Abstract
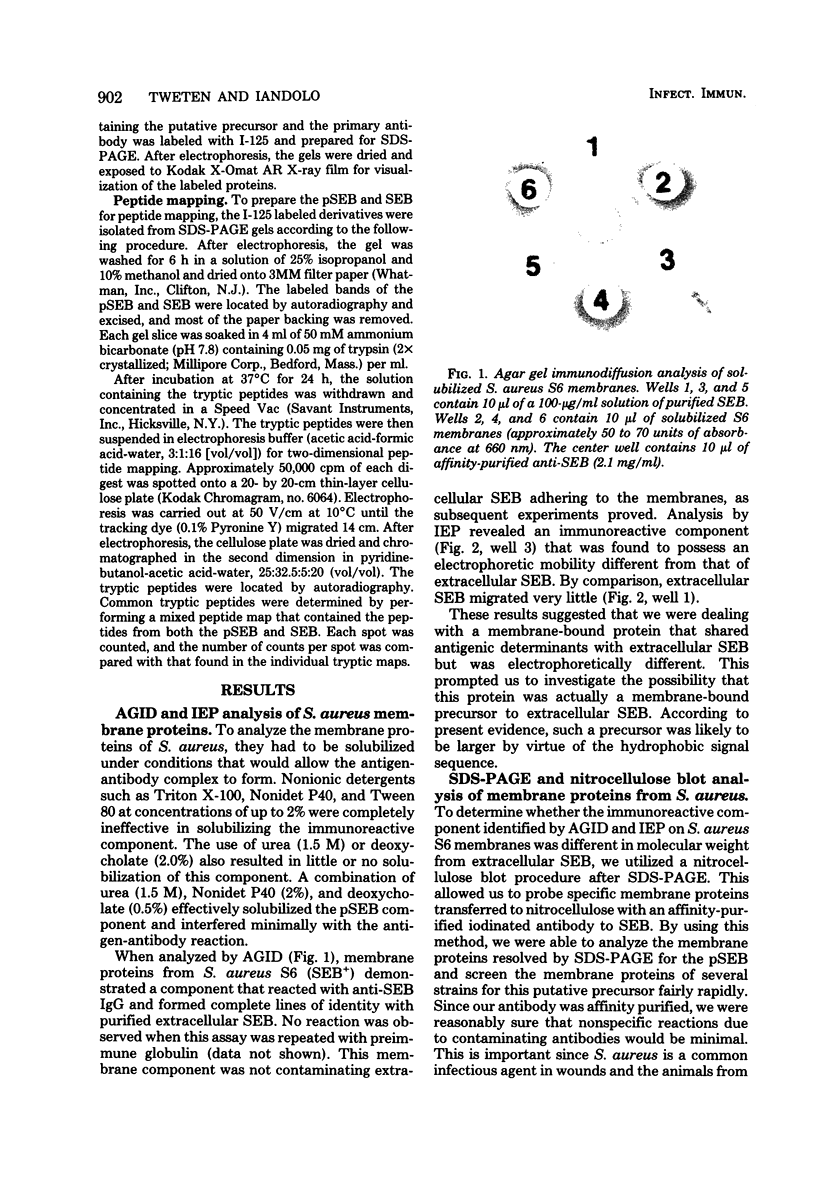
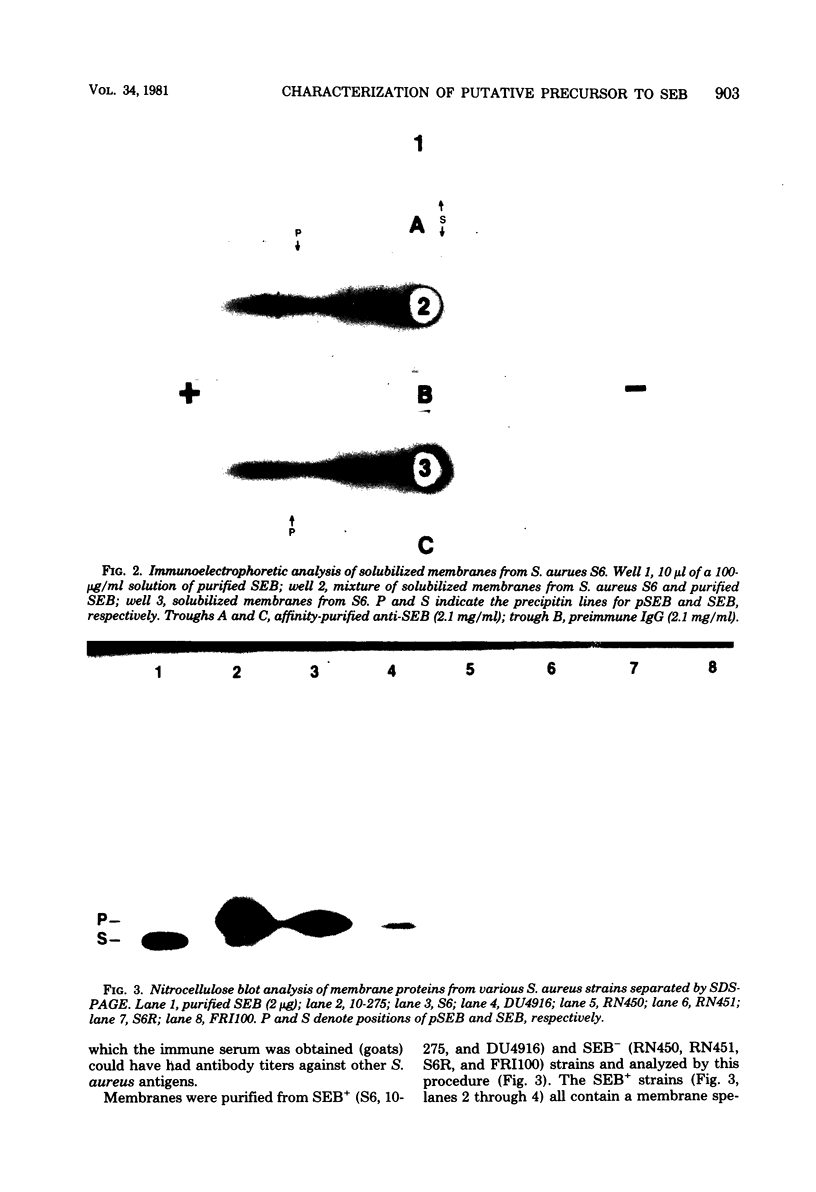
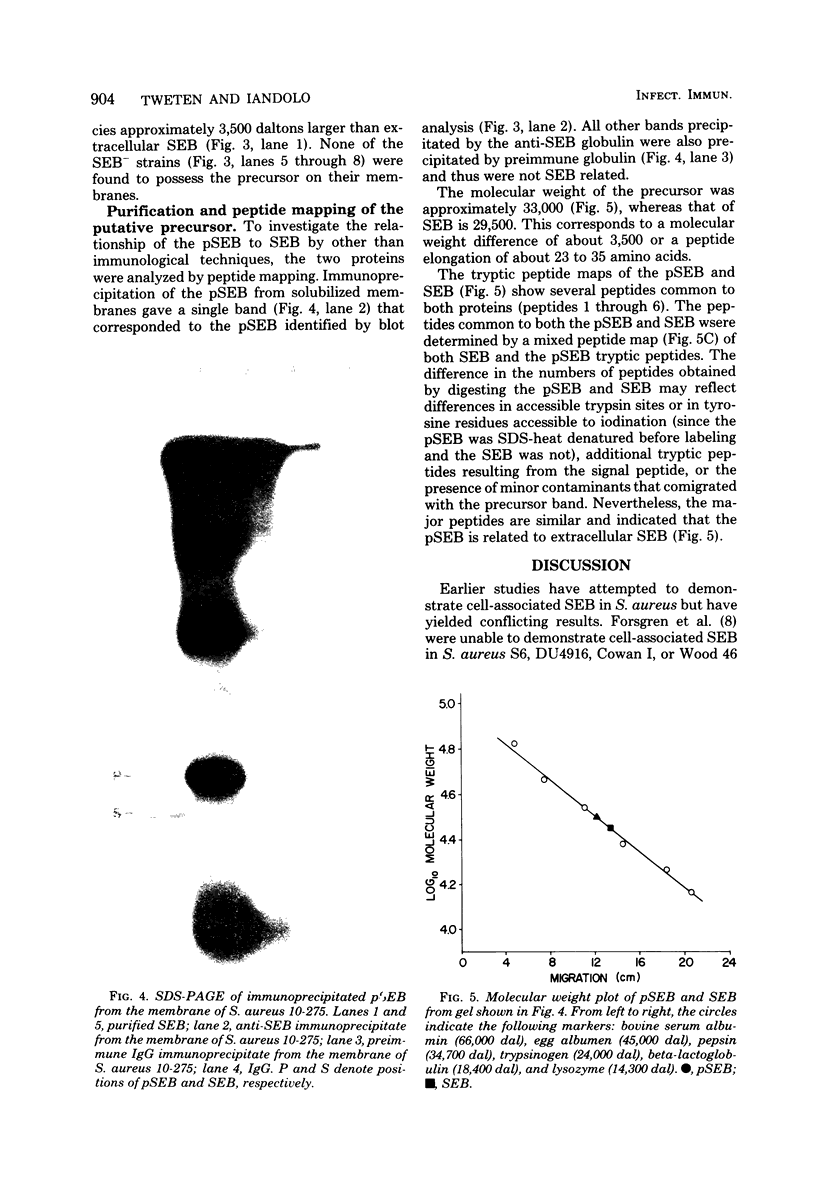
A putative precursor to staphylococcal enterotoxin B (SEB) has been identified as a component of purified membranes from Staphylococcus aureus S6. Agarose gel immunodiffusion analysis of the solubilized membranes demonstrated an immunoreactive protein that formed complete lines of identity with purified extracellular SEB. This putative precursor (pSEB) also had a different electrophoretic mobility from that of extracellular SEB when analyzed by immunoelectrophoresis. When membrane proteins from S6 were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred to nitrocellulose sheets and probed with I-125 labeled, affinity-purified anti-SEB, the pSEB band was identified. The pSEB was approximately 3,500 daltons larger than extracellular SEB. This component was purified by immunoprecipitation and sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Two-dimensional peptide maps of the putative SEB precursor revealed that most of the tryptic peptides were identical to those of mature extracellular SEB. When purified membranes of other SEB+ (DU4916 and 10-275) and SEB- (RN450, RN451, S6R, and FR1100) S. aureus strains were analyzed by the nitrocellulose blot procedure, only the SEB+ strains contained this putative SEB precursor on their membranes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowen B., Steinberg J., Laemmli U. K., Weintraub H. The detection of DNA-binding proteins by protein blotting. Nucleic Acids Res. 1980 Jan 11;8(1):1–20. doi: 10.1093/nar/8.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emr S. D., Hedgpeth J., Clément J. M., Silhavy T. J., Hofnung M. Sequence analysis of mutations that prevent export of lambda receptor, an Escherichia coli outer membrane protein. Nature. 1980 May 8;285(5760):82–85. doi: 10.1038/285082a0. [DOI] [PubMed] [Google Scholar]
- Ferrazza D., Levy S. B. Biosynthesis of a plasmid-encoded outer membrane surface exclusion protein involves processing from a precursor polypeptide. J Biol Chem. 1980 Oct 10;255(19):8955–8958. [PubMed] [Google Scholar]
- Forsgren A., Forsum U., Hallander H. O. Failure to detect cell-associated enterotoxin B in Staphylococcus aureus by immunofluorescence. Appl Microbiol. 1972 Mar;23(3):559–564. doi: 10.1128/am.23.3.559-564.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossard F., Seidah N. G., Crine P., Routhier R., Chrétien M. Partial N-terminal amino acid sequence of pro-opio-melanocortin (ACTH/beta-LPH precursor) from rat pars intermedia. Biochem Biophys Res Commun. 1980 Feb 12;92(3):1042–1051. doi: 10.1016/0006-291x(80)90807-4. [DOI] [PubMed] [Google Scholar]
- Hasilik A., Neufeld E. F. Biosynthesis of lysosomal enzymes in fibroblasts. Synthesis as precursors of higher molecular weight. J Biol Chem. 1980 May 25;255(10):4937–4945. [PubMed] [Google Scholar]
- Huang I. Y., Bergdoll M. S. Primary structure of staphylococcal enterotoxin B. I. Isolation, composition, and sequence of tryptic peptides from oxidized enterotoxin B. J Biol Chem. 1970 Jul 25;245(14):3493–3510. [PubMed] [Google Scholar]
- Huang I. Y., Bergdoll M. S. The primary structure of staphylococcal enterotoxin B. 3. The cyanogen bromide peptides of reduced and aminoethylated enterotoxin B, and the complete amino acid sequence. J Biol Chem. 1970 Jul 25;245(14):3518–3525. [PubMed] [Google Scholar]
- Huang I. Y., Bergdoll M. S. The primary structure of staphylococcal enterotoxin B. II. Isolation, composition, and sequence of chymotryptic peptides. J Biol Chem. 1970 Jul 25;245(14):3511–3517. [PubMed] [Google Scholar]
- Inouye H., Beckwith J. Synthesis and processing of an Escherichia coli alkaline phosphatase precursor in vitro. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1440–1444. doi: 10.1073/pnas.74.4.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis A. W., Lawrence R. C. Production of extracellular enzymes and enterotoxins A, B, and C by Staphylococcus aureus. Infect Immun. 1971 Aug;4(2):110–115. doi: 10.1128/iai.4.2.110-115.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRAUSE R. M., McCARTY M. Studies on the chemical structure of the streptococcal cell wall. II. The composition of group C cell walls and chemical basis for serologic specificity of the carbohydrate moiety. J Exp Med. 1962 Jan 1;115:49–62. doi: 10.1084/jem.115.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin A. S., Gregor I., Mason T. L., Nelson N., Schatz G. Cytoplasmically made subunits of yeast mitochondrial F1-ATPase and cytochrome c oxidase are synthesized as individual precursors, not as polyproteins. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3998–4002. doi: 10.1073/pnas.77.7.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccecchini M. L., Rudin Y., Schatz G. Transport of proteins across the mitochondrial outer membrane. A precursor form of the cytoplasmically made intermembrane enzyme cytochrome c peroxidase. J Biol Chem. 1979 Aug 25;254(16):7468–7471. [PubMed] [Google Scholar]
- Mandel G., Wickner W. Translational and post-translational cleavage of M13 procoat protein: extracts of both the cytoplasmic and outer membranes of Escherichia coli contain leader peptidase activity. Proc Natl Acad Sci U S A. 1979 Jan;76(1):236–240. doi: 10.1073/pnas.76.1.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlebrook J. L., Spero L., Argos P. The secondary structure of staphylococcal enterotoxins A, B and C. Biochim Biophys Acta. 1980 Feb 27;621(2):233–240. doi: 10.1016/0005-2795(80)90175-0. [DOI] [PubMed] [Google Scholar]
- Miller R. D., Fung D. Y. The occurrence of cell-associated enterotoxin B in Staphylococcus aureus. Can J Microbiol. 1976 Sep;22(9):1215–1221. doi: 10.1139/m76-180. [DOI] [PubMed] [Google Scholar]
- Milstein C., Brownlee G. G., Harrison T. M., Mathews M. B. A possible precursor of immunoglobulin light chains. Nat New Biol. 1972 Sep 27;239(91):117–120. doi: 10.1038/newbio239117a0. [DOI] [PubMed] [Google Scholar]
- Moreno F., Fowler A. V., Hall M., Silhavy T. J., Zabin I., Schwartz M. A signal sequence is not sufficient to lead beta-galactosidase out of the cytoplasm. Nature. 1980 Jul 24;286(5771):356–359. doi: 10.1038/286356a0. [DOI] [PubMed] [Google Scholar]
- Movva N. R., Nakamura K., Inouye M. Amino acid sequence of the signal peptide of ompA protein, a major outer membrane protein of Escherichia coli. J Biol Chem. 1980 Jan 10;255(1):27–29. [PubMed] [Google Scholar]
- Palmiter R. D., Gagnon J., Walsh K. A. Ovalbumin: a secreted protein without a transient hydrophobic leader sequence. Proc Natl Acad Sci U S A. 1978 Jan;75(1):94–98. doi: 10.1073/pnas.75.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall L. L., Hardy S. J., Josefsson L. G. Precursors of three exported proteins in Escherichia coli. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1209–1212. doi: 10.1073/pnas.75.3.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekizawa J., Inouye S., Halegoua S., Inouye M. Precursors of major outer membrane proteins of Escherichia coli. Biochem Biophys Res Commun. 1977 Aug 8;77(3):1126–1133. doi: 10.1016/s0006-291x(77)80095-8. [DOI] [PubMed] [Google Scholar]
- Sero L., Metzger J. F., Warren J. R., Griffin B. Y. Biological activity and complementation of the two peptides of staphylococcal enterotoxin B formed by limited tryptic hydrolysis. J Biol Chem. 1975 Jul 10;250(13):5026–5032. [PubMed] [Google Scholar]
- Shields D. In vitro biosynthesis of fish islet preprosomatostatin: evidence of processing and segregation of a high molecular weight precursor. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4074–4078. doi: 10.1073/pnas.77.7.4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore G. C., Carignan P., Raymond Y. In vitro synthesis of a putative precursor to the mitochondrial enzyme, carbamyl phosphate synthetase. J Biol Chem. 1979 May 10;254(9):3141–3144. [PubMed] [Google Scholar]
- Spero L., Warren J. R., Metzger J. F. Effect of single peptide bond scission by trypsin on the structure and activity of staphylococcal enterotoxin B. J Biol Chem. 1973 Nov 10;248(21):7289–7294. [PubMed] [Google Scholar]
- Strauss A. W., Zimmerman M., Boime I., Ashe B., Mumford R. A., Alberts A. W. Characterization of an endopeptidase involved in pre-protein processing. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4225–4229. doi: 10.1073/pnas.76.9.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodore T. S., Popkin T. J., Cole R. M. The separation and isolation of plasma membranes and mesosomal vesicles from Staphylococcus aureus. Prep Biochem. 1971;1(3):233–248. doi: 10.1080/00327487108081942. [DOI] [PubMed] [Google Scholar]
- von Heijne G., Blomberg C. Trans-membrane translocation of proteins. The direct transfer model. Eur J Biochem. 1979 Jun;97(1):175–181. doi: 10.1111/j.1432-1033.1979.tb13100.x. [DOI] [PubMed] [Google Scholar]