SUMMARY
Internal nutrient sensors play important roles in feeding behavior, yet their molecular structure and mechanism of action are poorly understood. Using Ca2+ imaging and behavioral assays, we show that the Gustatory Receptor 43a functions as a narrowly tuned fructose receptor in taste neurons. Remarkably, GR43a also functions as a fructose receptor in the brain. Interestingly, hemolymph fructose levels are tightly linked to feeding status: after nutritious carbohydrate consumption, fructose levels rise several fold and reach a concentration sufficient to activate GR43a in the brain. By using different feeding paradigms and artificial activation of Gr43a-expressing brain neurons, we show that GR43a is both necessary and sufficient to sense hemolymph fructose and promote feeding in hungry flies, but suppress feeding in satiated flies. Thus, our studies indicate that the Gr43a-expressing brain neurons function as a nutrient sensor for hemolymph fructose and assign opposing valence to feeding experiences in a satiation-dependent manner.
INTRODUCTION
The taste sensory system plays a central role in identifying and evaluating potential foods by discriminating between nutritious chemicals that promote feeding, and structurally diverse, harmful or even toxic compounds, that inhibit feeding. Despite the distinct evolutionary origin of taste receptors of mammals and invertebrates, the cellular organization underlying taste discrimination is largely conserved (Scott, 2005). In Drosophila, gustatory receptor neurons (GRNs) express distinct sets of gustatory receptors (GRs), providing the basis for discrimination between sweet and bitter taste, respectively (Dahanukar et al., 2007; Dunipace et al., 2001; Jiao et al., 2007; Moon et al., 2006; Scott et al., 2001; Slone et al., 2007; Thorne et al., 2004; Wang et al., 2004). Specifically, the sweet taste of sugars is thought to be exclusively mediated by members of a small conserved subfamily of eight putative sugar Gr (psGr) genes (Gr5a, Gr61a and Gr64a-f), which are partially co-expressed in a single GRN of each taste sensillum, the “sweet” neuron (Dahanukar et al., 2007; Jiao et al., 2007; Slone et al., 2007). Conversely, the bitter taste of alkaloids, terpenoids and phenols is mediated by receptors encoded by most other Gr genes, including Gr66a, Gr33a, and Gr93a, which are partially co-expressed in a second GRN of each sensillum, the “bitter/high salt” neuron (Lee et al., 2009; Moon et al., 2006; Moon et al., 2009; Weiss et al., 2011). Most sensillae have two additional GRNs not associated with any characterized Gr gene and are thought to detect low salt solutions and water, respectively (Cameron et al., 2011; Dethier, 1976; Liu et al., 2003).
In addition to evaluating external chemicals by the taste sensory system, cells located in internal organs, including the gut, liver/fat body and the brain, express receptors that detect nutrients or their metabolically processed derivates to regulate energy homeostasis and feeding behaviors. Interestingly, internal nutrient sensing in the gut of rodents is in part mediated by taste receptors (Dyer et al., 2005; Jang et al., 2007; Janssen et al., 2011; Margolskee et al., 2007; Rozengurt, 2006). Moreover, some bitter taste receptors were shown to be expressed in the mammalian brain (Singh et al., 2011) and glucose sensing neurons were identified in the hypothalamus (Karnani and Burdakov, 2010). In Drosophila, evidence of an internal nutrient sensor was recently suggested by work of several laboratories. Two groups showed that flies are able to evaluate tasteless carbohydrates based solely on their nutritional content (Burke and Waddell, 2011; Fujita and Tanimura, 2011). Suh and co-workers reported that hungry flies with severely impaired sugar-sensing ability can still discriminate sweet tasting sugars based on their nutritious content (Dus et al., 2011). However, the molecular identity of the proposed nutrient sensor, the anatomical structure in which it resides, and its ligand, are not known.
Here, we report that Gr43a, one of the most conserved insect gustatory receptor genes, is expressed in the brain. Using a Ca2+ imaging assay, we find that GR43a is a narrowly tuned fructose receptor. Although circulating fructose is approximately 100 times less abundant than the main hemolymph sugars glucose/trehalose, it rises to levels high enough after a sugar meal to activate the Gr43a-expressing brain neurons. Feeding experiments reveal that these neurons promote feeding in hungry flies, but suppress feeding in satiated flies. Finally, we show that artificial activation of Gr43a expressing brain neurons assign positive valence in hungry flies, but negative valence in satiated flies. Thus, our work establishes a precedent of a new nutrient sensing system that regulates food consumption in a satiation-dependent manner.
RESULTS
Gr43a expression is not restricted to the taste sensory system
Only a few of the 68 Gr genes appear to have orthologs in most insect species (Robertson and Wanner, 2006; Wanner and Robertson, 2008). These genes are likely to have conserved and important functions in chemical sensing. We generated a GAL4 knock-in allele in one of these Gr genes, Gr43a (Gr43aGAL4; Supplemental Figure 1), and investigated its expression and function.
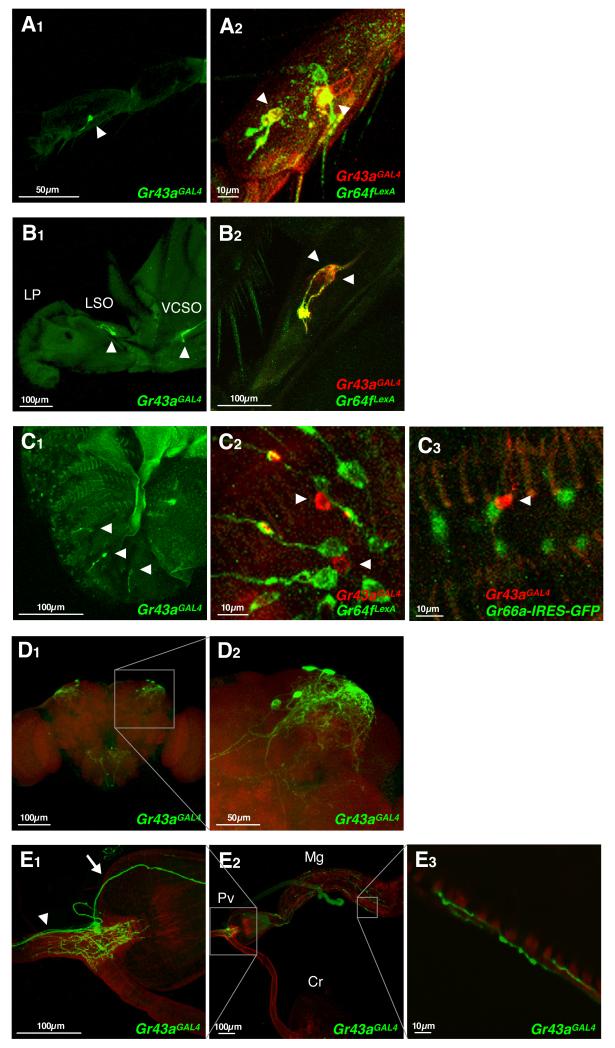
We first analyzed expression of Gr43aGAL4 in taste neurons using a UAS-mCD8-GFP reporter. Live GFP imaging and immunohistochemistry demonstrate that GFP is expressed in all major taste organs, including GRNs located in taste sensillae of the legs (tarsi), the pharynx and the labial palps (Figure 1A to 1C). To determine the neuron type expressing Gr43a, we combined cell markers for sweet and bitter/high salt neurons with the Gr43aGAL4 allele. In the 5th tarsal segment of the foreleg, Gr43aGAL4 is co-expressed with Gr64f: two of these neurons reside in sensillae located in the middle of the segment and express high levels of Gr43a, while weaker expression was occasionally observed in two tip neurons (Figure 1A2). However, numerous Gr64f expressing neurons do not express Gr43aGAL4 (Slone et al, in preparation). Gr43aGAL4 is also expressed in sweet neurons in the labral sense organs (LSO) of the pharynx (Figure 1B1). In the labial palps, Gr43aGAL4 is not co-expressed with either Gr64f or Gr66a, and expression is much weaker when compared to other taste organs (Figure 1B1) or other Gr genes expressed in the labial palps (data not shown). Taken together, these experiments suggest that Gr43a is expressed in distinct types of sensory neurons in the different taste organs: in tarsi and the LSO, it is expressed in a subset of sweet neurons, while in the labial palp, it is expressed in a few water and/or low salt neurons.
Figure 1. Gr43aGAL4 is expressed in chemosensory organs, the brain and the proventriculus.
(A-C) Expression of Gr43a in chemosensory organs. Gr43aGAL4 drives strong UAS-mCD8GFP expression in neurons located in the fifth tarsal segment of the foreleg (A1, live GFP), the LSO and the VCSO (B1; live GFP), but only weak expression in the LPs (C1; immunostaining). Co-expression analysis in sweet neurons was performed in flies containing Gr43aGAL4 and Gr64fLexA, driving expression of UAS-mCD8RFP (detected with anti-CD8 antibody) and lexAop-rCD2GFP (detected with anti-GFP antibody), respectively. Co-expression analysis in bitter neurons was performed in flies containing Gr43aGAL4 driving expression of UAS-mCD8RFP (detected with anti-CD8 antibody) and Gr66a-gfp (detected with anti-GFP antibody). LP, labial palp; LSO, labral sensory organ; VCSO, ventral cibarial sense organ. Arrowheads indicate Gr43aGAL4 neurons.
(D)Gr43aGAL4 is expressed in 2-4 neurons/hemisphere in the posterior superior lateral protocerebrum.
(E)Gr43aGAL4 is expressed in approximately four neurons in the proventricular ganglion. Gr43aGAL4 neurons innervate the lumen of the foregut, but not the crop duct (E2, left inset; E1). Some neurons send projections to the mid gut (E2, right inset; E1, arrow; E3), others to the SOG (E1, arrowhead). Pv, proventriculus; Cr, crop; Mg, mid gut.
We next examined Gr43aGAL4 expression in non-taste structures by performing anti-GFP antibody staining on whole mount preparations. This analysis showed that Gr43aGAL4 is expressed in two to four neurons of the posterior superior lateral protocerebrum in each brain hemisphere, in about four neurons associated with the proventriculus, and about four neurons located in the uterus (Figure 1D and 1E, and data not shown). Interestingly, the proventricular neurons fall into two distinct groups: two send axons into the brain and terminate in the subesophageal ganglion (SOG), while the other two extend axons posteriorly into the musculature of the mid gut (Figure 1E and Supplemental Figure 2A and 2B). All neurons extend dendritic processes in the foregut lumen (Supplemental Figure 2C).
GR43a is a fructose receptor
Expression of Gr43a in tarsal sweet neurons led us to investigate whether Gr43a encodes a novel sugar receptor. To do so, we developed a Ca2+ imaging preparation of the GRNs in the foreleg (Figure 2). Intracellular Ca2+ changes were recorded from the Gr43aGAL4 neurons located in the middle of the last tarsal segment using the calcium sensor GCaMP3.0 (Tian et al., 2009). When tarsal neurons were stimulated with sugar solutions, dose-dependent increases in intracellular Ca2+ was observed (Figure 2A to 2C; Supplemental Movie 1). The magnitude of the response was dependent on the sugar, with maltose and sucrose eliciting slightly higher responses than fructose and glucose, followed by arabinose and trehalose (Figure 2C). Solutions containing bitter-tasting compounds and salts failed to induce any Ca2+ fluxes, albeit a weak response was observed occasionally with quinine (Figure 2C). Likewise, no Ca2+ response was observed with either citric acid (ph 2.5) or NaOH (ph 12). Taken together, our observations show that Gr43a is expressed in broadly tuned sweet neurons, which is consistent with the observation that these neurons co-express the sugar-receptor gene Gr64f.
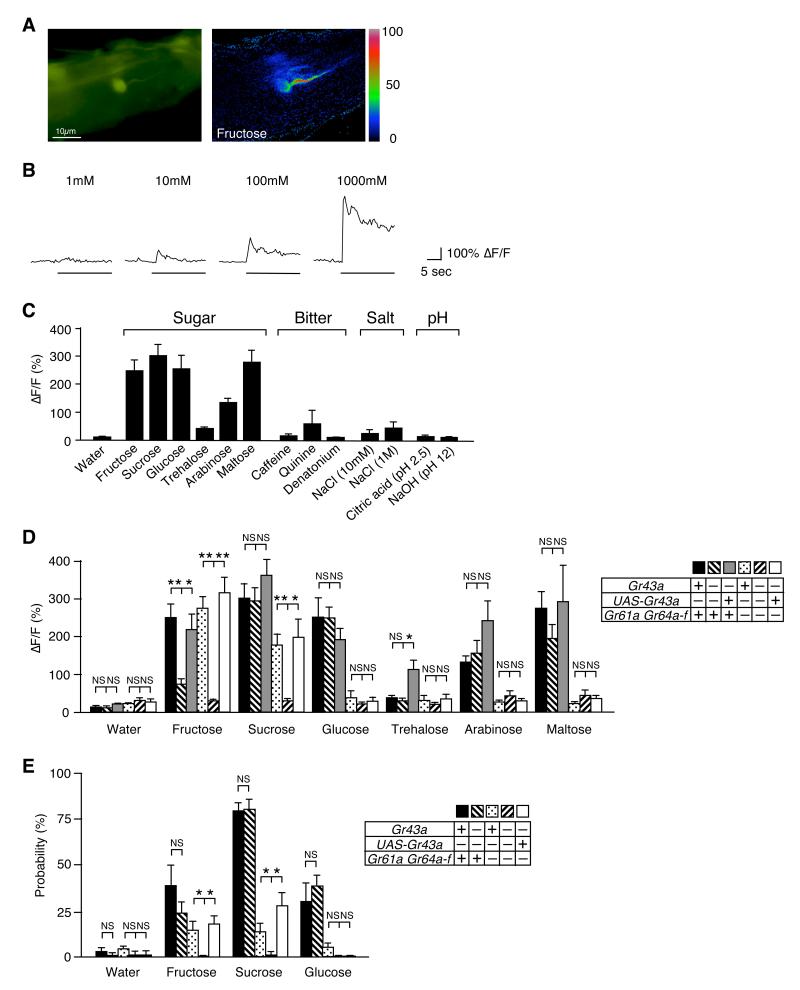
Figure 2. GR43a is a fructose receptor.
(A) Tarsal neuron expressing G-CaMP3.0 under control of Gr43aGAL4:ΔF pseudocolor fluorescence image was taken 1.5 seconds after application of 100mM fructose (right).
(B) G-CaMP3.0 (ΔF/F) fluorescence is dose-dependent (fructose was used as ligand).
(C)Gr43aGAL4 neurons responded to sugars, but not to bitter compounds, acid and base. Max ΔF/F within 30 seconds of applications is shown. Sugars and caffeine were at 100mM, quinine and denatonium at 10mM concentration. Error bars represent standard error. 3≤n≤8.
(D) Ca2+ response of Gr43aGAL4 neurons with/without Gr43a, Gr61a and Gr64a-f to various sugars. From left to right, genotypes are Gr43aGAL4/+, Gr43aGAL4/Gr43aGAL4, Gr43aGAL4/Gr43aGAL4;UAS-Gr43a, Gr43aGAL4/+;ΔGr61a ΔGr64a-f/ΔGr61a ΔGr64a-f, Gr43aGAL4/Gr43aGAL4;ΔGr61a ΔGr64a-f/ΔGr61a ΔGr64a-f, Gr43aGAL4/Gr43aGAL4;UAS-Gr43a/ΔGr61a ΔGr64a-f/ΔGr61a ΔGr64a-f. All sugars are 100mM. NS, not significant; *p < 0.05; **p < 0.0001; ANOVA. Error bars represent standard error. 8≤n≤9.
(E)Gr43a is sufficient to induce PER response to fructose. All sugars are 100mM. NS, not significant; *p < 0.05; ANOVA. Error bars represent standard error. 9≤n≤10 experiments (9-21 flies per experiment).
To correlate the sugar-induced responses with Gr43a and psGr genes, we performed Ca2+ imaging experiments in various mutant backgrounds (Figure 2D). Taste neurons of homozygous Gr43aGAL4 mutant flies showed significantly reduced Ca2+ response to fructose, whereas responses to all other sugars were unaffected. This phenotype was rescued by a UAS-Gr43a transgene (Figure 2D). In contrast, the Ca2+ response to fructose remained high in flies lacking the function of all eight psGr genes (ΔGr61a ΔGr64; Gr5a is not expressed in these neurons; Supplemental Figure 3). When homozygous Gr43aGAL4; ΔGr61a ΔGr64 mutant flies were assayed, response to all sugars was completely abolished, and adding back the UAS-Gr43a transgene rescued the response only to fructose and sucrose (a disaccharide of fructose and glucose). These observations indicate that Gr43a encodes a narrowly tuned fructose receptor, while the psGr genes encode receptors for glucose, trehalose, arabinose and maltose, as well as a low affinity receptor for fructose.
Previous studies have shown that ΔGr64 mutant flies completely lack a behavioral response to most sugars, with the exception of fructose and sucrose (Slone et al., 2007). To investigate whether Gr43a is necessary for the behavioral response to sweet tasting chemicals, we performed proboscis extension reflex (PER) assays by stimulating tarsal taste neurons with different sugars in wild type and various mutant flies (Figure 2E). Absence of Gr43a alone did slightly, albeit not significantly, reduce PER responses only to fructose, while ΔGr61a ΔGr64 double mutant flies showed a significantly reduced response to fructose and sucrose and failed to respond to glucose. However, when homozygous Gr43aGAL4; ΔGr61a ΔGr64 mutant flies were tested, PER responses to fructose and sucrose were lost completely, a phenotype that was rescued with the UAS-Gr43a transgene. These behavioral experiments and the Ca2+ imaging studies indicate that Gr43a encodes a fructose receptor.
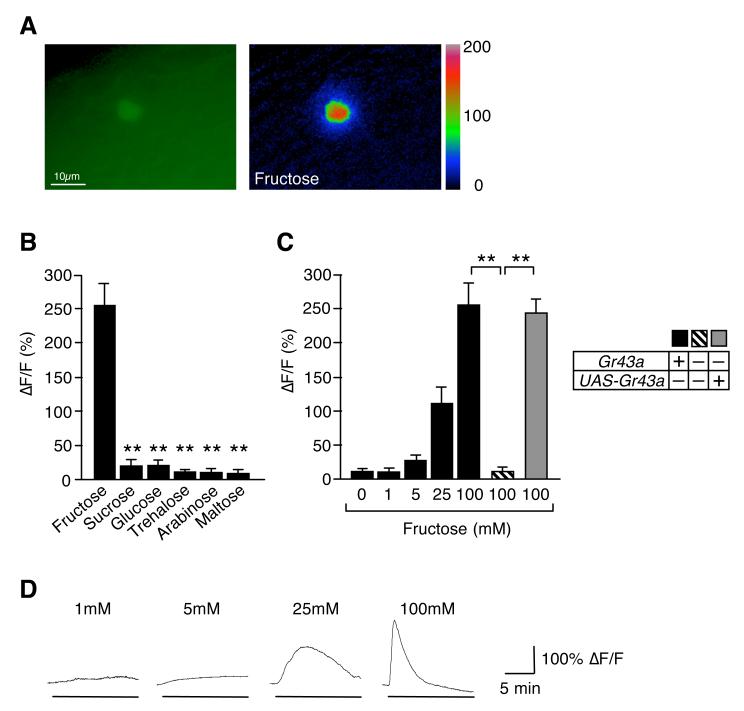
We next investigated the cellular function of the Gr43a gene in central neurons by performing Ca2+ imaging experiments using an ex-vivo brain preparation (Figure 3). Strikingly, fructose, but none of the other sugars, elicited a strong response at 100 and 25 mM (Figure 3A and 3B). A modest but significant response was also observed with 5 mM, but not 1 mM fructose (Figure 3C and 3D). Importantly, responses were lost in Gr43aGAL4 mutants, and completely restored in the presence of the UAS-Gr43a transgene (Figure 3C). These experiments show that GR43a functions also as a fructose receptor in the brain. Because the CNS has been suggested to harbor an internal nutrient sensor (Burke and Waddell, 2011; Dus et al., 2011; Fujita and Tanimura, 2011), we focused our subsequent analysis on the Gr43aGAL4 brain neurons.
Figure 3. GR43a functions as a fructose sensor in the brain.
(A) Brain neuron expressing G-CaMP3.0 under control of Gr43aGAL4: ΔF pseudocolor fluorescence image was taken 24 seconds after application of 100mM fructose (right).
(B)Gr43aGAL4 neurons specifically respond to fructose. Max ΔF/F within 15 minutes of application is shown. All sugars are 100 mM. Flies contained two genomic copies of Gr43a. **p < 0.0001; ANOVA. Error bars represent standard error. 6≤n≤7.
(C) Response of Gr43aGAL4 neurons to fructose is dose- and Gr43a dependent. **p < 0.0001; ANOVA. Error bars represent standard error. 8≤n≤9.
(D) Time-course of G-CaMP3.0 fluorescence changes in Gr43aGAL4 neurons stimulated with different concentrations of fructose.
Fructose is a low abundant hemolymph sugar that reflects carbohydrate consumption
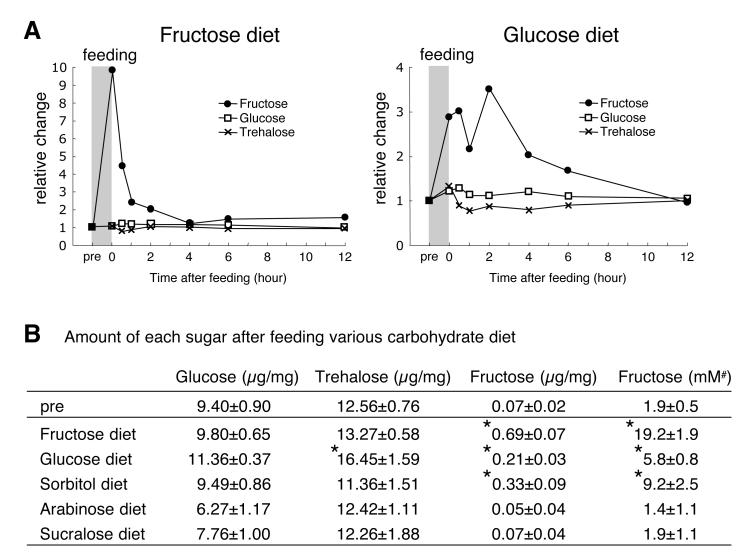
Trehalose and glucose are the main Drosophila hemolymph (insect blood) sugars (Kohyama-Koganeya et al., 2008; Lee and Park, 2004; Thompson, 2003)(see below). However, if GR43a plays a role in nutrient sensing, fructose should also be present in hemolymph. We therefore determined carbohydrate content in heads of flies kept under various feeding conditions (Figure 4). Consistent with previous findings, glucose and trehalose were the most abundant carbohydrates (9.4 +/-0.9 and 12.6 +/-0.7 g/mg, respectively), but an appreciable amount of fructose was also observed (0.07 g/mg or ~1.9 mM)(Figure 4B). When flies were provided with a sugar meal, the levels of glucose and trehalose remained relatively stable, but fructose levels sharply increased between three and ten fold (5.8 +/-0.8 mM to 19.2 +/-1.9 mM), which is sufficient to activate brain neurons in Ca2+ imaging experiments (Figures 3 and 4). The increase of circulating fructose after glucose feeding is most likely mediated by the polyol pathway, in which glucose is converted into fructose via sorbitol (Harvey, 2011). This idea was further supported by measurements of circulating fructose after feeding of sorbitol and non-nutritious sugars (Figure 4B). Sorbitol feeding led to a 4.5 fold fructose increase, whereas non-nutritious arabinose and sucralose failed to affect fructose levels (Figure 4B). We note that sucrose, which is efficiently broken down into fructose and glucose, and fructose itself, are the main sugars in most fruits. Hence, under natural feeding conditions, elevation of hemolymph fructose levels are likely to be similar to those observed with fructose solution. Taken together, these findings suggest that fructose serves as an indicator for the consumption of nutritious sugars.
Figure 4. Metabolic dynamics of circulating sugars.
Flies were starved for 24 hours (pre), followed by 40 minutes of feeding. Measurements were performed at indicated times after feeding (see Experimental Procedures).
(A) Relative change of internal glucose, trehalose and fructose over time.
(B) Amount of glucose, trehalose and fructose (per mg of head tissue) was measured immediately after feeding. *p < 0.05; ANOVA. 5≤n≤12. Last column (#) shows concentration of fructose (converted to mM) using the conservative estimate that 1/5 of the insect mass constitutes hemolymph (Chapman, 1998).
GR43a functions as an internal nutrient sensor in the brain
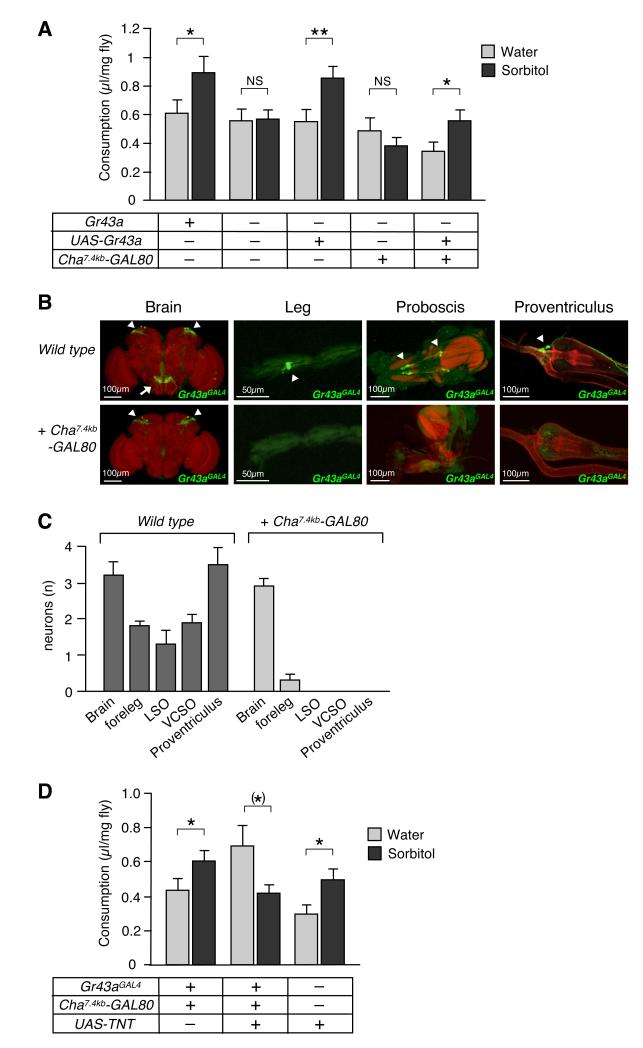
If GR43a functions as an internal nutrient sensor, lack of Gr43a might affect the fly’s ability to evaluate nutritious content of carbohydrates. To test this, we employed a modified capillary feeding assay (CAFE)(Ja et al., 2007), giving flies the choice between water and 100 mM sorbitol (Figure 5A). Sorbitol is a nutritious sugar alcohol, which is not detected by the taste sensory organs (Supplemental Figure 4) (Burke and Waddell, 2011; Fujita and Tanimura, 2011), eliminating confounding taste preference in the CAFE assay. Gr43a+ control flies consumed significantly more sorbitol than water, while Gr43aGAL4 mutants showed no preference, a phenotype that was rescued by a UAS-Gr43a transgene (Figure 5A). Thus, GR43a is essential to evaluate nutritional content of sorbitol.
Figure 5. GR43a functions as an internal nutrient sensor.
(A) GR43a evaluates nutritional content of carbohydrates. Single flies were subjected to the CAFÉ assay by presenting them with two capillaries containing water and 100mM sorbitol, respectively, for 24 hours. NS, not significant; *p < 0.05; **p < 0.01; ANOVA. Error bars represent standard error. 50≤n≤74.
(B)Cha7.4kb-GAL80 restricts Gr43aGAL4 expression to the brain neurons. GFP expression (arrowhead) is missing in all sensory neurons, but remains robust in the brain neurons. Likewise, all projections of sensory neurons to the SOG (arrow) disappeared.
(C) Average number of Gr43aGAL4 neurons in different tissues. 3≤n≤11 (without Cha7.4kb-GAL80), 6≤n≤15 (with Cha7.4kb-GAL80). Note that a few legs of flies with Cha7.4kb-GAL80 show very weak GFP expressions.
(D)Gr43aGAL4 brain neurons are necessary to evaluate nutritional content of carbohydrates. Flies with silenced Gr43aGAL4 brain neurons (Gr43aGAL4/UAS-TNT; Cha7.4kb-GAL80) lack sorbitol preference, in contrast to control flies. *p < 0.05; ANOVA. Error bars represent standard error. 64≤n≤83.
Because sorbitol is likely converted into fructose after absorption (Harvey, 2011)(Figure 4B), the feeding experiments described above suggest that the brain neurons are responsible for sensing circulating fructose. To eliminate a potential role for other Gr43aGal4 neurons in fructose sensing, we took advantage of a Cha7.4kb-GAL80 suppressor line (Sakai et al., 2009). GAL80 binds to and suppresses GAL4 transcriptional activity when co-expressed in the same cells (Suster et al., 2004). Cha7.4kb-GAL80 is expressed in all Gr43aGAL4 neurons except those in the brain, and hence suppresses GAL4 activity in these organs (Figure 5B and 5C). Indeed, flies with Gr43a expression restricted to the brain (Gr43aGAL4/Gr43aGal4;UAS-Gr43a/Cha7.4kb-GAL80) were able to discriminate sorbitol from water as well as Gr43a+ control flies (Figure 5A), indicating that Gr43a expression in the brain neurons is sufficient to evaluate nutritious carbohydrates.
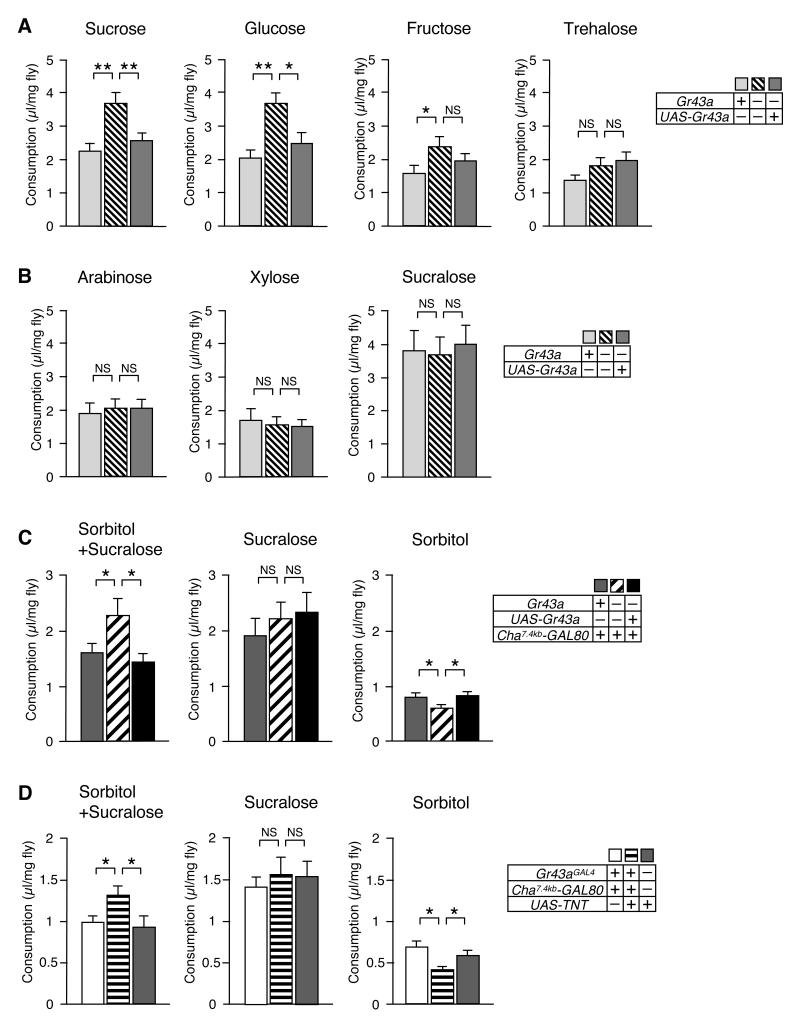
Because sorbitol has no sweet taste, flies used in the feeding experiments described above ingest only small amounts (<1 μl/mg fly/day) and become increasingly hungry (they eventually die of starvation; data not shown). Thus, these experiments established a role for GR43a under non-satiating conditions. To test whether GR43a may have a function under satiating conditions, we performed CAFE assays with single, sweet tasting sugars (Figure 6A). Gr43a+ control flies consumed these sugars based largely, but not entirely, on their sweetness as evaluated by PER. Sucrose and glucose were consumed at 2 μl/mg fly/day or more, while fructose and trehalose were consumed at moderate levels (~1.6 and ~1.4 μl/mg fly/day, respectively). Interestingly, Gr43aGAL4 mutant flies consumed 60 to 80% more of the highly desirable sugars, while fructose was only slightly over consumed, and no difference could be observed for trehalose intake (Figure 6A). Importantly, the overconsumption phenotype could be rescued by a UAS-Gr43a transgene. Based on these findings, we hypothesized that Gr43aGAL brain neurons suppress feeding of carbohydrates in these flies, once satiety is reached (> ~1.6 μl/mg fly/day). If this hypothesis is correct, we would predict that consumption of very sweet, but non-nutritious sugars is not suppressed by GR43a, because such sugars cannot be converted into fructose. Indeed, Gr43aGAL4 mutant and Gr43a+ flies consumed equal amounts of the non-nutritious sugars arabinose, xylose and sucralose (Figure 6B).
Figure 6. Gr43a function in brain neurons is sufficient to suppress feeding in satiated flies and promote feeding in hungry flies.
Single flies were subjected to the CAFÉ assay for 24 hours by presenting them a single capillary containing the indicated solution.
(A)Gr43a suppresses nutritious sugar consumption under satiated conditions. NS, not significant; *p < 0.05; **p < 0.01; ANOVA. Error bars represent standard error. 21≤n≤34. All sugars were used at 100 mM concentration, except sucrose, which was used at 50 mM, to obtain equal nutritional value for mono- and dissacharides.
(B) Non-nutritious sugars arabinose (100mM), xylose (100mM) and sucralose (50mM) were consumed in equal amounts by control and Gr43aGAL4 mutant flies. NS, not significant; ANOVA. 24≤n≤27.
(C)Gr43aGAL4 brain neurons are sufficient to suppress and promote feeding in satiated and hungry flies, respectively. Cha7.4kb-GAL80 restricts expression of Gr43aGAL4 to the brain (see Figure 5B and 5C). 50mM sucralose was added to “sweeten” sorbitol (100mM), enhance feeding and achieve satiation. For hungry state, sorbitol (100mM) alone was used. NS, not significant; *p < 0.05; ANOVA. 35≤n≤84.
(D)Gr43aGAL4 brain neurons are necessary to suppress and promote feeding in satiated and hungry flies, respectively. Flies with silenced Gr43aGAL4 brain neurons (Gr43aGAL4/UAS-TNT; Cha7.4kb-GAL80) consume more nutritious food (sucralose + sorbitol) under satiating conditions, but less under non-satiating conditions (sorbitol alone). NS, not significant; *p < 0.05; ANOVA. 41≤n≤88.
To test whether feeding suppression is also mediated by Gr43aGAL4 brain neurons, we examined consumption of nutritious sugar in Gr43aGAL4/Gr43aGAL4;UAS-Gr43a/Cha7.4kb-GAL80 flies and corresponding controls. The Cha7.4kb-GAL80 strain (as well as the UAS-TNT strain; see below) exhibited reduced nutritional demand and/or reduced attraction to most sugars and ingested them in lower quantities (data not shown). Thus, we used sucralose, complemented with nutritious sorbitol, to achieve satiation in these experiments (Figure 6C). As expected, Gr43aGal4 mutant flies ingested significantly more of this solution than control flies. Importantly, flies with Gr43aGAL4 expression restricted to the brain showed complete rescue of the overeating phenotype, indicating that feeding suppression in satiated flies is mediated by the Gr43aGAL4 brain neurons (Figure 6C, left panel). Non-nutritious sucralose alone was ingested equally by all strains, as expected (Figure 6C, middle panel). Interestingly, when flies become increasingly hungry (by providing them with tasteless sorbitol alone), Gr43aGAL4 mutants consumed significantly less than control flies or flies in which Gr43aGAL4 expression was restored in the brain (Figure 6C, right panel). These observations not only confirm that GR43a functions as a sensor for nutritious carbohydrates in the brain (Figure 5A), but they suggest that these neurons affect feeding behavior in a satiation-dependent manner; in hungry flies, GR43a promotes food intake, while in satiated flies, it suppresses feeding.
The data presented thus far indicate that GR43a expression in the brain is sufficient to regulate consumption of nutritious carbohydrates. However other Gr43aGAL4 expressing neurons (i.e. proventricular neurons, or LSO/VSCO) may provide the same function. To determine whether the brain neurons are the only nutrient sensor, we carried out feeding experiments with flies in which only the brain neurons, but none of the other Gr43aGAL4 neurons, were silenced by tetanus toxin (Sweeney et al., 1995). We first assayed the ability of hungry flies for feeding preference of sorbitol vs water (Figure 5D). Indeed, flies with silenced Gr43aGAL4 neurons in the brain, but not control flies, lacked sorbitol preference. We note that in the absence of GR43a in the brain, some flies consume equal amounts of sorbitol and water (Gr43aGAL4/Gr43aGAL4), while others have a preference for water (Gr43aGAL4/Gr43aGAL4;Cha7.4kb-GAL80 and Gr43aGAL4/UAS-TNT;Cha7.4kb-GAL80). Distinct water preference of these flies may have a neural basis in water sensing neurons (Cameron et al., 2011; Dethier, 1976; Liu et al., 2003), which is likely associated with the Cha7.4kb-GAL80 strain. Next, we measured consumption under satiating conditions using the CAFE assay with sucralose/sorbitol as food; Indeed, flies with silenced Gr43aGAL4 brain neurons consumed ~30% more of this diet than controls (Figure 6D). Importantly, nutritious content was critical for this overconsumption, as sucralose alone was ingested equally. However, when sorbitol alone was provided (which is ingested in very small amounts and leads to increased hunger; see above), flies with silenced Gr43aGAL4 brain neurons consumed significantly less than control flies. Taken together, these observations indicate that the Gr43aGAL4 brain neurons are necessary and sufficient to evaluate nutritious content of sorbitol and to promote or suppress feeding in hungry and satiated flies, respectively.
Activation of Gr43aGAL4 brain neurons evokes opposite motivational valence that is dependent on satiation status
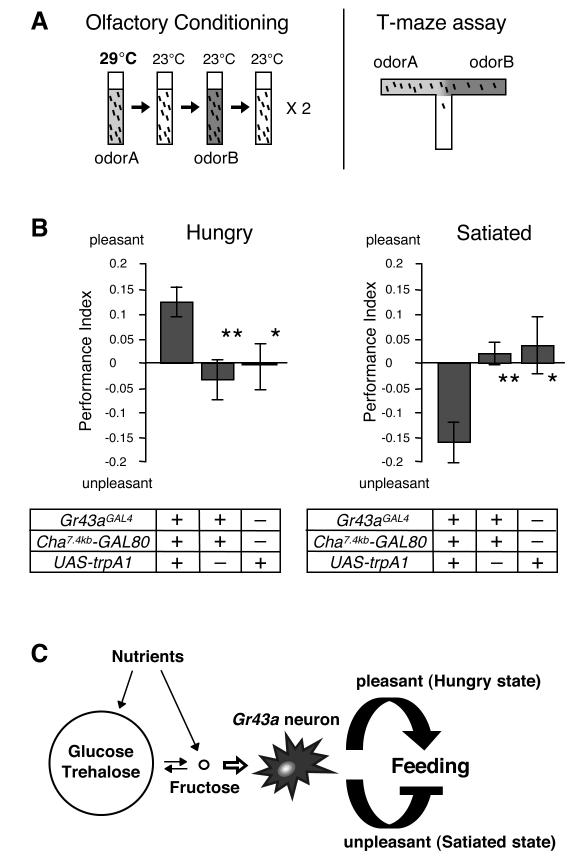
The behavioral experiments described thus far imply that a single group of neurons might assign positive or negative valence to a feeding experience, based on the satiation state. Specifically, activation of Gr43aGAL4 neurons in hungry flies may be perceived as pleasant and reinforce feeding behavior, while in satiated flies, it may be perceived as unpleasant, leading to feeding termination. To test this idea, we subjected flies in which activation of Gr43aGAL4 brain neurons is externally controlled through TRPA1 activation to a classical odor-conditioning paradigm (Quinn et al., 1974)(Figure 7A and 7B). TRPA1 is a temperature dependent ion channel that is activated above 25°C, while remaining inactive at 23°C and below (Hamada et al., 2008). Thus, shifting Gr43aGAL4/UAS-trpA1; Cha7.4kb-GAL80/+ flies from ambient (23°C) temperature to 29°C will lead to activation of Gr43aGAL4 brain neurons, independent of hemolymph fructose levels. We first entrained hungry flies by exposing them to a neutral odor A at 29°C, followed by a neutral odor B at 23°C (Figure 7A). The flies were then tested in a T-maze odor choice assay, which revealed a preference for odor A (Figure 7B, left panel). Intriguingly, when we entrained satiated flies in this paradigm, they avoided odor A (Figure 7B, right panel). Importantly, control flies lacking the UAS-trpA1 or the Gr43aGAL4 allele showed no preference or avoidance after the same entrainment procedures. These results indicate that under deprived nutrient conditions, activation of Gr43aGAL4 neurons is perceived as pleasant, reinforcing the associated behavior (odor A preference in T-maze/feeding in the natural context). In contrast, Gr43aGAL4 neuron activation in satiated flies causes an aversive sensation (odor A avoidance/feeding termination). Thus, activation of Gr43aGAL4 brain neurons evokes diametrically opposite, satiation-dependent perceptions.
Figure 7. Activation of Gr43aGAL4 brain neurons assigns satiety-dependent valence.
(A) Schematic diagram of the olfactory conditioning assay. Flies are exposed to odor A, while their Gr43aGAL4 brain neurons are activated using trpA1 (29°C). After a brief rest period in an odorless vial, they are exposed to odor B in the absence of neural activation (23°C), followed by another rest period in an odorless vial. This training session is repeated once more before the flies are tested in a T-Maize assay for acquired odor preference.
(B) Olfactory conditioning assay of starved and satiated flies. Prior conditioning, flies were kept in agarose or agarose containing 250 mM sucrose for 18-24 hours to induce starvation and satiation, respectively. Gr43aGAL4/UAS-trpA1;Cha7.4kb-GAL80/+ flies assign positive valence when hungry (pleasant; left graph), but negative valence when satiated (unpleasant; right graph). Control flies were Gr43aGAL4/+; Cha7.4kb-GAL80/+ and UAS-trpA1/+. *p < 0.05; **p<0.01; ANOVA. 12≤n≤18.
(C) Model: The fly’s major blood sugars, glucose and trehalose are kept at a fairly constant, relatively high level. Conversely, internal fructose level is very low, but fluctuates in response to feeding of nutritious sugars. Since nutritious carbohydrates can be converted into fructose, the activity of Gr43aGAL4 brain neurons depends on the nutritious value of the ingested food. Activation of Gr43aGAL4 brain neurons, in combination with the state of satiety, leads either to a pleasant sensation in hungry flies, reinforcing feeding behavior, or is perceived as unpleasant, thereby terminating feeding behavior.
DISCUSSION
The regulation of food intake is a complex process in higher animals; it is not only dependent on the palatability of food components as evaluated by the external taste sensory system, but also on nutritional content determined by internal sensors. Three recent reports have provided evidence that Drosophila possesses internal nutrient sensors that regulate food consumption based on the nutritious value of carbohydrates (Burke and Waddell, 2011; de Araujo et al., 2008; Dus et al., 2011; Fujita and Tanimura, 2011). In this paper, we have identified the molecular basis of such a sensor in the form of GR43a. This receptor evaluates hemolymph fructose levels, derived either directly from dietary fructose or from other nutritious sugars broken down or metabolized from glucose, and exerts opposite effects on feeding behavior in a satiation-depending manner.
Two distinct receptors sense food fructose
Fructose is the most abundant dietary carbohydrate in many fruits, including apples, grapes and blueberries, major food sources of many Drosophila species. We have shown that flies have two receptors for this sugar, GR43a, and one encoded by the psGr genes (Figure 2). While Ca2+ imaging experiments indicate that GR43a is more potent in sensing fructose (Figure 2D), it has a minor role as an external sugar receptor in adult flies (Figure 2E). However, Gr43a plays a prominent role in sugar sensing during the larval stage (Mishra et al, in preparation). Recent patch-clamp recordings in heterologous expression systems showed that the bombyx mori gustatory receptor BmGr-9 and its Drosophila ortholog GR43a function as a ligand-gated ion channel that is selectively activated by fructose (Sato et al., 2011). Curiously, BmGr-9 was not activated by sucrose (GR43a was not tested), and it will be interesting to see whether this difference is due to sequence variation between the two orthologs, or a reflection of suboptimal conditions in a heterologous expression system.
Fructose-sensing brain neurons function as an internal nutrient sensor
Like blood glucose in mammals, levels of glucose and trehalose, the main hemolymph sugars in Drosophila (Lee and Park, 2004; Thompson, 2003), are kept relatively stable, regardless of feeding state (Figure 4). Analogous to the insulin and glucagon pathways in mammals, flies counteract rising or decreasing glucose/trehalose in the hemolymph by secreting insulin-like peptides or adipokinetic hormone to maintain energy homeostasis (Bharucha et al., 2008; Buch et al., 2008; Lee and Park, 2004). Interestingly, glucose responsive neurons have been identified in the hypothalamus (Karnani and Burdakov, 2010; Marty et al., 2007), and it has been proposed that an internal sugar sensor in mice modulates feeding behavior via the dopamine reward system (de Araujo et al., 2008). However, the neural circuits and the molecular nature of internal nutrient sensors are unknown, both in mammals and insects.
To our knowledge, the internal fructose sensing system described here establishes a precedent. We have shown that about six Gr43aGAL4 neurons in the posterior superior lateral protocerebrum are specifically activated by fructose. Although GR43a functions independently of any of the known psGRs, ectopic expression of Gr43a in other brain neurons does not render them fructose sensitive (data not shown), implying that this receptor acts in concert with another GR protein, or requires a cell-specific transducer. Regardless, our observations indicate that Gr43aGAL4 brain neurons detect fructose in the hemolymph that derive directly from dietary fructose, or indirectly from other nutritious carbohydrates (Burke and Waddell, 2011; Dus et al., 2011; Fujita and Tanimura, 2011).
Why do flies use fructose, rather glucose (or its disaccharide, trehalose), which is 100 fold more abundant than fructose in the hemolymph, as a signal for an internal nutrient sensor? Our data indicate that a glucose sensor would be difficult to activate in a robust fashion because a single sugar meal, even if completely absorbed and converted into glucose would increase overall amount of hemolymph glucose by less than 1/3. Specifically, the amount of hemolymph glucose in a fly (1 mg weight) is ~9.4 μg, compared to ~3 μg of glucose or ~2.4 μg of fructose present in a single meal (Figures 4 and 6; see also Experimental Procedures). In contrast, the amount of hemolymph fructose is very low and therefore increases several fold after a single sugar meal. For example, the ~2.4 μg of fructose in a meal is more than 30 times the amount of fructose present in the hemolymph (0.07 μg). The actual increase observed in the hemolymph sugar assay is ~10 fold (Figure 4B), which nevertheless is sufficient for strong activation of the fructose sensor GR43a (Figure 3). Therefore, the internal fructose sensor described here provides a robust system that takes advantage of a steep increase in hemolymph fructose after a sugar meal to evaluate the nutritious content of food.
Activation of Gr43aGAL4 brain neurons evokes opposite, satiety-dependent perceptions
A remarkable property of the Gr43aGAL4 brain neurons is their ability to both promote feeding in hungry flies and suppress feeding in satiated flies (Figure 5 and 6). This was directly demonstrated in the odor-conditioning paradigm, which revealed that these neurons assign diametrically opposite valence that is dependent on the satiation status (Figure 7B). These observations lead us to propose the following model (Figure 7C): Ingestion and conversion of nutritious carbohydrates leads to an increase of hemolymph fructose, resulting in activation of Gr43aGAL4 brain neurons. In hungry flies, this activation is perceived positively, thereby reinforcing feeding behavior. In contrast, the activation in satiated flies is perceived negatively and leads to feeding termination. How can the satiation status so drastically change the behavioral output through a single group of neurons? At least two distinct mechanisms could account for these opposite effects. The first mechanism invokes direct modulation of some, but not all of the Gr43aGAL4 brain neurons. For example a subset of these neurons may respond to a factor present in hemolymph of satiated, but not hungry flies. Such a factor may act on a second receptor co-expressed in these neurons and modulate GR43a-mediated activity. Alternatively, Gr43aGAL4 brain neurons may target distinct regions of the brain. Consistent with this idea is the observation that axons of Gr43aGAL4 brain neurons project along two separate paths, connecting to distinct, spatially segregated neural ensembles (Supplemental Figure 5). Distinct output of Gr43aGAL4 brain neurons could be achieved if one of these neural ensembles is regulated by a satiation-dependent signal such as dopamine or octopamine, which are required for aversive and appetitive learning, respectively (Krashes et al., 2009; Schroll et al., 2006; Schwaerzel et al., 2003; Tomchik and Davis, 2009). Even though Gr43aGAL4 neurons are neither dopaminergic nor octopaminergic, it is possible that their downstream targets use these neurotransmitters to convert output from Gr43aGAL4 neurons into positive or negative valence.
In both scenarios, the satiety signal may derive from the fat cells or neurosecretory cells, such as the Drosophila insulin like peptide (DILP) expressing cells in the brain. Interestingly, TOR signaling of larvae fed with a protein rich diet was shown to induce secretion of a humoral signal from fat cells that acts on the DILP expressing cells to control growth and glucose homeostasis (Geminard et al., 2009). The same signaling pathway might be used here to regulate the intake of carbohydrates in adult flies by imposing positive and negative valence via the Gr43aGAL4 brain neurons. Alternatively, satiation-dependent factors, such as neuropeptide F (NPF), may modulate the Gr43aGAL4 brain circuitry. NPF and the mammalian ortholog, neuropeptide Y (NPY) are known to increase food consumption (Nassel and Winther, 2010; Valassi et al., 2008; Wu et al., 2003; Wu et al., 2005)(Wu et al., 2005). Moreover, the NPF/NPF receptor system also provides the neural framework that integrates the state of satiety and appetitive memory in adult flies (Krashes et al., 2009).
Additional Roles for GR43a in the digestive and reproductive systems
Gr43a is likely to have additional roles, due to its highly specific expression in proventriculus- and uterus-associated neurons. For example, detection of dietary fructose in the foregut may induce digestive processes, such as peristaltic movements of the gut musculature, or activation of metabolic enzymes in secretory cells. Gr43a expression in the uterus suggests a role for this receptor in female physiology and/or behavior that are linked to mating or reproduction. Fructose, like sex-peptide and other male-specific proteins (Hasemeyer et al., 2009; Kubli, 2008; Yapici et al., 2008), might be present in seminal fluid and serve as a ligand to modulate female behaviors associated with reproduction. Whatever the roles of Gr43a may be, we predict that the biological functions of this receptor are conserved across insect species.
EXPERIMENTAL PROCEDURE
Molecular Biology
GAL4 knock-in vector was constructed in CMC105 (Larsson et al., 2004). Gene targeting was performed according to (Rong and Golic, 2000). Details on cloning and homologous recombination are described in Supplemental Material.
Immunostaining
UAS-mCD8::GFP or mCD8::RFP was driven by Gr43aGAL4 to visualize neural structure. Antibody staining was conducted as described previously (Miyamoto and Amrein, 2008).
Calcium imaging
For the leg preparation, the foreleg was cut between the femur and the tibia. The tibia and the first three tarsal segments were dipped in silicone oil on a double-sided tape in a glass bottom dish (MatTek corp.). The leg was covered by 1% agarose, leaving the fourth and fifth tarsal segments exposed. The preparation was covered by 100μl of water and 100μl test solutions was administered through a pipette. Application of this Ca2+ imaging method to characterize both bitter/high salt and sweet neurons will be described elsewhere (Miyamoto et al., submitted). Images for data analysis were acquired from axons near the cell body for 10s before and 30s after application (1 frame/500 ms). Adjacent regions were used to determine autofluorescence background. Each leg was tested with 2-4 different compounds. Imaging was performed with a Nikon eclipse Ti inverted microscope with 20x water objective. The light source was a Lumen 200 lamp (Prior Scientific Inc). Samples were excited at 488 nm (metal halide lamp), and emitted light was collected through a 515-555 nm filter. Data acquisition was performed with NIS-Elements software (Nikon). Average of 5 frames taken immediately before the application were used to define base fluorescence.
Whole brains were dissected in a sugar-free ringer solution (5 mM HEPES (pH 7.2, 130 mM NaCl, 5 mM KCl, 2 mM CaCl2, 2mM MgCl2). The brains were placed in a glass bottom dish and covered with 100 μl of ringer solution. Images used for data analysis were acquired for 60s before and 900s after application (1 frame/3 s).
Behavioral Analyses
For behavioral experiments, mutations were crossed into a w+ background and flies were aged for two to eight days prior to behavioral analysis. PER assay (Slone et al., 2007) was carried out by stimulating the forelegs, instead of the labial palps.
CAFE assay was carried out as described (Ja et al., 2007), with the following modifications: Experimental vials contained 0.5% agarose as a water source. Three to five males, aged for 3-7 days on standard food, were weighed, individually transferred to an experimental vial containing a glass micropipette (VWR international, Cat No.53432-706) and allowed to feed on the specified solutions. The amount consumed, minus evaporation (determined from a vial without a fly) was measured after 24 hrs and normalized to the fly’s weight. In the two-choice CAFÉ assay, two capillaries were provided with the specified solutions, instead of a single capillary.
The olfactory conditioning assay (Quinn et al., 1974) was carried out as follows: flies were kept in 14 ml Falcon tubes with agarose (hungry condition) or with agarose containing 250mM sucrose (satiated condition) for 18-24 hours. 4-methlcyclohexanol (MCH) and 3-octanol (OCT) were diluted at 1:100 in paraffin oil. For conditioning, 5 μl of either odor was applied on a small piece (10mm × 5mm) of Whatman paper and placed in the middle of the tube. Approximately 50 flies were transferred into tube A and kept at 29°C for 5 minutes. Flies were then replaced into a tube containing clean air for 15 minutes (rest; 23°C), before they were transferred into tube B containing the second odor for 5 minutes at 23°C. This cycle was repeated once after 15 minutes of rest time. Flies were given 60 seconds to choose odor A or B. The performance index (PI) was calculated as follows: PI= [(Ncs+ - Ncs-)/Ntotal], whereby Ncs+ and Ncs- represent the number of flies choosing the odor associated with TRPA1 activation (tube A/29°C) and the odor not associated with TRPA1 activation (tube B/23°C), respectively. To rule out odor bias, odor and heat shock were presented reciprocally.
Internal sugar measurements
Fructose was measured using the Seliwanoff’s test (Nunes et al., 2008). Glucose and trehalose were measured using the InfinityTM Glucose Hexokinase Reagent (Thermo Scientific). Adult male heads were used for measurement (for details, see Supplemental Material). The amount of sugar consumed in a meal was based on the daily intake of 100 mM sugar solutions (Figure 6), divided by 12 (1 meal/2 hrs). The weight of a fly was assumed to be 1 mg.
Supplementary Material
GR43a is a fructose receptor expressed in the Drosophila brain
Circulatory fructose rises several fold after a sugar meal
GR43a in the brain functions as a nutrient sensor
Gr43a expressing brain neurons regulate feeding in a satiation-dependent manner
ACKNOWLEDGEMENTS
We would like to thank Drs. S. Fujii and Y. Chen for stimulating discussions while conducting these studies, and Drs. S. Waddell, R. Sitcheran and two anonymous reviewers for comments and suggestions on the manuscript. We thank Drs. L Looger, T Lee, T Kitamoto and J Carlson for Drosophila strains G-CaMP3.0, lexAop-rCD2GFP, Cha7.4kb-GAL80 and ΔGr61a, respectively, and Dr. LB Vosshall for a LexA plasmid. This work was supported by grants from the National Institute of Health, RO1-DC009014 and RO1-DC005606, to HA. TM conducted all experiments, except the PER analysis. JS and XS performed the PER analysis, and JS generated the Gr64fGAL4 knock-in strain. TM and HA conceived the experiments and wrote the paper. This paper is dedicated to the memory of Isabel Sofia Sitcheran Amrein, who is an enduring inspiration to HA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bharucha KN, Tarr P, Zipursky SL. A glucagon-like endocrine pathway in Drosophila modulates both lipid and carbohydrate homeostasis. J Exp Biol. 2008;211:3103–3110. doi: 10.1242/jeb.016451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buch S, Melcher C, Bauer M, Katzenberger J, Pankratz MJ. Opposing effects of dietary protein and sugar regulate a transcriptional target of Drosophila insulin-like peptide signaling. Cell Metab. 2008;7:321–332. doi: 10.1016/j.cmet.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Burke CJ, Waddell S. Remembering nutrient quality of sugar in Drosophila. Curr Biol. 2011;21:746–750. doi: 10.1016/j.cub.2011.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron P, Hiroi M, Ngai J, Scott K. The molecular basis for water taste in Drosophila. Nature. 2011;465:91–95. doi: 10.1038/nature09011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman R. The Insects: Structure and Function. 4th edn Cambridge University Press; Cambridge, New York, Melbourne, Madrid, Cape Town: 1998. [Google Scholar]
- Dahanukar A, Lei YT, Kwon JY, Carlson JR. Two Gr genes underlie sugar reception in Drosophila. Neuron. 2007;56:503–516. doi: 10.1016/j.neuron.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Araujo IE, Oliveira-Maia AJ, Sotnikova TD, Gainetdinov RR, Caron MG, Nicolelis MA, Simon SA. Food reward in the absence of taste receptor signaling. Neuron. 2008;57:930–941. doi: 10.1016/j.neuron.2008.01.032. [DOI] [PubMed] [Google Scholar]
- Dethier VG. The Hungry Fly: A Physiological Study of the Behavior Associated with Feeding. Harvard University Press; Cambridge MA: 1976. [Google Scholar]
- Dunipace L, Meister S, McNealy C, Amrein H. Spatially restricted expression of candidate taste receptors in the Drosophila gustatory system. Current Biology: Cb. 2001;11:822–835. doi: 10.1016/s0960-9822(01)00258-5. [DOI] [PubMed] [Google Scholar]
- Dus M, Min S, Keene AC, Lee GY, Suh GS. Taste-independent detection of the caloric content of sugar in Drosophila. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1017096108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer J, Salmon KS, Zibrik L, Shirazi-Beechey SP. Expression of sweet taste receptors of the T1R family in the intestinal tract and enteroendocrine cells. Biochem Soc Trans. 2005;33:302–305. doi: 10.1042/BST0330302. [DOI] [PubMed] [Google Scholar]
- Fujita M, Tanimura T. Drosophila evaluates and learns the nutritional value of sugars. Curr Biol. 2011;21:751–755. doi: 10.1016/j.cub.2011.03.058. [DOI] [PubMed] [Google Scholar]
- Geminard C, Rulifson EJ, Leopold P. Remote control of insulin secretion by fat cells in Drosophila. Cell Metab. 2009;10:199–207. doi: 10.1016/j.cmet.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Hamada FN, Rosenzweig M, Kang K, Pulver SR, Ghezzi A, Jegla TJ, Garrity PA. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454:217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey RA, Ferrier DR, editors. Biochemistry (Lippincott’s Illustrated Reviews) 5th edition Williams and Wilkins; Philadelphia, PA, Lippincott: 2011. [Google Scholar]
- Hasemeyer M, Yapici N, Heberlein U, Dickson BJ. Sensory neurons in the Drosophila genital tract regulate female reproductive behavior. Neuron. 2009;61:511–518. doi: 10.1016/j.neuron.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Ja WW, Carvalho GB, Mak EM, de la Rosa NN, Fang AY, Liong JC, Brummel T, Benzer S. Prandiology of Drosophila and the CAFE assay. Proc Natl Acad Sci U S A. 2007;104:8253–8256. doi: 10.1073/pnas.0702726104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang HJ, Kokrashvili Z, Theodorakis MJ, Carlson OD, Kim BJ, Zhou J, Kim HH, Xu X, Chan SL, Juhaszova M, et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci U S A. 2007;104:15069–15074. doi: 10.1073/pnas.0706890104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen S, Laermans J, Verhulst PJ, Thijs T, Tack J, Depoortere I. Bitter taste receptors and α-gustducin regulate the secretion of ghrelin with functional effects on food intake and gastric emptying. Proc Natl Acad Sci U S A. 2011;108:2094–2099. doi: 10.1073/pnas.1011508108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Moon SJ, Montell C. A Drosophila gustatory receptor required for the responses to sucrose, glucose, and maltose identified by mRNA tagging. Proc Natl Acad Sci U S A. 2007;104:14110–14115. doi: 10.1073/pnas.0702421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnani M, Burdakov D. Multiple hypothalamic circuits sense and regulate glucose levels. Am J Physiol Regul Integr Comp Physiol. 2010;300:R47–55. doi: 10.1152/ajpregu.00527.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohyama-Koganeya A, Kim YJ, Miura M, Hirabayashi Y. A Drosophila orphan G protein-coupled receptor BOSS functions as a glucose-responding receptor: loss of boss causes abnormal energy metabolism. Proc Natl Acad Sci U S A. 2008;105:15328–15333. doi: 10.1073/pnas.0807833105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, DasGupta S, Vreede A, White B, Armstrong JD, Waddell S. A neural circuit mechanism integrating motivational state with memory expression in Drosophila. Cell. 2009;139:416–427. doi: 10.1016/j.cell.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubli E. Sexual behaviour: a receptor for sex control in Drosophila females. Curr Biol. 2008;18:R210–212. doi: 10.1016/j.cub.2007.12.047. [DOI] [PubMed] [Google Scholar]
- Larsson MC, Domingos AI, Jones WD, Chiappe ME, Amrein H, Vosshall LB. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004;43:703–714. doi: 10.1016/j.neuron.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Lee G, Park JH. Hemolymph sugar homeostasis and starvation-induced hyperactivity affected by genetic manipulations of the adipokinetic hormone-encoding gene in Drosophila melanogaster. Genetics. 2004;167:311–323. doi: 10.1534/genetics.167.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Moon SJ, Montell C. Multiple gustatory receptors required for the caffeine response in Drosophila. Proc Natl Acad Sci U S A. 2009;106:4495–4500. doi: 10.1073/pnas.0811744106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Johnson WA, Welsh MJ. Drosophila DEG/ENaC pickpocket genes are expressed in the tracheal system, where they may be involved in liquid clearance. Proc Natl Acad Sci U S A. 2003;100:2128–2133. doi: 10.1073/pnas.252785099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolskee RF, Dyer J, Kokrashvili Z, Salmon KS, Ilegems E, Daly K, Maillet EL, Ninomiya Y, Mosinger B, Shirazi-Beechey SP. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci U S A. 2007;104:15075–15080. doi: 10.1073/pnas.0706678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty N, Dallaporta M, Thorens B. Brain glucose sensing, counterregulation, and energy homeostasis. Physiology (Bethesda) 2007;22:241–251. doi: 10.1152/physiol.00010.2007. [DOI] [PubMed] [Google Scholar]
- Miyamoto T, Amrein H. Courtship Suppression by a Drosophila pheromone recpetor. Nature Neuroscience. 2008 doi: 10.1038/nn.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon SJ, Kottgen M, Jiao Y, Xu H, Montell C. A taste receptor required for the caffeine response in vivo. Curr Biol. 2006;16:1812–1817. doi: 10.1016/j.cub.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Moon SJ, Lee Y, Jiao Y, Montell C. A Drosophila gustatory receptor essential for aversive taste and inhibiting male-to-male courtship. Curr Biol. 2009;19:1623–1627. doi: 10.1016/j.cub.2009.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassel DR, Winther AM. Drosophila neuropeptides in regulation of physiology and behavior. Prog Neurobiol. 2010;92:42–104. doi: 10.1016/j.pneurobio.2010.04.010. [DOI] [PubMed] [Google Scholar]
- Quinn WG, Harris WA, Benzer S. Conditioned behavior in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1974;71:708–712. doi: 10.1073/pnas.71.3.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson HM, Wanner KW. The chemoreceptor superfamily in the honey bee, Apis mellifera: expansion of the odorant, but not gustatory, receptor family. Genome Res. 2006;16:1395–1403. doi: 10.1101/gr.5057506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong YS, Golic KG. Gene targeting by homologous recombination in Drosophila. Science. 2000;288:2013–2018. doi: 10.1126/science.288.5473.2013. [DOI] [PubMed] [Google Scholar]
- Rozengurt E. Taste receptors in the gastrointestinal tract. I. Bitter taste receptors and alpha-gustducin in the mammalian gut. Am J Physiol Gastrointest Liver Physiol. 2006;291:G171–177. doi: 10.1152/ajpgi.00073.2006. [DOI] [PubMed] [Google Scholar]
- Sakai T, Kasuya J, Kitamoto T, Aigaki T. The Drosophila TRPA channel, Painless, regulates sexual receptivity in virgin females. Genes Brain Behav. 2009;8:546–557. doi: 10.1111/j.1601-183X.2009.00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Tanaka K, Touhara K. Sugar-regulated cation channel formed by an insect gustatory receptor. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1019622108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroll C, Riemensperger T, Bucher D, Ehmer J, Voller T, Erbguth K, Gerber B, Hendel T, Nagel G, Buchner E, et al. Light-induced activation of distinct modulatory neurons triggers appetitive or aversive learning in Drosophila larvae. Curr Biol. 2006;16:1741–1747. doi: 10.1016/j.cub.2006.07.023. [DOI] [PubMed] [Google Scholar]
- Schwaerzel M, Monastirioti M, Scholz H, Friggi-Grelin F, Birman S, Heisenberg M. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J Neurosci. 2003;23:10495–10502. doi: 10.1523/JNEUROSCI.23-33-10495.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott K. Taste recognition: food for thought. Neuron. 2005;48:455–464. doi: 10.1016/j.neuron.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Scott K, Brady R, Cravchik A, Morozov P, Rzhetsky A, Zuker C, Axel R. A chemosensory gene family encoding candidate gustatory and olfactory receptors in Drosophila. Cell. 2001;104:661–673. doi: 10.1016/s0092-8674(01)00263-x. [DOI] [PubMed] [Google Scholar]
- Singh N, Vrontakis M, Parkinson F, Chelikani P. Functional bitter taste receptors are expressed in brain cells. Biochem Biophys Res Commun. 2011;406:146–151. doi: 10.1016/j.bbrc.2011.02.016. [DOI] [PubMed] [Google Scholar]
- Slone J, Daniels J, Amrein H. Sugar receptors in Drosophila. Curr Biol. 2007;17:1809–1816. doi: 10.1016/j.cub.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suster ML, Seugnet L, Bate M, Sokolowski MB. Refining GAL4-driven transgene expression in Drosophila with a GAL80 enhancer-trap. Genesis. 2004;39:240–245. doi: 10.1002/gene.20051. [DOI] [PubMed] [Google Scholar]
- Sweeney ST, Broadie K, Keane J, Niemann H, O’Kane CJ. Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron. 1995;14:341–351. doi: 10.1016/0896-6273(95)90290-2. [DOI] [PubMed] [Google Scholar]
- Thompson SN. Trehalose - The Insect ‘Blood” sugar. Advances in Insect Physiology. 2003;31:205–285. [Google Scholar]
- Thorne N, Chromey C, Bray S, Amrein H. Taste perception and coding in Drosophila. Curr Biol. 2004;14:1065–1079. doi: 10.1016/j.cub.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Tian L, Hires SA, Mao T, Huber D, Chiappe ME, Chalasani SH, Petreanu L, Akerboom J, McKinney SA, Schreiter ER, et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods. 2009;6:875–881. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomchik SM, Davis RL. Dynamics of learning-related cAMP signaling and stimulus integration in the Drosophila olfactory pathway. Neuron. 2009;64:510–521. doi: 10.1016/j.neuron.2009.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valassi E, Scacchi M, Cavagnini F. Neuroendocrine control of food intake. Nutr Metab Cardiovasc Dis. 2008;18:158–168. doi: 10.1016/j.numecd.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Wang Z, Singhvi A, Kong P, Scott K. Taste representations in the Drosophila brain. Cell. 2004;117:981–991. doi: 10.1016/j.cell.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Wanner KW, Robertson HM. The gustatory receptor family in the silkworm moth Bombyx mori is characterized by a large expansion of a single lineage of putative bitter receptors. Insect Mol Biol. 2008;17:621–629. doi: 10.1111/j.1365-2583.2008.00836.x. [DOI] [PubMed] [Google Scholar]
- Weiss LA, Dahanukar A, Kwon JY, Banerjee D, Carlson JR. The molecular and cellular basis of bitter taste in Drosophila. Neuron. 2011;69:258–272. doi: 10.1016/j.neuron.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Wen T, Lee G, Park JH, Cai HN, Shen P. Developmental control of foraging and social behavior by the Drosophila neuropeptide Y-like system. Neuron. 2003;39:147–161. doi: 10.1016/s0896-6273(03)00396-9. [DOI] [PubMed] [Google Scholar]
- Wu Q, Zhao Z, Shen P. Regulation of aversion to noxious food by Drosophila neuropeptide Y- and insulin-like systems. Nat Neurosci. 2005;8:1350–1355. doi: 10.1038/nn1540. [DOI] [PubMed] [Google Scholar]
- Yapici N, Kim YJ, Ribeiro C, Dickson BJ. A receptor that mediates the post-mating switch in Drosophila reproductive behaviour. Nature. 2008;451:33–37. doi: 10.1038/nature06483. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.