and Event Occurrence. New York: Oxford University Press; 2003. [Google Scholar]
- 55.Ball SA, Martino S, Nich C, Frankforter TL, Van Horn D, Crits-Christoph P, et al. Site matters: multisite randomized trial of motivational enhancement therapy in community drug abuse clinics. J Consult Clin Psychol. 2007;75:556–567. doi: 10.1037/0022-006X.75.4.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Budney AJ, Moore BA, Rocha HL, Higgins ST. Clinical trial of abstinence-based vouchers and cognitive–behavioral therapy for cannabis dependence. J Consult Clin Psychol. 2006;74:307–316. doi: 10.1037/0022-006X.4.2.307. [DOI] [PubMed] [Google Scholar]
- 57.Deci FL, Koestner R, Ryan RM. A meta-analytic review of experiments examining the effects of extrinsic rewards on intrinsic motivation. Psychol Bull. 1999;128:627–668. doi: 10.1037/0033-2909.125.6.627. [DOI] [PubMed] [Google Scholar]
- 58.Messina N, Farabee D, Rawson RA. Treatment responsivity of cocaine-dependent patients with antisocial personality disorder to cognitive–behavioral and contingency management interventions. J Consult Clin Psychol. 2003;71:320–329. doi: 10.1037/0022-006x.71.2.320. [DOI] [PubMed] [Google Scholar]
- 59.Peters EN, Nich C, Carroll KM. Primary outcomes in two randomized controlled trials of treatments for cannabis use disorders. Drug Alcohol Depend. 2011;118:408–416. doi: 10.1016/j.drugalcdep.2011.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Donovan DM, Bigelow GE, Brigham GS, Carroll KM, Cohen AJ, Gardin JG, et al. Primary outcomes indices in illicit drug dependence treatment research: systematic approach to selection and measurement of drug use end-points in clinical trials. Addiction. 107:694–708. doi: 10.1111/j.1360-0443.2011.03473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]