Abstract
Traditional molecular techniques have been used in research in discovering the genes and enzymes that are involved in aflatoxin formation and genetic regulation. We cloned most, if not all, of the aflatoxin pathway genes. A consensus gene cluster for aflatoxin biosynthesis was discovered in 2005. The factors that affect aflatoxin formation have been studied. In this report, the author summarized the current status of research progress and future possibilities that may be used for solving aflatoxin contamination.
Keywords: aflatoxins, mycotoxins, Aspergillus flavus, gene cluster, gene regulation, biocontrol, food contaminants
1. Introduction on Aspergillus flavus, Aflatoxins, and the Importance for the Economy
Aspergilli belong to the class of imperfect filamentous fungi. Among the approximately 250 known species, many are producers of beneficial secondary metabolites, such as antibiotics and other pharmaceuticals [1]. For example, Aspergillus terreus, produces lovastatin, a potent cholesterol-lowering drug. Other Aspergilli secrete antibiotics (penicillin and cephalosporin), antifungals (griseofulvin), and anti-tumor drugs (terrequinone A) [2,3]. Many uncharacterized compounds are produced by Aspergilli through various metabolic pathways. These compounds include pathway end products and pathway intermediates or shunt metabolites formed along these pathways and may also have beneficial pharmaceutical properties that can be a potential source of new drugs. However, there are many secondary metabolites produced by Aspergillus species, however, they are not always beneficial. Some of them are even toxic and/or carcinogenic and called mycotoxins. Mycotoxins are structurely very diverse chemical compounds with diverse toxic effects and a variety of biological activities [4].
Within the genus Aspergillus, Aspergillus flavus is the most important economically and most notorious because it produces aflatoxins. A. flavus fungus, one of the most abundant soil-borne molds on earth, is a saprobe that is capable of surviving on many organic nutrient sources like plant debris, animal fodder, cotton, compost piles, dead insects and animal carcasses, stored grains, and even immunocompromised humans and animals [5]. It has the ability to survive temperatures ranging from 12 °C to 48 °C, but the optimal growth temperature ranges from 28 °C to 37 °C. Its ability to grow at relatively high temperatures contributes to its pathogenicity toward humans and other warm-blooded animals. For most of its lifecycle, the fungus exists in the form of mycelium or asexual spores known as conidia. Under adverse conditions such as lack of adequated nutrients or water, the fungal mycelium will transform to resistant structures called sclerotia, which can survive in extremely harsh environmental conditions. The fungus overwinters either as spores, sclerotia, or as mycelium in debris. When conditions become favorable, the sclerotia germinate directly to produce new colonies or conidiophores with conidia [6,7,8].
The fungus A. flavus is a weak and opportunistic plant pathogen, affecting many agricultural crops such as maize (corn), cotton, groundnuts (peanuts), as well as tree nuts such as Brazil nuts, pecans, pistachio nuts, and walnuts. Preharvest contamination of these crops with aflatoxins is common. A. flavus also causes the spoilage of post harvest grains during storage. Because A. flavus lacks host specificity [9], it can attack seeds of both monocots and dicots, and seeds produced both above ground (corn) as well as below the ground (peanuts). Under weather conditions favorable for its growth, A. flavus can cause ear rot on maize, resulting in significant economic losses to farmers [10,11,12].
The A. flavus toxins were first identified as the cause of a severe animal poisoning incident in England in 1960 called the Turkey X disease [13,14]. Most A. flavus produces aflatoxins B1 and B2 whereas Aspergillus parasiticus, produces aflatoxins B1, B2, G1, and G2. These four major aflatoxins are named based on their blue (B) or green (G) fluorescence under ultraviolet light, and their relative mobility by thin-layer chromatography on silica gel. Aflatoxin M1 is a hydroxylated derivative metabolized from aflatoxin B1 by cows and secreted in milk [15]. In addition to aflatoxins B1 and B2, A. flavus also produces many other mycotoxins such as cyclopiazonic acid, kojic acid, beta-nitropropionic acid, aspertoxin, aflatrem and aspergillic acid [16].
Aflatoxin B1, among the four major types of aflatoxins, is the most toxic and the most potent carcinogen in humans and animals including nonhuman primates, birds, fish, and rodents. Chronic exposure can result in suppressed immune response, malnutrition, proliferation of the bile duct, centrilobular necrosis and fatty infiltration of the liver, hepatic lesions, and even hepatomas. In animal models, aflatoxin B1 is modified into a more toxic and carcinogenic by-product during detoxification by a cytochrome P450 monooxygenase in liver [17,18,19,20]. The epoxide form of aflatoxin binds to guanine residues in DNA, forms guanyl-N7 adducts, and induces mutations. One mutation, a G to T transversion [21,22] at the third base of codon 249, a mutation hot spot of the p53 tumor suppressor gene and is generally believed to be the mechanism for initiating hepatocarcinoma formation [23,24,25,26]. The p53 gene encodes a transcription factor involved in cell cycle regulation. It is commonly mutated in human liver cancers [27]. Aflatoxin B1 is also a potential immunosuppressive agent [28]. Chronic low level exposure of growing vertebrates to aflatoxins may enhance their susceptibility to infection and tumorigenesis [28]. Aflatoxin B1 (AFB1) also affects other organs and tissues, such as the lungs and the entire respiratory system [29]. Human hepatocarcinomas are also associated with hepatitis B virus (HBV) and C virus (HCV) infections [18,30,31]. Together with aflatoxins these viruses significantly increased the risk of hepatoma in hepatitis patients [32,33,34,35].
Food and feed contamination by aflatoxins is a significant food safety issue in the developing countries sometimes because of lack of detection, monitoring and regulating measures to safe guard the food supply. It is estimated that approximately 4.5 billion people living in developing countries are chronically exposed to largely uncontrolled amounts of aflatoxin that severely results in changes in immunity and nutrition [36]. Major outbreaks of acute aflatoxicosis from contaminated food in humans have been documented in developing countries [20]. For example, in western India in 1974, 108 persons among 397 people affected, died from aflatoxin poisoning [37]. A more recent incident of aflatoxin poisoning occurred in Kenya in July 2004 leading to the death of 125 people among the 317 reported illnesses due to consumption of aflatoxin contaminated maize (corn) [20,37]. In the Kenia case, the aflatoxins were produced by A. parvisclerotigenus instead of A. flavus. Acute toxicosis is not the only concern. The world health authorities warn that low doses with long-term dietary exposure to aflatoxins is also a major risk as they can lead to hepatocellular carcinoma [22,24,38,39]. International Agency for Research on Cancer (IARC) has designated aflatoxin as a human liver carcinogen [39,40]. This food poisoning problem is rarely observed in the U.S. in humans but does occasionally occur in animals. The most notable recent case involved the reported death of over 100 dogs in 2006 that had consumed tainted dog feed [41].
To minimize potential exposure to aflatoxins, maximum levels of aflatoxins in many commodities have been set at levels below 20 ppb by most countries [15,42,43]. Regulatory guidelines of the U.S. Food and Drug Administration (FDA) specifically prevent the sale of commodities if contamination by aflatoxins exceeds 20 ppb total aflatoxins for interstate commerce of food and feedstuff and 0.5 ppb aflatoxin M1 in milk. The European Commission has set the limits on groundnuts subject to further processing at 15 ppb for total aflatoxins and 8 ppb for aflatoxin B1, and for nuts and dried fruits subject to further processing at 10 ppb for total aflatoxins and 5 ppb for aflatoxin B1. The aflatoxin standards for cereals, dried fruits, and nuts intended for direct human consumption are even more stringent, and the limit for total aflatoxins is 4 ppb and 2 ppb for aflatoxin B1 [43].
Due to restrictions limiting the trade of contaminated crops, aflatoxin contamination of agricultural commodities is not only a serious food safety concern [10,14,44,45,46,47,48,49,50,51], but it has significant economic implications for the agricultural industry worldwide.
2. Genetics and Molecular Biology of Aflatoxin Biosynthesis
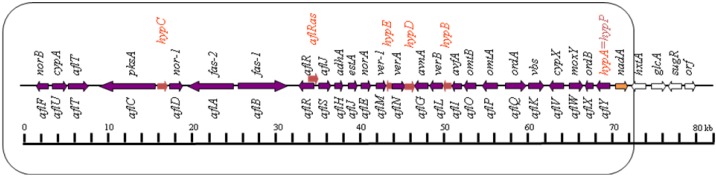
Since the identification of aflatoxins, extensive efforts have been made and expenses incurred worldwide to monitor aflatoxin occurrence and to develop control strategies [52,53,54,55,56,57]. The hallmark discovery of a color mutant that accumulates the brick-red pigment, norsolorinic acid (NOR), in A. parasiticus marked a milestone in the understanding the chemistry of aflatoxin biosynthesis [58,59,60,61]. Since NOR is the earliest and the first stable aflatoxin precursor in the aflatoxin biosynthetic pathway [62,63,64], this discovery led to the identification of other key aflatoxin intermediates and established the early step metabolites in the aflatoxin pathway. It provided the opportunity to isolate the first aflatoxin pathway gene that encoding a reductase for the conversion from NOR to eventually aflatoxins [63,65,66]. After the cloning of several important aflatoxin pathway genes, the aflatoxin pathway gene cluster was discovered in A. parasiticus and A. flavus [67]. The knowledge of the cluster promoted renewed interest in understanding aflatoxin biosynthesis by scientists all over the world. Significant progress has been made in elucidating the biosynthetic pathway, the pathway intermediates, genes, corresponding enzymes, and regulatory mechanisms [44,46,68,69,70,71,72,73,74,75,76,77,78,79,80,81]. As many as 30 genes are potentially involved in aflatoxin biosynthesis (Figure 1). In A. flavus and A. parasiticus the aflatoxin pathway genes are clustered within a 75-kb region of the fungal genome on chromosome III roughly 80 kb away from telomere [67,81,82,83,84,85,86,87].
Figure 1.
Aflatoxin pathway gene cluster in A. flavus. This figure shows the order and location of the 30 aflatoxin pathway genes plus an aflR antisense gene clustered together in about 80 kb DNA region. The old gene names are labeled on top of the line and the new gene names sysmatically renamed according to gene convention are labeled below the line [81]. The transcripts of hypA, hypB, hypC, hypD, hypE and aflRas are identified through Aspergillus flavus EST. Arrows indicate the direction of gene transcription.
2.1. Conversion of Acetate to Norsolorinic Acid (NOR)
The first stable aflatoxin precursor was confirmed to be norsolorinic acid (NOR) [59,60,88] (Figure 2). A hexanoyl starter unit is the initial substrate for aflatoxin formation [65]. Two fatty acid synthases (FAS) and a polyketide synthase (NR-PKS, PksA) are involved in the synthesis of the polyketide from a hexanoyl starter unit. Seven iterative, malonyl-derived ketide extensions are required to produce norsolorinic acid anthrone (noranthrone) [76,83,84,85,89,90,91,92,93,94,95,85,89]. Mahanti et al. [ 96] cloned, by genetic complementation, a 7.5-kb large transcript which is required for NOR formation in a blocked A. parasiticus mutant. Its protein has high degree of similarity (67%) and identity (48%) to the beta-subunit of FASs (FAS1) of Saccharomyces cerevisiae and Yarrowia lipolytica. Metabolite feeding and gene disruption experiments further confirmed that uvm8 encodes a subunit of a novel fatty acid synthase (FAS) directly involved in the backbone formation of the polyketide precursor of NOR during aflatoxin biosynthesis, therefore, on the basis of its function, the uvm8 gene was renamed fas-1A. In the revised naming scheme, the fas-1A gene was renamed as fas-1, it encodes fatty acid synthase-1 in the aflatoxin biosynthetic pathway gene cluster (Figure 1). Another large transcript (fas-2A) which encodes an alpha-subunit of fatty acid synthase in the aflatoxin gene cluster was reported [96]. The gene fas-1A and fas-2A were renamed as fas-1 and fas-2. They encode two fatty acid synthases (FASα and FASβ) [97]. In A. nidulans the involvement of FASs in sterigmatocystin (ST) biosynthesis was also confirmed and were named stcJ and stcK in the ST cluster [90,95]. The biochemical evidence for the role of a fatty acid synthase and a polyketide synthase (PKS) in the biosynthesis of aflatoxin was demonstrated [98]. Further details on the early stage of aflatoxin biosynthesis involving fatty acid synthases and polyketide synthases were reported [89,91,92,99]. The N-acetylcysteamine thioester of hexanoic acid was incorporated into NOR in a fas-1 disrupted transformant. A polyketide synthase gene (pksA) in A. parasiticus was demonstrated by gene disruption to be required for aflatoxin biosynthesis [73]. The predicted amino acid sequences of these PKSs contain the typical four conserved domains commonly found in other known PKS proteins: β-ketoacyl synthase (KS), acyltransferase (AT), acyl carrier protein (ACP), and thioesterase (TE) [73]. Townsend’s group has dissected the functional domains of the PKS for aflatoxin biosynthesis [76,93,100]. These include domains for the starter unit acyl transferase (SAT) which recognizes hexanoyl CoA and the N-acetylcysteamine thioester of hexanoic acid, the acyl carrier protein (ACP), ketosynthase (KS), malonyl-CoA:ACP transacylase (MAT), product template (PT) allowing the iterative steps in forming the polyketide, and a thioesterase/Claisen-like cyclase (TE/CLC) [76]. The predicted product converted by PksA is noranthrone. The conversion of noranthrone to NOR, the first stable intermediate in the pathway [53,60,62,69,101,102,103], is poorly defined, but it has been proposed to be catalyzed by a noranthrone oxidase, a monooxygenase, or to occur spontaneously [64]. Sequence analysis and enzymatic studies support the contention that the hypC (a gene in the intergenic region of pksA and nor-1) gene product is the required noranthrone oxidase involved in the catalysis of the orxidation of norsolorinic acid anthrone to NOR [80] The fas-1, fas-2, and pksA genes were renamed as aflA, aflB, and aflC respectively [81,83,87] (Figure 1). The aflA, aflB and aflC gene homologues in A. nidulans are stcJ, stcK, and stcA, respectively [90].
2.2. Conversion of Norsolorinic Acid (NOR) to Averantin (AVN)
The first stable aflatoxin (AF) intermediate was identified as NOR produced in A. parasiticus uv-generated disruption mutants [60,69,103,104] and in A. flavus [53,102]. The NOR-accumulating mutants are leaky mutants whose aflatoxin biosynthesis is not completely blocked. By genetic complementation, the gene, aflD (nor-1), encoding a reductase was cloned [105]. A recombinant Nor-1 protein expressed in E. coli catalyzed the reduction of NOR. Therefore, aflD (nor-1) encodes the ketoreductase needed for the conversion of the 1'-keto group in NOR to the 1'-hydroxyl group of AVN [106]. Disruption of the aflD (nor-1) gene also confirmed its involvement in conversion of NOR to AVN in aflatoxin biosynthesis [107]. The aflD (nor-1) homologous gene in A. nidulans is stcE [90]. Genes homologous to aflD (nor-1), in the AF cluster, such as aflE (norA) and aflF (norB) are predicted to encode short chain aryl alcohol dehydrogenases. These proteins may also be able to catalyze the reduction of NOR to AVN depending on the reductive environment of the cell and may explain the leakiness of the nor-1 mutation if they are able to complement Nor-1’s function [108].
Figure 2.
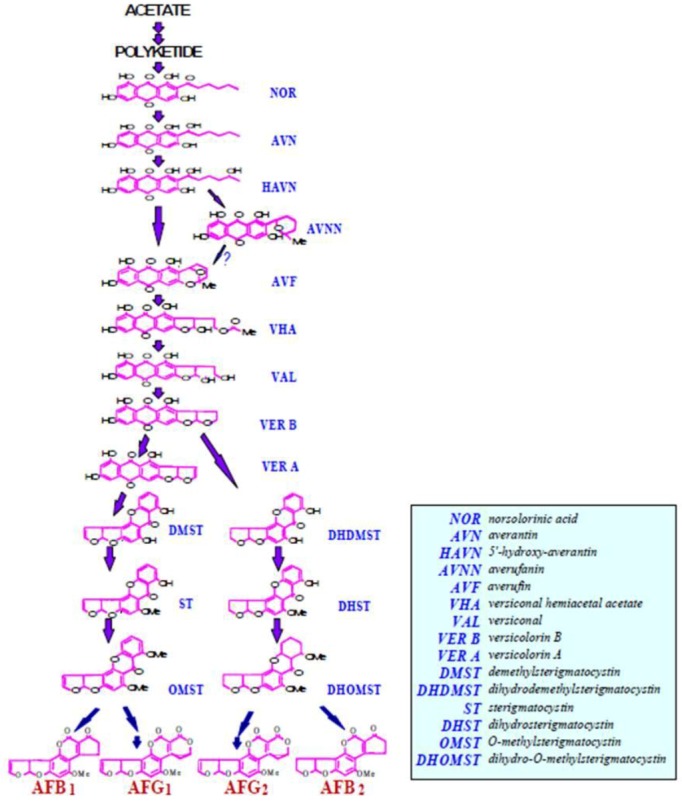
The schematic aflatoxin biosynthetic pathway is presented. The arrows indicate the pathway steps from previous precursor to the next intermediate towards the formation of aflatoxins. The abbreviations are shown on the right.
2.3. Conversion of Averantin (AVN) to 5'-Hydroxyaverantin (HAVN)
Radioisotope incorporation experiments provide the earliest evidence in establishing the conversion of AVN to HAVN [109,110]. There are three enzymatic steps that account for the conversion of NOR to averufin (AVF) [111]: (i) NOR to AVN catalyzed by a reductase, (ii) NOR to HAVN catalyzed by a monooxygenase, and (iii) HAVN to AVF catalyzed by a second dehydrogenase. It was also proposed that the oxidation reactions are reversible and that NADPH was the preferred cofactor [112]. The gene previously named ord-1 encoding a P-450 monooxygenase was cloned and disrupted [113]. Substrate feeding studies of the ord-1 mutant confirmed that HAVN is the intermediate in the conversion of AVN to AVF. The ord-1 gene, which has a high degree of sequence similarity to A. nidulans stcF [90], was renamed aflG (avnA).
2.4. Conversion of 5'-Hydroxyaverantin (HAVN) to Oxoaverantin (OAVN), and Averufin (AVF)
Averufin is one of the key intermediates in aflatoxin formation [103,114,115,116,117,118,119]. Several intermediates were reported to be involved in the conversion from AVN to AVF [70,118]. One of these is averufanin (AVNN). Studies later demonstrated that it is a shunt metabolite and not a genuine aflatoxin intermediate [94,120]. The cluster gene aflH (adhA) in A. parasiticus was characterized to encode an alcohol dehydrogenase [103,118,121]. It was shown that adhA deletion mutants accumulated predominantly HAVN and after prolonged growth, the mutants were able to produce small amounts of AVNN consistant with AVNN being a shunt metabolite. Thus, HAVN might be converted directly to AVF or indirectly to AVF by an additional cytosolic enzyme. Sakuno et al. [120] characterized two cytosolic enzymes and a new aflatoxin intermediate named 5'-oxoaverantin (OAVN) as an intermediate between HAVN and AVF. The enzyme for the conversion from HAVN to OAVN is encoded by the aflH (adhA) gene. The adhA gene deletion mutant is leaky indicating that additional enzyme(s) or gene(s) may be involved in the conversion from OAVN to AVF. The enzymatic steps for aflatoxin biosynthesis and the possible involvement of additional enzymes have also been proposed [80,86,122]. The aflH (adhA) gene in A. flavus and the adhA gene in A. parasiticus share no significant homology at either the DNA or the amino acid level.
2.5. Conversion of Averufin (AVF) to Versiconal Hemiacetal Acetate (VHA)
The conversion of AVF to VHA involves the cytochrome P450 monooxidase, CypX, and another gene, aflI (avfA). Although aflI is required for the conversion, its oxidative role is unclear [123]. A. nidulans also has an aflI gene homolog (stcO) [90,123]. Complementation of an averufin-accumulating mutant, A. parasiticus SRRC 165, with the aflI gene of A. flavus restored the strain’s ability to convert AVF to VHA and to produce aflatoxins [123]. It is likely that the aflI (avfA) encoded protein along with CypX gene product is involved in the ring-closure step in the formation of hydroxyversicolorone. It is possible that the avfA gene product is assocated with the P450 monooxygenase to carry out the conversion as no additional intermediates other that AVF result from the disruption of either gene.
2.6. Conversion of Versiconal Hemiacetal Acetate (VHA) to Versiconal (VHOH, Also Abbreviated as VAL)
It has been demonstrated that an esterase is involvement in the conversion of VHA to VHOH (VAL) [57,111,112,124,125,126,127,128]. The esterase was purified in A. parasiticus [125,126]. An esterase gene, aflJ (estA), in the aflatoxin gene cluster was identified [129]. The homologous gene in the A. nidulans ST biosynthetic gene cluster is stcI. In the A. parasiticus aflJ (estA) deletion mutants, the accumulated metabolites were mainly VHA and versicolorin A (VERA) [130]. A small amount of versiconol acetate (VOAc) and other downstream aflatoxin intermediates, including VHOH and versicolorin B also accumulated. A metabolic grid containing VHA, VOAc, VHOH, and versiconol (VOH) was previously described and it was suggested that the reactions from VHA to VHOH and from VOAc to VOH are catalyzed by the same esterase [111]. Later, another metabolic grid containing versicolorone (VONE), VOAc, and VHA was identified [131]. Indeed, it has now been proven that the estA-encoded esterase catalyzes the conversion of both VHA to VHOH and VOAc to VOH during aflatoxin biosynthesis [130].
2.7. Conversion of Versiconal (VHOH) to Versicolorin B (VER B)
The enzymatic evidence that VHOH is converted toVER B by a cyclase was first provided by Lin and Anderson [132]. This enzyme was identified as versicolorin B synthase and was studied intensively by Townsend’s laboratory [114,133,134,135,136]. The gene was cloned and named vbs [114,134,136]. The expected cyclase activity was demonstrated by the expressed recombinant protein of the vbs gene [134,135]. The VHOH cyclase [132] and VER B synthase [133] were independently isolated from A. parasiticus. The enzyme catalyzes the side chain cyclodehydration of racemic VHA to VER B. This is another key step in aflatoxin formation since it closes the bisfuran ring of aflatoxin, the moiety ultimately responsible for aflatoxin’s toxicity and carcinogenicity. The vbs gene was re-named aflK (vbs) [81]. The homologous gene in the A. nidulans ST biosynthetic gene cluster is stcN.
2.8. Conversion of Versicolorin B (VER B) to Versicolorin A (VER A)
The critical branch point leading to the formation of either AFB1/AFG1 or AFB2/AFG2 is VER B. Similar to AFB2/AFG2, VER B contains a tetrahydrobisfuran ring and, like AFB1/AFG1, VER A contains a dihydrobisfuran ring. The conversion of VER B to VER A requires desaturation of the bisfuran ring of VER B by an unstable microsomal enzyme that requires NADPH [137]. Disruption of stcL in A. nidulans [138] abolished ST synthesis and resulted in the accumulation of VER B. The stcL gene encodes a cytochrome P-450 monooxygenase. The homologue, aflL (verB), is present in the aflatoxin gene cluster of A. parasiticus and A. flavus strains. Cultural conditions appear to markedly affect the activity of VER B desaturase and thereby, the final ratio of AFB1 to AFB2 and AFG1 to AFG2[94].
2.9. Conversion of Versicolorin A (VER A) to Demethylsterigmatocystin (DMST) and Versicolorin B (VER B) to Demethyldihydrosterigmatocystin (DMDHST)
The biochemical conversion steps from VER A to DMST (and VerB to DHDMST) have been described in great detail [139]. The aflM (ver-1) gene [140], cloned by genetic complementation of VER A-accumulating A. parasiticus CS10 (a mutant strain created by knocking out the verA gene in aflatoxin-producing strain A. parasiticus SU-1), was shown to be responsible for the conversion of VER A to an intermediate that has not been isolated. The aflM (ver-1) gene was predicted to encode a ketoreductase, similar Nor-1. The ver-1 homologue, stcU, (previously named verA) was identified in A. nidulans [141]. Double mutation of stcU and stcL resulted in accumulation of only VER A [141]. The stcS gene(previously named verB), another cytochrome P-450 monooxygenase gene, was also identified and studies showed that it is also involved in the conversion of VER A to an intermediate in the formation of DMST (possibly the first intermediate, which is then acted upon by Ver-1). Disruption of stcS resulted in the accumulation of VER A as did disruption of Ver-1 [142]. Thus, both stcU and stcS are required for the conversion of VER A to DMST. The stcS homologue in A. parasiticus, named aflN (verA), has also been identified [81,87]. A third enzyme is required for the conversion: hypA (aflY). This gene is predicted to encode a Baeyer-Villiger monooxygenase. Disruption of this gene also led to accumulation of VERA suggesting that, like VER-1, it acts as part of an enzyme complex without allowing the formation of an intermediate. A fourth enzyme, OrdB has also been implicated in the conversion, and like AvfA, its homolog, may be a helper protein for the monooxygenase, CypX.
2.10. Conversion of Demethylsterigmatocystin (DMST) to Sterigmatocystin (ST) and Dihydrodemethylsterigmatocystin (DHDMST) to Dihydrosterigmatocystin (DHST)
Two O-methyltransferases, (I and II), are confirmed to be involved in aflatoxin biosynthesis [143]. O-methyltransferase I catalyzes the transfer of the methyl from S-adenosylmethionine (SAM) to the hydroxyls of DMST and DHDMST to produce ST and DHST, respectively. This 43-kDa enzyme was purified from A. parasiticus and characterized [144,145]. The corresponding gene, dmtA, was isolated from A. parasiticus based on a partial amino acid sequence of the purified enzyme [146]. Yu et al. [123] concurrently isolated the same gene but named it aflO (omtB) (for O-methyltransferase B) from A. parasiticus, A. flavus and A. sojae. The predicted dmtA-encoded protein contains a consensus SAM-binding motif [146]. The aflO (omtB) homolog in A. nidulans was identified as stcP this gene is required for the conversion of DMST to ST in A. nidulans as shown by gene disruption [147].
2.11. Conversion of Sterigmatocystin (ST) to O-Methylsterigmatocystin (OMST) and Dihydrosterigmatocystin (DHST) to Dihydro-O-methylsterigmatocystin (DHOMST)
The gene for O-methyltransferase required for the conversion of ST to OMST and DHST to DHOMST was first cloned [148] from A. parasiticus by reverse genetics using antibodies raised against the purified A. parasiticus O-methyltransferase A [78]. This gene was initially named omt-1, then omtA and finally renamed aflP (omtA) [148]. The recombinant enzyme was expressed in E. coli and its activity to convert ST to OMST was demonstrated by substrate feeding studies [148]. O-methyltransferase A has strict substrate-specificity and cannot methylate DMST or DHDMST. Thus, the O-methyltransferases A encoded by aflP (omtA) is the enzyme responsible for the conversion of ST to OMST and DHST to DHOMST. The genomic DNA sequence of this gene (omtA) was cloned from A. parasiticus and A. flavus [149]. This aflP (omtA) gene homologue was also detected in other aflatoxigenic and non-aflatoxigenic Aspergillus species [150]. The absence of the aflP orthologue in A. nidulans is the reason that A. nidulans produces ST as the end product instead of aflatoxins.
2.12. Conversion of O-Methylsterigmatocystin (OMST) to Aflatoxin B1 (AFB1) and Aflatoxin G1 (AFG1) and Dihydro-O-methylsterigmatocystin (DHOMST) to Aflatoxin B2 (AFB2) and Aflatoxin G2 (AFG2)
Based on feeding experiments, the relationship between B-group and G-group aflatoxin formation was proposed [151]. A P-450 monooxygenase gene in A. flavus named ord-1 was shown to be necessory for this reaction [152,153]. This P-450 monooxygenase gene, aflQ (ordA), was cloned in A. parasiticus and demonstrated in a yeast system that it is involved in the conversion of OMST to AFB1/AFG1, and DHOMST to AFB2/AFG2 [154]. Whether aflQ (ordA) gene product, OrdA, catalyzes two successive monooxygenase reactions in the later steps of aflatoxin biosynthesis is not clear. Studies [154] suggested that additional enzyme(s) is required for the synthesis of G-group aflatoxins. After the cloning and characterization of the cypA gene, it is clear that cypA encoded a cytochrome P450 monooxygenase for the formation of G-group aflatoxins [155]. Most recently, the nadA gene, which was shown, by gene profiling studies using microarray, to be a member of the aflatoxin gene cluster [156,157] rather than belonging to the adjoining sugar utilization cluster as originally proposed [158], was found to play a role in AFG1/AFG2 formation. Yabe’s group recently disrupted the nadA gene and reported that NadA is a cytosolic enzyme for the conversion from a new aflatoxin intermediate named NADA, which is between OMST and AFG1, to AFG1 [159]. The aflE (norA) gene was initially believed to be involved in the conversion of NOR due to certain degree of sequence similarity to the aflD (nor-1) gene [108]. However, recent studies support the hypothesis that the aflE (norA) is involved in the final two steps in AFB1 formation [80]. In the same report, the transcript, hypB, a homolog of hypC, may be involved in one of the oxidation steps in the conversion of OMST to aflatoxins. A. flavus produces only AFB1 and AFB2, whereas A. parasiticus produces all four major aflatoxins, AFB1, AFB2, AFG1, and AFG2. Coincidently, only the G-group aflatoxin producer, A. parasiticus, has intact nadA and norB genes. Preliminary data suggests that norB encodes another enzyme predominantly involved in AFG1/AFG2 formation [160].
2.13. Aflatoxins M1 and M2
The aflatoxin M1 and M2 are mammalian bioconversion products of AFB1 and AFB2 respectively and are originally isolated and identified from bovine milk [161,162,163,164,165]. After entering the mammaliam body (human or animals), aflatoxins are metabolized by the liver cytochrome P450 enzymes to a reactive epoxide intermediate which becomes more carcinogenic, or be hydroxylated and become the less harmful aflatoxins M1 and M2. However, recent studies by feeding with aspertoxin (12c-hydroxy-OMST) [166] indicated that A. parasiticus produces the minor aflatoxins M1 (AFM1), M2 (AFM2), GM1 (AFGM1), and GM2 (AFGM2), as well as the major aflatoxins B1 (AFB1), B2 (AFB2), G1 (AFG1), and G2 (AFG2). It demonstrated that aspertoxin is a precursor of AFM1 and AFGM1. Feeding of the same fungus with O-methylsterigmatocystin (OMST), AFM1 and AFGM1 were formed together with AFB1 and AFG1; feeding with dihydro-OMST (DHOMST), AFM2 and AFGM2 were formed together with AFB2 and AFG2. It showed that the enzyme OrdA catalyzes both 12c-hydroxylation reaction from OMST to aspertoxin and the successive reaction from aspertoxin to AFM1 and the AFB1 is not a precursor of AFM1.
3. Genetic Regulation of Aflatoxin Biosynthesis
The aflatoxin pathway genes organized in cluster in the genome of A. flavus and A. parasiticus [67,81,87,167] are expressed concurrently. The positive-acting regulatory gene, aflR, is located in the middle of the gene cluster. Adjacent to aflR a divergently transcribed gene, aflS (aflJ), was found to be involved in the regulation of transcription [71,168]. Other physically unrelated genes, such as laeA and veA, have been shown to exhibit a “global” regulatory role on aflatoxin biosynthesis [169,170,171,172].
3.1. Genetic Control by aflR Gene, Encoding the Pathway-Specific Transcription Factor, AflR
A 47 kDa sequence-specific zinc-finger DNA-binding protein (AflR), encoded by the aflR gene, is required for transcriptional activation of most, if not all, the structural genes of the aflatoxin gene cluster [72,74,173,174,175,176,177,178,179,180]. Like other Gal4-type regulatory proteins that bind to palindromic sequences, functional AflR probably binds as a dimer. It binds to the palindromic sequence 5'-TCGN5CGR-3' in the promoter regions of the structural genes [77,181]. The AflR-binding motifs are found to be located from −80 to −600 positions, with the majority at the −100 to −200 positions, relative to the translation start site. AflR binds, in some cases, to a deviated sequence rather than the typical motif such as in the case of aflG (avnA). When there is more than one binding motif, only one of them is the preferred binding site such as in the case of aflC (pksA) [77,181]. The more upstream motif is found to belong to another gene for turning on the expression of hypC. Deletion of aflR in A. parasiticus abolishes the expression of other aflatoxin pathway genes [182]. Overexpression of aflR in A. flavus up-regulates aflatoxin pathway gene transcription and aflatoxin accumulation [176] in a fashion similar to that reported for A. parasiticus [173]. These results demonstrate that AflR is specifically involved in the regulation of aflatoxin biosynthesis. Indeed, all 23 upregulated genes, identified by transcription profiling using DNA microarray assays comparing wild-type and aflR-deleted A. parasiticus strains, have the consensus AflR binding motif in their promoter regions [156,168,183,184].
3.2. Genetic Control by aflS (aflJ) Gene, Encoding a Putative Transcriptional Co-activator, AflS
The aflS (aflJ) gene [168,185], bidirectionally transcribed from aflR, although not demonstrating significant homology with any other encoded proteins found in databases, is necessary for aflatoxin formation. The aflS and aflR share a 737 bp intergenic region. In the A. parasiticus aflR transformants, the production of aflatoxin pathway intermediates was significantly enhanced in transformants that contained an additional aflR plus aflS [173]. Quantitative PCR showed that in the aflS knockout mutants, the lack of aflS transcript is associated with 5- to 20-fold reduction of expression of some aflatoxin pathway genes such as aflC (pksA), aflD (nor-1), aflM (ver-1), and aflP (omtA). The mutants lost the ability to synthesize aflatoxin intermediates and no aflatoxins were produced [168]. However, deletion of aflS (aflJ) did not have a discernible effect on aflR transcription, and vice versa. Du et al. [186] showed that overexpression of A. flavus aflS (aflJ) did not result in elevated transcription of aflM (ver-1), aflP (omtA), or aflR, but it appears to have some effect on aflC (pksA), aflD (nor-1), aflA (fas-1), and aflB (fas-2) [186], which are required for the biosynthesis of the early aflatoxin pathway intermediates. The mechanism(s) by which aflS modulates transcription of these pathway genes in concert with aflR is under investigation.
3.3. Genetic Control on Secondary Metabolism by laeA Gene, Encoding a Global Regulator, LaeA
The novel global regulatory gene, laeA (for lack of aflR expression), was first identified from A. nidulans [169]. This gene is well conserved in fungi as shown by its presence in the genomes of all fungi so far sequenced. LaeA is a nuclear protein which contains an S-adenosylmethionine (SAM) binding motif and activates transcription of several other secondary metabolism gene clusters in addition to the AF cluster. Examples include the sterigmatocystin and penicillin clusters in A. nidulans, the gliotoxin cluster in A. fumigatus, and aflatoxin cluster in A. flavus [169,187]. It also regulates genes required for virulence of A. fumigatus [188]. Perrin et al. [170] carried out a whole-genome comparison of the transcriptional profiles of wild-type and laeA-deleted A. fumigatus strains and found that LaeA positively controls the expression of 20% to 40% of major classes of secondary metabolite biosynthesis genes. It also regulates some genes not associated with secondary metabolite clusters. Similar results were confirmed in gene expression profiling in A. flavus using microarrays to study the genetic mechanism of sclerotium formation. The exact mechanism of how LaeA regulates secondary metabolism gene clusters is not yet known. Interestingly, when an unrelated gene such as argB was placed within the boundary of the ST gene cluster, it was co-regulated with other genes in the cluster. But, when a gene in the cluster, such as aflR was placed elsewhere in the genome, its regulation was not affected by LaeA [189]. One proposed regulatory mechanism is that LaeA differentially methylates histone protein and it alters the chromatin structure for gene expression. Unlike the mentioned signaling factors, the primary role of LaeA is to regulate metabolic gene clusters, not sporulation, based on the obesevation that laeA-deleted strains produced conidia equivalent to wild-type level [169]. Most recent analyses of nonaflatoxigenic A. parasiticus sec- (for secondary metabolism negative) variants generated through serial transfer of mycelia of the sec+ parents show that laeA was expressed in both sec+ and sec- strains [190]. This result suggests that LaeA only exerts its effect on aflatoxin biosynthesis at a certain level and is independent of other regulatory pathways that are involved in fungal development.
3.4. Genetic Control on Fungal Development and Mycotoxin Formation by veA Gene, Encoding a Regulator, VeA
The veA gene in A. nidulans [191] is a gene initially found to be crucial for light-dependent conidiation. A comparison of the light effect on sterigmatocystin production by A. nidulans veA+ and veA1 strains showed that both strains produced sterigmatocystin but the highest amount was produced by the veA+ strain grown in darkness. However, veA-deleted A. flavus and A. parasiticus strains completely lost the ability to produce aflatoxin regardless of the illumination conditions [192,193]. Under normal growth conditions, some A. flavus and all A. parasiticus strains produce conidia in both dark and light conditions. Stinnett et al. [193] showed that VeA contains a bipartite nuclear localization signal (NLS) motif and its migration to the nucleus is light-dependent and requires the importin α carrier protein. In the dark VeA is located mainly in the nucleus; under light it is located both in cytoplasm and nucleus. VeA has no recognizable DNA-binding seuqences and likely exerts its effect on sterigmatosyctin and aflatoxin production through protein-protein interactions with other regulatory factors. Post-translational modifications such as phosphylation and dephosphorylation may modulate its activity. Lack of VeA production in the veA-deleted A. flavus and A. parasiticus strains consequently abolishes aflatoxin production because a threshold concentration of nuclear VeA might be necessary to initiate aflatoxin biosynthesis.
4. Factors that Affect Aflatoxin Biosynthesis
Many biotic and abiotic environmental factors influence aflatoxin biosynthesis [68,194,195,196,197] including nutritional factors such as carbon and nitrogen source; environmental effects such as water activity and temperature; physiological conditions such as pH [7] and bioreactive agents [198,199]. These studies would offer promise of devising control strategies to shut down aflatoxin production in aflatoxigenic A. flavus species through manipulations of environmental conditions for the fungal response to these factors.
4.1. Carbon
Carbon, nitrogen, amino acid, lipid, trace elements and other nutritional factors have long been observed to affect aflatoxin production [198,200,201]. The best-known nutritional factors affecting aflatoxin biosynthesis are carbon and nitrogen sources [202,203,204]. The relationship of carbon source and aflatoxin formation has been well established. Simple sugars such as glucose, sucrose, fructose, and maltose support aflatoxin formation, while peptone, sorbose, or lactose does not [198,205]. Woloshuk et al. reported the connection between alpha amylase activity and aflatoxin production in A. flavus [206]. Yu et al. identified a gene cluster related to sugar utilization in A. parasiticus next to the aflatoxin gene cluster [158]. A close physical linkage between the two gene clusters could point to a relationship between the clusters in the processing of carbohydrates leading to the induction of aflatoxin biosynthesis. Lipid substrate is a good carbon source to support aflatoxin production [207,208,209]. A lipase gene, lipA, was cloned in A. parasiticus and A. flavus [210]. The expression of lipA and subsequent aflatoxin production are induced by lipid substrate. The addition of 0.5% soybean oil to non-aflatoxin-conducive peptone medium induces lipase gene expression and leads to aflatoxin formation [210]. However, the molecular mechanism by which a carbon source is involved in the regulation of aflatoxin pathway gene expression is to be further investigated.
4.2. Nitrogen
Nitrogen is closely linked to aflatoxin production [198]. Asparagine, aspartate, alanine, ammonium nitrate, ammonium nitrite, ammonium sulfate, glutamate, glutamine, and proline containing media support aflatoxin production; while sodium nitrate and sodium nitrite containing media do not [211,212,213]. It was also suggested that nitrate represses averufin and aflatoxin formation [214,215]. Nitrate was reported to have a suppressive effect on aflatoxin production, and overexpression of aflR gene by additional copies of aflR overcomes the negative regulatory effect on aflatoxin pathway gene transcription [173]. Nitrogen utilization genes and a nitrogen regulator gene, areA from A. parasiticus were cloned [216,217]. In the intergenic region between aflR and aflS (aflJ), several AreA binding motifs have been identified [216,217]. The AreA binding could prevent AflR binding. Certain amino acids can have different effects on aflatoxin production. Recent studies show that tryptophan inhibits aflatoxin formation while tyrosine enhances aflatoxin production in A. flavus [183].
4.3. Temperature
Aflatoxin formation is directly affected by temperature [218,219,220,221]. Optimal aflatoxin production is observed at temperatures near 30 °C (28 °C to 35 °C) [221]. When temperature increases to above 36 °C, aflatoxin production is nearly completely inhibited. Genome wide gene profiling using microarray and RT-PCR verification [221] indicated that high temperature is associated with a decrease in the expression of the aflatoxin pathway genes. RT-PCR detected ample amount of transcripts of both regulatory genes aflR and aflS (aflJ) [221]. So it was hypothesized that temperature may affect the activity of AflR or some other unknown regulatory element. High resolution studies using next generation sequencing technologies on aflatoxin pathway gene expression in response to temperature clearly showed that temperature affects the level of aflR and aflS transcripts [222]. High temperature affects aflS more than aflR. Change in the ratio of aflS to aflR renders aflR unfunctional for transcription activation.
4.4. Water Activity
Severe aflatoxin outbreaks in corn have been documented to occur under hot weather and drought conditions [223,224]. The mechanism of A. flavus infestation in corn under these conditions is not well understood. The possible scenarios may include a combination of these factors: (a) the plant defense mechanism is weakened under water stress conditions; (b) higher insect feeding and associated injuries to plant tissues, thus providing entry opportunities for fungal invasion; and (c) more fungal spores dispersed into the air under dry climate.
4.5. Culture pH
Aflatoxin biosynthesis in A. flavus occurs in acidic media, but is inhibited in alkaline media [7]. The pacC gene is a major transcriptional regulatory factor for pH homeostasis [225]. In the aflR promoter region, at least one PacC binding site has been identified [77,181]. The presence of a putative PacC-binding site close to the aflR transcription start site may play some role in pH regulation on aflatoxin production [225,226]. In the non-aflatoxin-conducive peptone medium, this site was shown to be inhibitory to aflatoxin formation [7]. The regulatory mechanism might be due to the binding of pacC to this site at alkaline conditions to repress the transcription of acid-expressed gene aflR and thus aflatoxin formation [227,228]. The PacC and AreA [217] binding sites in the aflR-aflS (aflJ) intergenic region suggest that gene expression is regulated by environmental signals (pH and nitrate).
4.6. Developmental Stage
Sporulation and sclerotial formation are associated with secondary metabolism [6,8,229,230]. Spore formation and secondary metabolite formation occur at about the same time [85,229]. Some mutants that are deficient in sporulation are unable to produce aflatoxins [68] and some compounds that inhibit sporulation in A. parasiticus also inhibit aflatoxin formation [231]. The aflatoxin-producing ability was gradually decreased in response to a series subculturing. The changes in aflatoxin production ability were accompanied by marked morphological changes [232].
Moreover, chemicals that inhibit polyamine biosynthesis in A. parasiticus and A. nidulans inhibit both sporulation and aflatoxin/ST biosynthesis [233]. A more recent finding reveals that the regulation of sporulation and ST production is through a shared G-protein mediated signaling pathway in A. nidulans [229,234]. Mutations in A. nidulans flbA and fadA genes, early acting members of a G-protein signal transduction pathway, result in loss of ST gene expression, ST production, and sporulation [179,229,235]. The regulation is partially mediated through protein kinase A [179,235]. This G-protein signaling pathway involving FadA in the regulation of aflatoxin production also exists in A. parasiticus and A. flavus [229].
4.7. Oxidative Stress
Oxidative stress and aflatoxin biosynthesis are related in A. parasiticus [236,237,238,239]. Oxidative stress induces aflatoxin formation in A. parasiticus [240]. Treatment of A. flavus with tert-butyl hydroperoxide, or gallic acid induced significant increases in aflatoxin production [238]. Similar treatment of A. parasiticus also induced aflatoxin production [239,241]. Hydrolysable tannins significantly inhibit aflatoxin biosynthesis, with the main anti-aflatoxigenic constituents in these tannins being gallic acid [237]. Gallic acid reduces expression of structural genes within the aflatoxin biosynthetic cluster, but surprisingly not the aflatoxin pathway gene regulator, aflR. It appears that gallic acid disrupts signal transduction pathway(s) for aflatoxigenesis. When certain phenolics or other antioxidants, such as ascorbic acid, are added to oxidatively stressed A. flavus, aflatoxin production significantly declines, with no effect on fungal growth [238]. Caffeic acid is another antioxidant that inhibits aflatoxigenesis. Microarray analysis of A. flavus treated with caffeic acid identified a gene, named ahpC2,an alkyl hydroperoxide reductase that is potentially involved in quelling the signal for aflatoxin production. However, no notable effect on expression of laeA, a gene encoding a global regulator for secondary metabolism in Aspergillus [169] was observed when under caffeic acid treatment.
4.8. Plant Metabolites
Plant metabolites play some role on aflatoxin formation [242,243,244,245]. Wright et al. reported that, at certain conditions, n-decyl aldehyde reduces not only fungal growth of A. parasiticus but also aflatoxin production by over 95% compared with control [246]. Octanal reduces fungal growth by 60%, however, it increases aflatoxin production by 500%, while hexanal reduces fungal growth by 50%, but it has no effect on aflatoxin production. The 13(S)-hydroperoxide derivative of linoleic acid, the reaction product of lipoxygenase (encoded by L2 LOX gene from maize), is reported to reduce aflatoxin production [247]. Therefore, besides routine detection and screening, management of aflatoxin contamination in commodities must include pre-harvest as well as post-harvest control measures.
5. Future Perspective in Control of Aflatoxin Contamination
The economic implications of aflatoxins and their potential health threat to humans have clearly shown a need to eliminate or at least minimize its contamination in food and feedstuff. Efforts include monitoring, managing and controlling their levels in agricultural products from farm to market and from preharvest to post-harvest.
5.1. Detection and Screening
Surveillance programs have been established to reduce the risk of aflatoxin consumption in humans and animals. Analytical testing methods for rapid detection of a large number of samples of food stuffs have been developed [83]. Current analytical techniques for accurate characterization and quantitation of aflatoxins include thin layer chromatography (TLC), high pressure liquid chromatography (HPLC) and gas chromatography (GC). Rapid immuno-assay (RIA) and serum assay (ELISA) formats based on sera developed for major aflatoxins [83,248,249] are also available for rapid detection of aflatoxins at levels as low as one tenth of a nanogram per milliliter [250]. A dozen commercial test kits have been developed for field-testing of various agricultural commodities.
5.2. Preharvest Control
Aflatoxin contamination can be reduced somewhat by appropriate cultural practices such as irrigation, control of insect pests etc prior to harvest. In some cases, changes in cultural practices to minimize aflatoxin contamination are not feasible. For example, inclusion of extra irrigation regimes in desert cotton fields is not feasible because it would add significant cost to the grower even if they were available. However, effective control of aflatoxin contamination is expected to be dependent on a detailed understanding of the physiological and environmental factors that affect aflatoxin biosynthesis, the biology and ecology of the fungus, and the parameters of the host plant-fungal interactions. Efforts are underway to study these parameters, primarily by functional genomics approaches [251,252].
5.3. Postharvest Control
Aflatoxin contamination during storage after harvest is prevalent in most tropical countries due to hot, wet climates coupled with sub-adequate methods of harvesting, handling and storage practices, which often lead to severe fungal growth and thus contamination of food and feed [253,254]. Significant emphasis has been placed on improving storage conditions that are less favorable for fungal growth. Detoxification of aflatoxin contaminated grains to reduce aflatoxin to an acceptable level is another strategy in corn and peanuts [255].
5.4. Biological Control
Application of nonaflatoxigenic, biocompetitive, native A. flavus strains to outcompete toxigenic isolates in the fields has been effective in significantly reducing aflatoxins contamination [256]. Biocompetition may be most feasible in crops such as cotton where resistance against fungal infections is not available due to limited genetic diversity in the cotton germplasm. In the long term, however, significant control of the aflatoxin problem will likely be linked to introduction of resistant germplasms, which are resistant either to fungal invasion or toxin production or both. Naturally resistant germplasms are available and the identification of specific biochemical factors linked to resistance against A. flavus will assist in enhancing the observed resistance levels in the existing germplasms. Identification of a novel pool of germplasm that demonstrates the desired characteristics is also critical to the success of the current marker-assistance breeding programs. However, because the aflatoxin contamination is a complex problem and a combination of approaches will be required to control the preharvest contamination.
5.5. Breeding for Commercial Crops that Are Resistant to Fungal Growth and/or Aflatoxin Formation
The most economic and effective strategy for reducing and eliminating aflatocin contamination is to breed commercial crop varieties that show resistance to fungal infection and/or aflatoxin formation. Significant efforts are made in plant breeding programs in identifying resistance or tolerance to aflatoxin contamination in preharvest crops. Unfortunately, no highly resistant varieties or germplasm lines have been identified for the major crops such as corn, cotton, and peanut. Some low to medium resistant lines in corn are under testing and development [257,258]. Progress has been made in identifying genes in corn that shows resistance to aflatoxin producing fungus [259].
6. Conclusions
Scientists worldwide have extensively studied biosynthesis of aflatoxins for more than 50 years. The more we learn concerning the mechanisms of aflatoxin biosynthesis, the more we need to investigate its regulatory mechanisms. Regulation of aflatoxin gene expression occurs at multiple levels and by multiple regulatory components. There are genetic factors, biotic and abiotic elements that affect aflatoxin formation. Studies revealed the functions of the enzymes involved in each of the steps of aflatoxin biosynthesis, the genes encoding those enzymes, and the regulatory mechanisms of aflatoxin formation. With the rapid progress in fungal genomics, we will master a vast amount of new information on gene function, genetic regulation and signal transduction within this fungal system, as well as its interactions with the environment. It is now time to supplant the classic gene cloning strategy with the cutting-edge whole genome approach including the Next Generation Sequencing technologies. The genetic and genomic resources will significantly enhance our understanding of the mechanisms of aflatoxin production, pathogenicity of the fungus, and crop-fungus interactions. Better understanding of the mechanisms of gene regulation on aflatoxin biosynthesis will help us to identify natural inhibitors of fungal growth and aflatoxin formation. Eventually, we will be able to design effective and novel strategies to eliminate aflatoxin contamination for a safer, nutritious and sustainable food and feed supply.
References
- 1.Brakhage A.A., Schuemann J., Bergmann S., Scherlach K., Schroeckh V., Hertweck C. Activation of fungal silent gene clusters: A new avenue to drug discovery. Prog. Drug Res. 2008;66:3–12. doi: 10.1007/978-3-7643-8595-8_1. [DOI] [PubMed] [Google Scholar]
- 2.Hoffmeister D., Keller N.P. Natural products of filamentous fungi: Enzymes, genes, and their regulatio. Nat. Prod. Rep. 2007;24:393–416. doi: 10.1039/b603084j. [DOI] [PubMed] [Google Scholar]
- 3.Keller N.P., Turner G., Bennett J.W. Fungal secondary metabolism—From biochemistry to genomics. Nat. Rev. Microbiol. 2005;3:937–947. doi: 10.1038/nrmicro1286. [DOI] [PubMed] [Google Scholar]
- 4.Sweeney M.J., Dobson A.D. Mycotoxin production by Aspergillus, Fusarium and Penicillium species. Int. J. Food Microbiol. 1998;43:141–158. doi: 10.1016/S0168-1605(98)00112-3. [DOI] [PubMed] [Google Scholar]
- 5.Klich M.A. Soil fungi of some low-altitude desert cotton fields and ability of their extracts to inhibit Aspergillus flavus. Mycopathologia. 1998;142:97–100. doi: 10.1023/A:1006989712282. [DOI] [PubMed] [Google Scholar]
- 6.Bennett J.W., Leong P.M., Kruger S.J., Keyes D. Sclerotial and low aflatoxigenic morphological variants from haploid and diploid Aspergillus parasiticus. Experientia. 1986;42:848–851. doi: 10.1007/BF01941550. [DOI] [Google Scholar]
- 7.Cotty P. Aflatoxin and sclerotial production by Aspergillus flavus: Influence of pH. Phytopathol. 1988;78:1250–1253. doi: 10.1094/Phyto-78-1250. [DOI] [Google Scholar]
- 8.Chang P.K., Bennett J.W., Cotty P.J. Association of aflatoxin biosynthesis and sclerotial development in Aspergillus parasiticus. Mycopathologia. 2002;153:41–48. doi: 10.1023/A:1015211915310. [DOI] [PubMed] [Google Scholar]
- 9.St Leger R.J., Screen S.E., Shams-Pirzadeh B. Lack of host specialization in Aspergillus flavus. Appl. Environ. Microbiol. 2000;66:320–324. doi: 10.1128/AEM.66.1.320-324.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richard J.L., Payne G.A. Mycotoxins: Risks in Plant, Animal and Human Systems. Council for Agricultural Science and Technology (CAST); Ames, IA, USA: 2003. [Google Scholar]
- 11.Robens J.F., Cardwell K. The costs of mycotoxin management to the USA: Management of aflatoxins in the United States. J. Toxicol. 2003;22:139–152. [Google Scholar]
- 12.Robens J.F., Cardwell K. The Cost of Mycotoxin Management in the United States. In: Abbas H.K., editor. Aflatoxin and Food Safety. CRC Press; Boca Raton, FL, USA: 2005. pp. 1–12. [Google Scholar]
- 13.Allcroft R., Carnaghan R.B.A., Sargeant K., O’Kelly J. A toxic factor in Brazilian groundnut meal. Vet. Rec. 1961;73:428–429. [Google Scholar]
- 14.Lancaster M.D., Jenkins F.P., Philip J.M. Toxicity associated with certain samples of ground nuts. Nature. 1961;192:1095–1096. [Google Scholar]
- 15.Van Egmond H.P. Current situation on regulations for mycotoxins. Overview of tolerances and status of standard methods of sampling and analysis. J. Food Addit. Contam. 1989;6:139–188. doi: 10.1080/02652038909373773. [DOI] [PubMed] [Google Scholar]
- 16.Goto T., Wicklow D.T., Ito Y. Aflatoxin and cyclopiazonic acid production by a sclerotium-producing Aspergillus tamarii strain. Appl. Environ. Microbiol. 1996;62:4036–4038. doi: 10.1128/aem.62.11.4036-4038.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eaton D., Gallagher E. Mechanisms of aflatoxin carcinogenesis. Annu. Rev. Pharmacol. Toxicol. 1994;34:135–172. doi: 10.1146/annurev.pa.34.040194.001031. [DOI] [PubMed] [Google Scholar]
- 18.Hsieh D.P.H. Potential Human Health Hazards of Mycotoxins. In: Natori S., Hashimoto H., Ueno Y., editors. Mycotoxins and Phycotoxins. Elsevier; Amsterdam, The Netherlands: 1989. pp. 69–80. [Google Scholar]
- 19.Ngindu A., Johnson B.K., Kenya P.R., Ngira J.A., Ocheng D.M., Nandwa H., Omondi T.N., Jansen A.J., Ngare W., Kaviti J.N., et al. Outbreak of acute hepatitis caused by aflatoxin poisoning in Kenya. Lancet. 1982;1:1346–1348. doi: 10.1016/s0140-6736(82)92411-4. [DOI] [PubMed] [Google Scholar]
- 20.Lewis L., Onsongo M., Njapau H., Schurz-Rogers H., Luber G., Kieszak S., Nyamongo J., Backer L., Dahiye A.M., Misore A., et al. Aflatoxin contamination of commercial maize products during an outbreak of acute aflatoxicosis in eastern and central Kenya. Environ. Health Perspect. 2005;113:1763–1767. doi: 10.1289/ehp.7998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baertschi S.W., Raney K.D., Shimada T., Harris T.M., Guengerich F.P. Comparison rates of enzymatic oxidation of aflatoxin B1, aflatoxin G1, and sterigmatocystin and activities of the epoxides in forming guanyl-N adducts and inducing different genetic responses. Chem. Res. Toxicol. 1989;2:114–122. doi: 10.1021/tx00008a008. [DOI] [PubMed] [Google Scholar]
- 22.Bressac B., Kew M., Wands J., Ozturk M. Selective G to T mutations of p53 gene in hepatocellular carcinoma from southern Africa. Nature. 1991;350:429–431. doi: 10.1038/350429a0. [DOI] [PubMed] [Google Scholar]
- 23.Coursaget P., Depril N., Chabaud M., Nandi R., Mayelo V., LeCann P., Yvonnet B. High prevalence of mutations at codon 249 of the p53 gene in hyptocellular carcinomas from Senegal. Br. J. Cancer. 1993;67:1395–1397. doi: 10.1038/bjc.1993.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsu I.C., Metcalf R.A., Sun T., Welsh J.A., Wang N.J., Harris C.C. Mutational hotspot in the p53 gene in human hepatocellular carcinomas. Nature. 1991;350:427–428. doi: 10.1038/350427a0. [DOI] [PubMed] [Google Scholar]
- 25.Ozturk M. p53 mutation in hepatocellular carcinoma after aflatoxin exposure. Lancet. 1991;338:1356–1359. doi: 10.1016/0140-6736(91)92236-u. [DOI] [PubMed] [Google Scholar]
- 26.Busby W.F., Jr., Wogan G.N. Aflatoxins. In: Shank R.C., editor. Mycotoxins and N-Nitrosocompounds: Environmental Risks. Vol. 2. CRC Press; Boca Raton, FL, USA: 1981. pp. 3–45. [Google Scholar]
- 27.Groopman J.D., Wogan G.N., Roebuck B.D., Kensler T.W. Molecular biomarkers for aflatoxins and their application to human cancer prevention. Cancer Res. 1994;54:190–191. [PubMed] [Google Scholar]
- 28.Raisuddin S., Singh K.P., Zaidi S.I., Paul B.N., Ray P.K. Immunosuppressive effects of aflatoxin in growing rats. Mycopathologia. 1993;124:189–194. doi: 10.1007/BF01103737. [DOI] [PubMed] [Google Scholar]
- 29.Kelly J.D., Eaton D.L., Guengerich F.P., Coulombe R.A., Jr. Aflatoxin B1 activation in human lung. Toxicol. Appl. Pharmacol. 1997;144:88–95. doi: 10.1006/taap.1997.8117. [DOI] [PubMed] [Google Scholar]
- 30.Wild C.P., Shrestha S.M., Anwar W.A., Montesano R. Field studies of aflatoxin exposure, metabolism and induction of genetic alterations in relation to HBV infection and hepatocellular carcinoma in the Gambia and Thailand. Toxicol. Lett. 1992;64-65:455–461. doi: 10.1016/0378-4274(92)90219-A. [DOI] [PubMed] [Google Scholar]
- 31.Peers F., Bosch X., Kaldor J., Linsell A., Pluijmen M. Aflatoxin exposure, hepatitis B virus infection and liver cancer in Swaziland. Int. J.Cancer. 1987;39:545–553. doi: 10.1002/ijc.2910390502. [DOI] [PubMed] [Google Scholar]
- 32.McGlynn K.A., Hunter K., LeVoyer T., Roush J., Wise P., Michielli R.A., Shen F.M., Evans A.A., London W.T., Buetow K.H. Susceptibility to aflatoxin B1-related primary hepatocellular carcinoma in mice and humans. Cancer Res. 2003;63:4594–4601. [PubMed] [Google Scholar]
- 33.Arsura M., Cavin L.G. Nuclear factor-kappaB and liver carcinogenesis. Cancer Lett. 2005;229:157–169. doi: 10.1016/j.canlet.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 34.Chen C.J., Wang L.Y., Lu S.N., Wu M.H., You S.L., Zhang Y.J., Wang L.W., Santella R.M. Elevated aflatoxin exposure and increased risk of hepatocellular carcinoma. Hepatology. 1996;24:38–42. doi: 10.1002/hep.510240108. [DOI] [PubMed] [Google Scholar]
- 35.Chen C.J., Yu M.W., Liaw Y.F., Wang L.W., Chiamprasert S., Matin F., Hirvonen A., Bell D.A., Santella R.M. Chronic hepatitis B carriers with null genotypes of glutathione S transferase MI and TI polymorphisms who are exposed to aflatoxin are at increased risk of hepatocellular carcinoma. Am. J. Human Genet. 1996;59:128–134. [PMC free article] [PubMed] [Google Scholar]
- 36.Williams J.H., Phillips T.D., Jolly P.E., Stiles J.K., Jolly C.M., Aggarwal D. Human aflatoxicosis in developing countries: A review of toxicology, exposure, potential health consequences, and interventions. Am. J. Clin. Nutr. 2004;80:1106–1122. doi: 10.1093/ajcn/80.5.1106. [DOI] [PubMed] [Google Scholar]
- 37.Krishnamachari K.A., Bhat R.V., Nagarajan V., Tilak T.B. Hepatitis due to aflatoxicosis: An outbreak of hepatitis in parts of western India. Lancet. 1975;1:1061–1063. doi: 10.1016/s0140-6736(75)91829-2. [DOI] [PubMed] [Google Scholar]
- 38.Fung F., Clark R.F. Health effects of mycotoxins: A toxicological overview. J. Toxicol. Clin. Toxicol. 2004;42:217–234. doi: 10.1081/CLT-120030947. [DOI] [PubMed] [Google Scholar]
- 39.Wogan G.N. Aflatoxins as risk factors for hepatocellular carcinoma in humans. Cancer Res. 1992;52:2114–2118. [PubMed] [Google Scholar]
- 40.Wogan G.N. Impacts of chemicals on liver cancer risk. Semin. Cancer Biol. 2000;10:201–210. doi: 10.1006/scbi.2000.0320. [DOI] [PubMed] [Google Scholar]
- 41.The US Food and Drug Administration. Toxic pet food may have killed dozens of dogs. [(accessed on 10 May 2006)]. Available online: http://www.msnbc.msn.com/id/10771943/ns/health-pet_health/t/toxic-pet-food-may-have-killed-dozens-dogs/
- 42.Van Egmond H.P., Schothorst R.C., Jonker M.A. Regulations relating to mycotoxins in food: Perspectives in a global and European context. Anal. Bioanal. Chem. 2007;389:147–157. doi: 10.1007/s00216-007-1317-9. [DOI] [PubMed] [Google Scholar]
- 43.Van Egmond H.P., Jonker M.A. In: Worldwide Regulations on Aflatoxins. Abbas H.K., editor. CRC Press; Boca Raton, FL, USA: 2005. pp. 77–93. [Google Scholar]
- 44.Bennett J.W., Klich M. Mycotoxins. Clin. Microbiol. Rev. 2003;16:497–516. doi: 10.1128/CMR.16.3.497-516.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bennett J.W. Mycotoxins, mycotoxicoses, mycotoxicology and mycopathologia. Mycopathologia. 1987;100:3–5. doi: 10.1007/BF00769561. [DOI] [PubMed] [Google Scholar]
- 46.Bennett J.W., Lee L.S. Mycotoxins—Their biosynthesis in fungi: Aflatoxins and other bisfuranoids. J. Food Protection. 1979;42:805–809. doi: 10.4315/0362-028X-42.10.805. [DOI] [PubMed] [Google Scholar]
- 47.Bhatnagar D., Brown R., Ehrlich K., Cleveland T.E. Mycotoxins Contaminating Cereal Grain Crops: Their Occurrence and Toxicity. In: Khachatourians G.G., Arora D.K., editors. Applied Mycology and Biotechnology. Vol. 2. Elsevier; New York, NY, USA: 2002. pp. 171–196. [Google Scholar]
- 48.Cleveland T.E., Bhatnagar D. Molecular Strategies for Reducing Aflatoxin Levels in Crops Before Harvest. In: Bhatnagar D., Cleveland T.E., editors. Molecular Approaches to Improving Food Quality and Safety. Van Nostrand Reinhold; New York, NY, USA: 1992. pp. 205–228. [Google Scholar]
- 49.Eaton D.L., Groopman J.D. The Toxicology of Aflatoxins: Human Health, Veterinary, and Agricultural Significance. Academic Press; San Diego, CA, USA: 1994. [Google Scholar]
- 50.Hall A.J., Wild C.P. Epidemiology of Aflatoxin-Related Disease. In: Eaton D.L., Groopman J.D., editors. The Toxicology of Aflatoxins: Human Health, Veterinary, and Agricultural Significance. Academic Press; San Diego, CA, USA: 1994. pp. 233–258. [Google Scholar]
- 51.Jelinek C.F., Pohland A.E., Wood G.E. Worldwide occurrence of mycotoxins in food and feeds—An update. J. Assoc. Off. Anal. Chem. 1989;72:223–230. [PubMed] [Google Scholar]
- 52.Papa K.E. Linkage groups in Aspergillus flavus. Mycologia. 1976;68:159–165. doi: 10.2307/3758906. [DOI] [PubMed] [Google Scholar]
- 53.Papa K.E. Genetics of Aspergillus flavus: Complementation and mapping of aflatoxin mutants. Genet. Res. 1979;34:1–9. doi: 10.1017/S0016672300019236. [DOI] [PubMed] [Google Scholar]
- 54.Papa K.E. Genetics of Aspergillus flavus: Linkage of aflatoxin mutants. Can. J. Microbiol. 1984;30:68–73. doi: 10.1139/m84-012. [DOI] [PubMed] [Google Scholar]
- 55.Bennett J.W. Microbiological aspects of the aflatoxin problem. Am. Ass. Feed Microscop. Off. Proc. 1970;18:118–131. [Google Scholar]
- 56.Bennett J.W., Goldblatt L.A. The isolation of mutants of Aspergillus flavus and A. parasiticus with altered aflatoxin producing ability. Sabouraudia. 1973;11:235–241. doi: 10.1080/00362177385190471. [DOI] [PubMed] [Google Scholar]
- 57.Bennett J.W., Lee L.S., Cucullu A.F. Effect of dichlorvos on aflatoxin and versicolorin A production in Aspergillus parasiticus. Bot. Gaz. 1976;137:318–324. [Google Scholar]
- 58.Bennett J.W. Aflatoxins and anthraquinones from diploids of Aspergillus parasiticus. J. Gen. Microbiol. 1979;113:127–136. doi: 10.1099/00221287-113-1-127. [DOI] [PubMed] [Google Scholar]
- 59.Bennett J.W., Kronberg F.G., Goodman L.A., Seltman M.A. Isolation of an anthraquinoe-accumulating mutant of Aspergillus parasiticus and partial characterization by dry column chromatography. Mycologia. 1983;75:202–208. [Google Scholar]
- 60.Bennett J.W., Lee L.S., Vinnett C.H. The correlation of aflatoxin and norsolorinic acid production. J. Am. Oil Chem. Soc. 1971;48:368–370. doi: 10.1007/BF02890764. [DOI] [PubMed] [Google Scholar]
- 61.Bennett J.W., Kronberg F., Gougis G. Pigmented isolates from anthraquinone-producing mutants of Aspergillus parasiticus. Am. Soc. Microbiol. 1976;76:6. [Google Scholar]
- 62.Bennett J.W., Chang P.K., Bhatnagar D. One gene to whole pathway: The role of norsolorinic acid in aflatoxin research. Adv. Appl. Microbiol. 1997;45:1–15. doi: 10.1016/S0065-2164(08)70260-0. [DOI] [PubMed] [Google Scholar]
- 63.Hsieh D.P., Lin M.T., Yao R.C., Singh R. Biosynthesis of aflatoxin. Conversion of norsolorinic acid and other hypothetical intermediates into aflatoxin B1. J. Agric. Food Chem. 1976;24:1170–1174. doi: 10.1021/jf60208a018. [DOI] [PubMed] [Google Scholar]
- 64.Dutton M.F. Enzymes and aflatoxin biosynthesis. Microbiol. Rev. 1988;52:274–295. doi: 10.1128/mr.52.2.274-295.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hsieh D.P., Mateles R.I. The relative contribution of acetate and glucose to aflatoxin biosynthesis. Biochem. Biophys. Acta. 1970;208:482–486. doi: 10.1016/0304-4165(70)90222-9. [DOI] [PubMed] [Google Scholar]
- 66.Hsieh D.P., Lin M.T., Yao R.C. Conversion of sterigmatocystin to aflatoxin B1 by Aspergillus parasiticus. Biochem. Biophys. Res. Commun. 1973;52:992–997. doi: 10.1016/0006-291X(73)91035-8. [DOI] [PubMed] [Google Scholar]
- 67.Yu J., Chang P.K., Cary J.W., Wright M., Bhatnagar D., Cleveland T.E., Payne G.A., Linz J.E. Comparative mapping of aflatoxin pathway gene clusters in Aspergillus parasiticus and Aspergillus flavus. Appl. Environ. Microbiol. 1995;61:2365–2371. doi: 10.1128/aem.61.6.2365-2371.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bennett J.W., Papa K.E. The aflatoxigenic Aspergillus spp. Adv. Plant Pathol. 1988;6:265–279. [Google Scholar]
- 69.Bennett J.W., Silverstein R.B., Kruger S.J. Isolation and characterization of two nonaflatoxigenic classes of morphological variants of Aspergillus parasiticus. J. Am. Oil Chem. Soc. 1981;58:A952–A955. doi: 10.1007/BF02679298. [DOI] [Google Scholar]
- 70.Bhatnagar D., Ehrlich K.C., Cleveland T.E. Oxidation-Reduction Reactions in Biosynthesis of Secondary Metabolites. In: Bhatnagar D., Lillehoj E.B., Arora D.K., editors. Mycotoxins in Ecological Systems. Vol. 10. Marcel Dekker; New York, NY, USA: 1992. pp. 255–285. [Google Scholar]
- 71.Chang P.K. Lack of interaction between AFLR and AFLJ contributes to nonaflatoxigenicity of Aspergillus sojae. J. Biotechnol. 2004;107:245–253. doi: 10.1016/j.jbiotec.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 72.Chang P.K., Cary J.W., Bhatnagar D., Cleveland T.E., Bennett J.W., Linz J.E., Woloshuk C.P., Payne G.A. Cloning of the Aspergillus parasiticus apa-2 gene associated with the regulation of aflatoxin biosynthesis. Appl. Environ. Microbiol. 1993;59:3273–3279. doi: 10.1128/aem.59.10.3273-3279.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chang P.K., Cary J.W., Yu J., Bhatnagar D., Cleveland T.E. The Aspergillus parasiticus polyketide synthase gene pksA, a homolog of Aspergillus nidulans wA, is required for aflatoxin B1 biosynthesis. Mol. Gen. Genet. 1995;248:270–277. doi: 10.1007/BF02191593. [DOI] [PubMed] [Google Scholar]
- 74.Chang P.K., Yu J., Bhatnagar D., Cleveland T.E. The carboxy-terminal portion of the aflatoxin pathway regulatory protein AFLR of Aspergillus parasiticus activates GAL1: lacZ gene expression in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 1999;65:2508–2512. doi: 10.1128/aem.65.6.2508-2512.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cleveland T.E., Lax A.R., Lee L.S., Bhatnagar D. Appearance of enzyme activities catalyzing conversion of sterigmatocystin to aflatoxin B1 in late-growth-phase Aspergillus parasiticus cultures. Appl. Environ. Microbiol. 1987;53:1711–1713. doi: 10.1128/aem.53.7.1711-1713.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Crawford J.M., Thomas P.M., Scheerer J.R., Vagstad A.L., Kelleher N.L., Townsend C.A. Deconstruction of iterative multidomain polyketide synthase function. Science. 2008;320:243–246. doi: 10.1126/science.1154711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ehrlich K.C., Cary J.W., Montalbano B.G. Characterization of the promoter for the gene encoding the aflatoxin biosynthetic pathway regulatory protein AFLR. Biochim. Biophys. Acta. 1999;1444:412–417. doi: 10.1016/S0167-4781(99)00022-6. [DOI] [PubMed] [Google Scholar]
- 78.Keller N.P., Dischinger H.C., Jr., Bhatnagar D., Cleveland T.E., Ullah A.H. Purification of a 40-kilodalton methyltransferase active in the aflatoxin biosynthetic pathway. Appl. Environ. Microbiol. 1993;59:479–484. doi: 10.1128/aem.59.2.479-484.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ehrlich K.C., Yu J. Aflatoxin-Like Gene Clusters and How They Evolved. In: Varma A.K., Rai M.K., editors. Mycotoxins in Food, Feed, and Bioweapons. Springer Verlag; Heidelberg, Germany: 2009. pp. 65–76. [Google Scholar]
- 80.Ehrlich K.C. Predicted roles of uncharacterized clustered genes in aflatoxin biosynthesis. Toxins. 2009;1:37–58. doi: 10.3390/toxins1010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yu J., Chang P.K., Ehrlich K.C., Cary J.W., Bhatnagar D., Cleveland T.E., Payne G.A., Linz J.E., Woloshuk C.P., Bennett J.W. Clustered pathway genes in aflatoxin biosynthesis. Appl. Environ. Microbiol. 2004;70:1253–1262. doi: 10.1128/AEM.70.3.1253-1262.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chang P.K., Horn B.W., Dorner J.W. Sequence breakpoints in the aflatoxin biosynthesis gene cluster and flanking regions in nonaflatoxigenic Aspergillus flavus isolates. Fungal Genet. Biol. 2005;42:914–923. doi: 10.1016/j.fgb.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 83.Wilson D.M. Analytical methods for aflatoxins in corn and peanuts. Arch. Environ. Contam. Toxicol. 1989;18:308–314. doi: 10.1007/BF01062353. [DOI] [PubMed] [Google Scholar]
- 84.Trail F., Mahanti N., Rarick M., Mehigh R., Liang S.H., Zhou R., Linz J.E. Physical and transcriptional map of an aflatoxin gene cluster in Aspergillus parasiticus and functional disruption of a gene involved early in the aflatoxin pathway. Appl. Environ. Microbiol. 1995;61:2665–2673. doi: 10.1128/aem.61.7.2665-2673.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Trail F., Mahanti N., Linz J. Molecular biology of aflatoxin biosynthesis. Microbiol. 1995;141:755–765. doi: 10.1099/13500872-141-4-755. [DOI] [PubMed] [Google Scholar]
- 86.Townsend C.A. Progress towards a biosynthetic rationale of the aflatoxin pathway. Pure Appl. Chem. 1997;58:227–238. doi: 10.1351/pac198658020227. [DOI] [Google Scholar]
- 87.Yu J., Bhatnagar D., Cleveland T.E. Completed sequence of aflatoxin pathway gene cluster in Aspergillus parasiticus. FEBS Lett. 2004;564:126–130. doi: 10.1016/S0014-5793(04)00327-8. [DOI] [PubMed] [Google Scholar]
- 88.Bennett J.W. Loss of norsolorinic acid and aflatoxin production by a mutant of Aspergillus parasiticus. J. Gen. Microbiol. 1981;124:429–432. [Google Scholar]
- 89.Watanabe C.M., Wilson D., Linz J.E., Townsend C.A. Demonstration of the catalytic roles and evidence for the physical association of type I fatty acid synthases and a polyketide synthase in the biosynthesis of aflatoxin B1. Chem. Biol. 1996;3:463–469. doi: 10.1016/S1074-5521(96)90094-0. [DOI] [PubMed] [Google Scholar]
- 90.Brown D.W., Yu J.H., Kelkar H.S., Fernandes M., Nesbitt T.C., Keller N.P., Adams T.H., Leonard T.J. Twenty-five coregulated transcripts define a sterigmatocystin gene cluster in Aspergillus nidulans. Proc. Natl. Acad. Sci. USA. 1996;93:1418–1422. doi: 10.1073/pnas.93.4.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Watanabe C.M., Townsend C.A. Initial characterization of a type I fatty acid synthase and polyketide synthase multienzyme complex NorS in the biosynthesis of aflatoxin B1. Chem. Biol. 2002;9:981–988. doi: 10.1016/S1074-5521(02)00213-2. [DOI] [PubMed] [Google Scholar]
- 92.Crawford J.M., Dancy B.C., Hill E.A., Udwary D.W., Townsend C.A. Identification of a starter unit acyl-carrier protein transacylase domain in an iterative type I polyketide synthase. Proc. Natl. Acad. Sci. USA. 2006;103:16728–16733. doi: 10.1073/pnas.0604112103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Crawford J.M., Vagstad A.L., Ehrlich K.C., Townsend C.A. Starter unit specificity directs genome mining of polyketide synthase pathways in fungi. Bioorg. Chem. 2008;36:16–22. doi: 10.1016/j.bioorg.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yabe K., Nakajima H. Enzyme reactions and genes in aflatoxin biosynthesis. Appl. Microbiol. Biotechnol. 2004;64:745–755. doi: 10.1007/s00253-004-1566-x. [DOI] [PubMed] [Google Scholar]
- 95.Brown D.W., Adams T.H., Keller N.P. Aspergillus has distinct fatty acid synthases for primary and secondary metabolism. Proc. Natl. Acad. Sci. USA. 1996;93:14873–14877. doi: 10.1073/pnas.93.25.14873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mahanti N., Bhatnagar D., Cary J.W., Joubran J., Linz J.E. Structure and function of fas-1A, a gene encoding a putative fatty acid synthetase directly involved in aflatoxin biosynthesis in Aspergillus parasiticus. Appl. Environ. Microbiol. 1996;62:191–195. doi: 10.1128/aem.62.1.191-195.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Payne G.A. Process of Contamination by Aflatoxin-Producing Fungi and Their Impacts on Crops. In: Sinha K.K., Bhatnagar D., editors. Mycotoxins in Agriculture and Food Safety. Marcel Dekker; New York, NY, USA: 1998. pp. 279–306. [Google Scholar]
- 98.Watanabe C.M., Townsend C.A. Incorporation of molecular oxygen in aflatoxin B1 biosynthesis. J. Org. Chem. 1996;61:1990–1993. doi: 10.1021/jo952056v. [DOI] [Google Scholar]
- 99.Hitchman T.S., Schmidt E.W., Trail F., Rarick M.D., Linz J.E., Townsend C.A. Hexanoate synthase, a specialized type I fatty acid synthase in aflatoxin B1 biosynthesis. Bioorg. Chem. 2001;29:293–307. doi: 10.1006/bioo.2001.1216. [DOI] [PubMed] [Google Scholar]
- 100.Crawford J.M., Vagstad A.L., Whitworth K.P., Ehrlich K.C., Townsend C.A. Synthetic strategy of nonreducing Iterative polyketide synthases and the origin of the classical “Starter-Unit Effect”. Chembiochem. 2008;9:1019–1023. doi: 10.1002/cbic.200700702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bennett J.W., Bhatnagar D., Chang P.K. The molecular genetics of aflatoxin biosynthesis. FEMS Symp. 1994:51–58. [Google Scholar]
- 102.Papa G. Norsolorinic acid mutant of Aspergillus flavus. J. Gen. Microbiol. 1982;128:1345–1348. [Google Scholar]
- 103.Lee L.S., Bennett J.W., Goldblatt L.A., Lundin R.E. Norsolorinic acid from a mutant strain of Aspergillus parasiticus. J. Am. Oil Chem. Soc. 1971;48:93–94. doi: 10.1007/BF02635696. [DOI] [PubMed] [Google Scholar]
- 104.Detroy R.W., Freer S., Ciegler A. Aflatoxin and anthraquinone biosynthesis by nitrosoguanidine-derived mutants of Aspergillus parasiticus. Can. J. Microbiol. 1973;19:1373–1378. doi: 10.1139/m73-221. [DOI] [PubMed] [Google Scholar]
- 105.Chang P.K., Skory C.D., Linz J.E. Cloning of a gene associated with aflatoxin B1 biosynthesis in Aspergillus parasiticus. Curr. Genet. 1992;21:231–233. doi: 10.1007/BF00336846. [DOI] [PubMed] [Google Scholar]
- 106.Zhou R., Linz J.E. Enzymatic function of the nor-1 protein in aflatoxin biosynthesis in Aspergillus parasiticus. Appl. Environ. Microbiol. 1999;65:5639–5641. doi: 10.1128/aem.65.12.5639-5641.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Trail F., Chang P.-K., Cary J., Linz J.E. Structural and functional analysis of the nor-1 gene involved in the biosynthesis of aflatoxins by Aspergillus parasiticus. Appl. Environ. Microbiol. 1994;60:4078–4085. doi: 10.1128/aem.60.11.4078-4085.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cary J.W., Wright M., Bhatnagar D., Lee R., Chu F.S. Molecular characterization of an Aspergillus parasiticus dehydrogenase gene, norA, located on the aflatoxin biosynthesis gene cluste. Appl. Environ. Microbiol. 1996;62:360–366. doi: 10.1128/aem.62.2.360-366.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bennett J.W., Lee L.S., Shoss S.M., Boudreaux G.H. Identification of averantin as an aflatoxin B1 precursor: Placement in the biosynthetic pathway. Appl. Environ. Microbiol. 1980;39:835–839. doi: 10.1128/aem.39.4.835-839.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.McCormick S.P., Bhatnagar D., Lee L.S. Averufanin is an aflatoxin B1 precursor between averantin and averufin in the biosynthetic pathway. Appl. Environ. Microbiol. 1987;53:14–16. doi: 10.1128/aem.53.1.14-16.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yabe K., Ando Y., Hamasaki T. A metabolic grid among versiconal hemiacetal acetate, versiconol acetate, versiconol and versiconal during aflatoxin biosynthesis. J. Gen. Microbiol. 1991;137:2469–2475. doi: 10.1099/00221287-137-10-2469. [DOI] [PubMed] [Google Scholar]
- 112.Yabe K., Nakamura Y., Nakajima H., Ando Y., Hamasaki T. Enzymatic conversion of norsolorinic acid to averufin in aflatoxin biosynthesis. Appl. Environ. Microbiol. 1991;57:1340–1345. doi: 10.1128/aem.57.5.1340-1345.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yu J., Chang P.K., Cary J.W., Bhatnagar D., Cleveland T.E. avnA, a gene encoding a cytochrome P-450 monooxygenase, is involved in the conversion of averantin to averufin in aflatoxin biosynthesis in Aspergillus parasiticus. Appl. Environ. Microbiol. 1997;63:1349–1356. doi: 10.1128/aem.63.4.1349-1356.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hsieh D.P. Inhibition of aflatoxin biosynthesis of dichlorvos. J. Agric. Food Chem. 1973;21:468–470. doi: 10.1021/jf60187a035. [DOI] [PubMed] [Google Scholar]
- 115.Singh R., Hsieh D.P. Aflatoxin biosynthetic pathway: Elucidation by using blocked mutants of Aspergillus parasiticus. Arch. Biochem. Biophys. 1977;178:285–292. doi: 10.1016/0003-9861(77)90193-X. [DOI] [PubMed] [Google Scholar]
- 116.Fitzell D.L., Hsieh D.P., Yao R.C., la Mar G.N. Biosynthesis of averufin from acetate by Aspergillus parasiticus. J. Agric. Food Chem. 1975;23:442–444. doi: 10.1021/jf60199a039. [DOI] [PubMed] [Google Scholar]
- 117.Lin M.T., Hsieh D.P., Yao R.C., Donkersloot J.A. Conversion of averufin into aflatoxins by Aspergillus parasiticus. Biochemistry. 1973;12:5167–5171. doi: 10.1021/bi00749a023. [DOI] [PubMed] [Google Scholar]
- 118.Lin M.T., Hsieh D.P. Averufin in the biosynthesis of aflatoxin B. J. Am Chem. Soc. 1973;95:1668–1669. doi: 10.1021/ja00786a056. [DOI] [PubMed] [Google Scholar]
- 119.Keller N.P., Watanabe C.M., Kelkar H.S., Adams T.H., Townsend C.A. Requirement of monooxygenase-mediated steps for sterigmatocystin biosynthesis by Aspergillus nidulans. Appl. Environ. Microbiol. 2000;66:359–362. doi: 10.1128/aem.66.1.359-362.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sakuno E., Yabe K., Nakajima H. Involvement of two cytosolic enzymes and a novel intermediate, 5'-oxoaverantin, in the pathway from 5'-hydroxyaverantin to averufin in aflatoxin biosynthesis. Appl. Environ. Microbiol. 2003;69:6418–6426. doi: 10.1128/AEM.69.11.6418-6426.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chang P.K., Yu J., Ehrlich K.C., Boue S.M., Montalbano B.G., Bhatnagar D., Cleveland T.E. adhA in Aspergillus parasiticus is involved in conversion of 5'-hydroxyaverantin to averufin. Appl. Environ. Microbiol. 2000;66:4715–4719. doi: 10.1128/aem.66.11.4715-4719.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ehrlich K.C., Chang P.-K., Scharfenstein J.S.L., Cary J.W., Crawford J.M., Townsend C.A. Absence of the aflatoxin biosynthesis gene, norA, allows accumulation of deoxyaflatoxin B1 in Aspergillus flavus cultures. FEMS Microbiol. Lett. 2010;305:65–70. doi: 10.1111/j.1574-6968.2010.01914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yu J., Woloshuk C.P., Bhatnagar D., Cleveland T.E. Cloning and characterization of avfA and omtB genes involved in aflatoxin biosynthesis in three Aspergillus species. Gene. 2000;248:157–167. doi: 10.1016/S0378-1119(00)00126-8. [DOI] [PubMed] [Google Scholar]
- 124.Yao R.C., Hsieh D.P. Step of dichlorvos inhibition in the pathway of aflatoxin biosynthesis. Appl. Microbiol. 1974;28:52–57. doi: 10.1128/am.28.1.52-57.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hsieh D.P., Wan C.C., Billington J.A. A versiconal hemiacetal acetate converting enzyme in aflatoxin biosynthesis. Mycopathologia. 1989;107:121–126. doi: 10.1007/BF00707548. [DOI] [PubMed] [Google Scholar]
- 126.Kusumoto K.I., Hsieh D.P.H. Purification and characterization of the esterases involved in aflatoxin biosynthesis in Aspergillus parasiticus. Can. J. Microbiol. 1996;42:804–810. doi: 10.1139/m96-101. [DOI] [PubMed] [Google Scholar]
- 127.Schroeder H.W., Cole R.J., Grigsby R.D., Hein H., Jr. Inhibition of aflatoxin production and tentative identification of an aflatoxin intermediate “versiconal acetate” from treatment with dichlorvos. Appl. Microbiol. 1974;27:394–399. doi: 10.1128/am.27.2.394-399.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fitzell D.L., Singh R., Hsieh D.P., Motell E.L. Nuclear magnetic resonance identification of versiconal hemiacetal acetate as an intermediate in aflatoxin biosynthesis. J. Agric. Food. Chem. 1977;25:1193–1197. doi: 10.1021/jf60213a024. [DOI] [PubMed] [Google Scholar]
- 129.Yu J., Chang P.K., Bhatnagar D., Cleveland T.E. Cloning and functional expression of an esterase gene in Aspergillus parasitcus. Mycopathologia. 2002;156:227–234. doi: 10.1023/a:1023353025330. [DOI] [PubMed] [Google Scholar]
- 130.Chang P.K., Yabe K., Yu J. The Aspergillus parasiticus estA-encoded esterase converts versiconal hemiacetal acetate to versiconal and versiconol acetate to versiconol in aflatoxin biosynthesis. Appl. Environ. Microbiol. 2004;70:3593–3599. doi: 10.1128/AEM.70.6.3593-3599.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yabe K., Chihaya N., Hamamatsu S., Sakuno E., Hamasaki T., Nakajima H., Bennett J.W. Enzymatic conversion of averufin to hydroxyversicolorone and elucidation of a novel metabolic grid involved in aflatoxin biosynthesis. Appl. Environ. Microbiol. 2003;69:66–73. doi: 10.1128/AEM.69.1.66-73.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lin B.K., Anderson J.A. Purification and properties of versiconal cyclase from Aspergillus parasiticus. Arch. Biochem. Biophys. 1992;293:67–70. doi: 10.1016/0003-9861(92)90366-5. [DOI] [PubMed] [Google Scholar]
- 133.McGuire S.M., Silva J.C., Casillas E.G., Townsend C.A. Purification and characterization of versicolorin B synthase from Aspergillus parasiticus. Catalysis of the stereodifferentiating cyclization in aflatoxin biosynthesis essential to DNA interaction. Biochemistry. 1996;35:11470–11486. doi: 10.1021/bi960924s. [DOI] [PubMed] [Google Scholar]
- 134.Silva J.C., Minto R.E., Barry C.E., III, Holland K.A., Townsend C.A. Isolation and characterization of the versicolorin B synthase gene from Aspergillus parasiticus. Expansion of the aflatoxin B1 biosynthetic gene cluster. J. Biol. Chem. 1996;271:13600–13608. doi: 10.1074/jbc.271.23.13600. [DOI] [PubMed] [Google Scholar]
- 135.Silva J.C., Townsend C.A. Heterologous expression, isolation, and characterization of versicolorin B synthase from Aspergillus parasiticu. A key enzyme in the aflatoxin B1 biosynthetic pathway. J. Biol. Chem. 1997;272:804–813. doi: 10.1074/jbc.272.2.804. [DOI] [PubMed] [Google Scholar]
- 136.Zuckerman A.J., Rees K.R., Inman D., Petts V. Site of action of aflatoxin on human liver cells in culture. Nature. 1967;214:814–815. doi: 10.1038/214814b0. [DOI] [PubMed] [Google Scholar]
- 137.Yabe K., Matsuyama Y., Ando Y., Nakajima H., Hamasaki T. Stereochemistry during aflatoxin biosynthesis: Conversion of norsolorinic acid to averufin. Appl. Environ. Microbiol. 1993;59:2486–2492. doi: 10.1128/aem.59.8.2486-2492.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kelkar H.S., Skloss T.W., Haw J.F., Keller N.P., Adams T.H. Aspergillus nidulans stcL encodes a putative cytochrome P-450 monooxygenase required for bisfuran desaturation during aflatoxin/sterigmatocystin biosynthesis. J. Biol. Chem. 1997;272:1589–1594. doi: 10.1074/jbc.272.3.1589. [DOI] [PubMed] [Google Scholar]
- 139.Henry K.M., Townsend C.A. Ordering the reductive and cytochrome P450 oxidative steps in demethylsterigmatocystin formation yields general insights into the biosynthesis of aflatoxin and related fungal metabolites. J. Am. Chem. Soc. 2005;127:3724–3733. doi: 10.1021/ja0455188. [DOI] [PubMed] [Google Scholar]
- 140.Skory C.D., Chang P.K., Cary J., Linz J.E. Isolation and characterization of a gene from Aspergillus parasiticus associated with the conversion of versicolorin A to sterigmatocystin in aflatoxin biosynthesis. Appl. Environ. Microbiol. 1992;58:3527–3537. doi: 10.1128/aem.58.11.3527-3537.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Keller N.P., Kantz N.J., Adams T.H. Aspergillus nidulans verA is required for production of the mycotoxin sterigmatocystin. Appl. Environ. Microbiol. 1994;60:1444–1450. doi: 10.1128/aem.60.5.1444-1450.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Keller N.P., Segnar S., Bhatnagar D., Adams T.H. stcS, a putative P-450 monooxygenase, is required for the conversion of versicolorin A to sterigmatocystin in Aspergillus nidulans. Appl. Environ. Microbiol. 1995;61:3628–3632. doi: 10.1128/aem.61.10.3628-3632.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yabe K., Ando Y., Hashimoto J., Hamasaki T. Two distinct O-methyltransferases in aflatoxin biosynthesis. Appl. Environ. Microbiol. 1989;55:2172–2177. doi: 10.1128/aem.55.9.2172-2177.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yabe K., Matsushima K., Koyama T., Hamasaki T. Purification and characterization of O-methyltransferase I involved in conversion of demethylsterigmatocystin to sterigmatocystin and of dihydrodemethylsterigmatocystin to dihydrosterigmatocystin during aflatoxin biosynthesis. Appl. Environ. Microbiol. 1998;64:166–171. doi: 10.1128/aem.64.1.166-171.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yabe K., Nakamura M., Hamasaki T. Enzymatic formation of G-group aflatoxins and biosynthetic relationship between G- and B-group aflatoxins. Appl. Environ. Microbiol. 1999;65:3867–3872. doi: 10.1128/aem.65.9.3867-3872.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Motomura M., Chihaya N., Shinozawa T., Hamasaki T., Yabe K. Cloning and characterization of the O-methyltransferase I gene (dmtA) from Aspergillus parasiticus associated with the conversions of demethylsterigmatocystin to sterigmatocystin and dihydrodemethylsterigmatocystin to dihydrosterigmatocystin in aflatoxin biosynthesis. Appl. Environ. Microbiol. 1999;65:4987–4994. doi: 10.1128/aem.65.11.4987-4994.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kelkar H.S., Keller N.P., Adams T.H. Aspergillus nidulans stcP encodes an O-methyltransferase that is required for sterigmatocystin biosynthesis. Appl. Environ. Microbiol. 1996;62:4296–4298. doi: 10.1128/aem.62.11.4296-4298.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yu J., Cary J.W., Bhatnagar D., Cleveland T.E., Keller N.P., Chu F.S. Cloning and characterization of a cDNA from Aspergillus parasiticus encoding an O-methyltransferase involved in aflatoxin biosynthesis. Appl. Environ. Microbiol. 1993;59:3564–3571. doi: 10.1128/aem.59.11.3564-3571.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yu J., Chang P.K., Payne G.A., Cary J.W., Bhatnagar D., Cleveland T.E. Comparison of the omtA genes encoding O-methyltransferases involved in aflatoxin biosynthesis from Aspergillus parasiticus and A. flavus. Gene. 1995;163:121–125. doi: 10.1016/0378-1119(95)00397-O. [DOI] [PubMed] [Google Scholar]
- 150.Klich M.A., Yu J., Chang P.K., Mullaney E.J., Bhatnagar D., Cleveland T.E. Hybridization of genes involved in aflatoxin biosynthesis to DNA of aflatoxigenic and non-aflatoxigenic aspergilli. Appl. Microbiol. Biotechnol. 1995;44:439–443. doi: 10.1007/BF00169941. [DOI] [PubMed] [Google Scholar]
- 151.Yabe K., Ando Y., Hamasaki T. Biosynthetic relationship among aflatoxins B1, B2, G1, and G2. Appl. Environ. Microbiol. 1988;54:2101–2106. doi: 10.1128/aem.54.8.2101-2106.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Prieto R., Yousibova G.L., Woloshuk C.P. Identification of aflatoxin biosynthesis genes by genetic complementation in an Aspergillus flavus mutant lacking the aflatoxin gene cluster. Appl. Environ. Microbiol. 1996;62:3567–3571. doi: 10.1128/aem.62.10.3567-3571.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Prieto R., Woloshuk C.P. ord1, an oxidoreductase gene responsible for conversion of O-methylsterigmatocystin to aflatoxin in Aspergillus flavus. Appl. Environ. Microbiol. 1997;63:1661–1666. doi: 10.1128/aem.63.5.1661-1666.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yu J., Chang P.K., Ehrlich K.C., Cary J.W., Montalbano B., Dyer J.M., Bhatnagar D., Cleveland T.E. Characterization of the critical amino acids of an Aspergillus parasiticus cytochrome P-450 monooxygenase encoded by ordA that is involved in the biosynthesis of aflatoxins B1, G1, B2, and G2. Appl. Environ. Microbiol. 1998;64:4834–4841. doi: 10.1128/aem.64.12.4834-4841.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ehrlich K.C., Chang P.K., Yu J., Cotty P.J. Aflatoxin biosynthesis cluster gene cypA is required for G aflatoxin formation. Appl. Environ. Microbiol. 2004;70:6518–6524. doi: 10.1128/AEM.70.11.6518-6524.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Price M.S., Yu J., Nierman W.C., Kim H.S., Pritchard B., Jacobus C.A., Bhatnagar D., Cleveland T.E., Payne G.A. The aflatoxin pathway regulator AflR induces gene transcription inside and outside of the aflatoxin biosynthetic cluster. FEMS Microbiol. Lett. 2006;255:275–279. doi: 10.1111/j.1574-6968.2005.00084.x. [DOI] [PubMed] [Google Scholar]
- 157.Ehrlich K.C., Chang P.-K., Yu J., Cary J.W., Bhatnagar D. Control of Aflatoxin Biosynthesis in Aspergilli. In: Guevara-Gonzalez R.G., editor. Aflatoxins—Biochemistry and Molecular Biology. Intech; Rijeka, Croatia: 2011. pp. 21–40. [Google Scholar]
- 158.Yu J., Chang P.K., Bhatnagar D., Cleveland T.E. Cloning of a sugar utilization gene cluster in Aspergillus parasiticus. Biochim. Biophys. Acta. 2000;1493:211–214. doi: 10.1016/S0167-4781(00)00148-2. [DOI] [PubMed] [Google Scholar]
- 159.Cai J., Zeng H., Shima Y., Hatabayashi H., Nakagawa H., Ito Y., Adachi Y., Nakajima H., Yabe K. Involvement of the nadA gene in formation of G-group aflatoxins in Aspergillus parasiticus. Fungal Genet. Biol. 2008;45:1081–1093. doi: 10.1016/j.fgb.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 160.Ehrlich K.C., Scharfenstein J.S.L., Montalbano B.G., Chang P.-K. Are the genes nadA and norB involved in formation of aflatoxin G1. Int. J. Mol. Sci. 2008;9:1717–1729. doi: 10.3390/ijms9091717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Rice D.W., Hsieh D.P. Aflatoxin M1: In vitro preparation and comparative in vitro metabolism versus aflatoxin B1 in the rat and mouse. Res. Commun. Chem. Pathol. Pharmacol. 1982;35:467–490. [PubMed] [Google Scholar]
- 162.Garrido N.S., Iha M.H., Santos Ortolani M.R., Duarte Fávaro R.M. Occurrence of aflatoxin M1 and aflatoxin M2 in milk commercialized in Ribeirão Preto-SP, Brazil. J. Food Addit. Contam. 2003;20:70–73. doi: 10.1080/0265203021000035371. [DOI] [PubMed] [Google Scholar]
- 163.Hsieh D.P., Beltran L.M., Fukayama M.Y., Rice D.W., Wong J.J. Production and isolation of aflatoxin M1 for toxicological studies. J. Assoc. Off. Anal. Chem. 1986;69:510–512. [PubMed] [Google Scholar]
- 164.Hsieh D.P., Cullen J.M., Hsieh L.S., Shao Y., Ruebner B.H. Cancer risks posed by aflatoxin M1. Princess Takamatsu Symp. 1985;16:57–65. [PubMed] [Google Scholar]
- 165.Huang J.H., Hsieh D.P. Comparative study of aflatoxins M1 and B1 production in solid-state and shaking liquid cultures. Proc. Natl. Sci. Counc. Repub. China. 1988;12:34–42. [PubMed] [Google Scholar]
- 166.Yabe K., Chihaya N., Hatabayashi H., Kito M., Hoshino S., Zeng H., Cai J., Nakajima H. Production of M-/GM-group aflatoxins catalyzed by the OrdA enzyme in aflatoxin biosynthesis. Fungal Genet. Biol. 2012;49:744–754. doi: 10.1016/j.fgb.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 167.Woloshuk C.P., Prieto R. Genetic organization and function of the aflatoxin B1 biosynthetic genes. FEMS Microbiol. Lett. 1998;160:169–176. doi: 10.1111/j.1574-6968.1998.tb12907.x. [DOI] [PubMed] [Google Scholar]
- 168.Meyers D.M., Obrian G., Du W.L., Bhatnagar D., Payne G.A. Characterization of aflJ, a gene required for conversion of pathway intermediates to aflatoxin. Appl. Environ. Microbiol. 1998;64:3713–3717. doi: 10.1128/aem.64.10.3713-3717.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bok J.-W., Keller N.P. LaeA, a regulator of secondary metabolism in Aspergillus spp. Eukaryot. Cell. 2004;3:527–535. doi: 10.1128/EC.3.2.527-535.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Perrin R.M., Fedorova N.D., Bok J.W., Cramer R.A., Wortman J.R., Kim H.S., Nierman W.C., Keller N.P. Transcriptional regulation of chemical diversity in Aspergillus fumigatus by LaeA. PLoS Pathog. 2007;3:e50. doi: 10.1371/journal.ppat.0030050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kato N., Brooks W., Calvo A.M. The expression of sterigmatocystin and penicillin genes in Aspergillus nidulans is controlled by veA, a gene required for sexual development. Eukaryot. Cell. 2003;2:1178–1186. doi: 10.1128/EC.2.6.1178-1186.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Calvo A.M., Bok J.-W., Brooks W., Keller N.P. VeA is required for toxin and sclerotial production in Aspergillus parasiticus. Appl. Environ. Microbiol. 2004;70:4733–4739. doi: 10.1128/AEM.70.8.4733-4739.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chang P.K., Ehrlich K.C., Yu J., Bhatnagar D., Cleveland T.E. Increased expression of Aspergillus parasiticus aflR, encoding a sequence-specific DNA-binding protein, relieves nitrate inhibition of aflatoxin biosynthesis. Appl. Environ. Microbiol. 1995;61:2372–2377. doi: 10.1128/aem.61.6.2372-2377.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chang P.K., Yu J., Bhatnagar D., Cleveland T.E. Repressor-AFLR interaction modulates aflatoxin biosynthesis in Aspergillus parasiticus. Mycopathologia. 1999;147:105–112. doi: 10.1023/A:1007157309168. [DOI] [PubMed] [Google Scholar]
- 175.Ehrlich K.C., Montalbano B.G., Bhatnagar D., Cleveland T.E. Alteration of different domains in AFLR affects aflatoxin pathway metabolism in Aspergillus parasiticus transformants. Fungal Genet. Biol. 1998;23:279–287. doi: 10.1006/fgbi.1998.1045. [DOI] [PubMed] [Google Scholar]
- 176.Flaherty J.E., Payne G.A. Overexpression of aflR leads to upregulation of pathway gene expression and increased aflatoxin production in Aspergillus flavus. Appl. Environ. Microbiol. 1997;63:3995–4000. doi: 10.1128/aem.63.10.3995-4000.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Payne G.A., Nystrom G.J., Bhatnagar D., Cleveland T.E., Woloshuk C.P. Cloning of the afl-2 gene involved in aflatoxin biosynthesis from Aspergillus flavus. Appl. Environ. Microbiol. 1993;59:156–162. doi: 10.1128/aem.59.1.156-162.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Woloshuk C.P., Foutz K.R., Brewer J.F., Bhatnagar D., Cleveland T.E., Payne G.A. Molecular characterization of aflR, a regulatory locus for aflatoxin biosynthesis. Appl. Environ. Microbiol. 1994;60:2408–2414. doi: 10.1128/aem.60.7.2408-2414.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yu J.H., Wieser J., Adams T.H. The Aspergillus FlbA RGS domain protein antagonizes G protein signaling to block proliferation and allow development. EMBO J. 1996;15:5184–5190. [PMC free article] [PubMed] [Google Scholar]
- 180.Yu J.H., Butchko R.A., Fernandes M., Keller N.P., Leonard T.J., Adams T.H. Conservation of structure and function of the aflatoxin regulatory gene aflR from Aspergillus nidulans and A. flavus. Curr. Genet. 1996;29:549–555. doi: 10.1007/BF02426959. [DOI] [PubMed] [Google Scholar]
- 181.Ehrlich K.C., Montalbano B.G., Cary J.W. Binding of the C6-zinc cluster protein, AFLR, to the promoters of aflatoxin pathway biosynthesis genes in Aspergillus parasiticu. Gene. 1999;230:249–257. doi: 10.1016/S0378-1119(99)00075-X. [DOI] [PubMed] [Google Scholar]
- 182.Cary J.W., Ehrlich K.C., Wright M., Chang P.K., Bhatnagar D. Generation of aflR disruption mutants of Aspergillus parasiticus. Appl. Microbiol. Biotechnol. 2000;53:680–684. doi: 10.1007/s002530000319. [DOI] [PubMed] [Google Scholar]
- 183.Wilkinson J.R., Yu J., Bland J.M., Nierman W.C., Bhatnagar D., Cleveland T.E. Amino acid supplementation reveals differential regulation of aflatoxin biosynthesis in Aspergillus flavus NRRL 3357 and Aspergillus parasiticus SRRC 143. Appl. Microbiol. Biotechnol. 2007;74:1308–1319. doi: 10.1007/s00253-006-0768-9. [DOI] [PubMed] [Google Scholar]
- 184.Wilkinson J.R., Yu J., Abbas H.K., Scheffler B.E., Kim H.S., Nierman W.C., Bhatnagar D., Cleveland T.E. Aflatoxin formation and gene expression in response to carbon source media shift in Aspergillus parasiticus. J. Food Addit. Contam. 2007;24:1051–1060. doi: 10.1080/02652030701579454. [DOI] [PubMed] [Google Scholar]
- 185.Abnet C.C. Carcinogenic food contaminants. Cancer Invest. 2007;25:189–196. doi: 10.1080/07357900701208733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Du W., Obrian G.R., Payne G.A. Function and regulation of aflJ in the accumulation of aflatoxin early pathway intermediate in Aspergillus flavus. J. Food Addit. Contam. 2007;24:1043–1050. doi: 10.1080/02652030701513826. [DOI] [PubMed] [Google Scholar]
- 187.Bouhired S., Weber M., Kempf-Sontag A., Keller N.P., Hoffmeister D. Accurate prediction of the Aspergillus nidulans terrequinone gene cluster boundaries using the transcriptional regulator LaeA. Fungal Genet. Biol. 2007;44:1134–1145. doi: 10.1016/j.fgb.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 188.Sugui J.A., Pardo J., Chang Y.C., Zarember K.A., Nardone G., Galvez E.M., Mullbacher A., Gallin J.I., Simon M.M., Kwon-Chung K.J. Gliotoxin is a virulence factor of Aspergillus fumigatus: gliP deletion attenuates virulence in mice immunosuppressed with hydrocortisone. Eukaryot. Cell. 2007;6:1562–1569. doi: 10.1128/EC.00141-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Bok J.W., Noordermeer D., Kale S.P., Keller N.P. Secondary metabolic gene cluster silencing in Aspergillus nidulans. Mol. Microbiol. 2006;61:1636–1645. doi: 10.1111/j.1365-2958.2006.05330.x. [DOI] [PubMed] [Google Scholar]
- 190.Kale S.P., Cary J.W., Hollis N., Wilkinson J.R., Bhatnagar D., Yu J., Cleveland T.E., Bennett J.W. Analysis of aflatoxin regulatory factors in serial transfer-induced non-aflatoxigenic Aspergillus parasiticus. J. Food Addit. Contam. 2007;24:1061–1069. doi: 10.1080/02652030701564563. [DOI] [PubMed] [Google Scholar]
- 191.Mooney J.L., Yager L.N. Light is required for conidiation in Aspergillus nidulans. Genes Dev. 1990;4:1473–1482. doi: 10.1101/gad.4.9.1473. [DOI] [PubMed] [Google Scholar]
- 192.Duran R.M., Cary J.W., Calvo A.M. Production of cyclopiazonic acid, aflatrem, and aflatoxin by Aspergillus flavus is regulated by veA, a gene necessary for sclerotial formation. Appl. Microbiol. Biotechnol. 2007;73:1158–1168. doi: 10.1007/s00253-006-0581-5. [DOI] [PubMed] [Google Scholar]
- 193.Stinnett S.M., Espeso E.A., Cobeno L., Araujo-Bazan L., Calvo A.M. Aspergillus nidulans VeA subcellular localization is dependent on the importin alpha carrier and on light. Mol. Microbiol. 2007;63:242–255. doi: 10.1111/j.1365-2958.2006.05506.x. [DOI] [PubMed] [Google Scholar]
- 194.Kale S.P., Cary J.W., Bhatnagar D., Bennett J.W. Characterization of experimentally induced, nonaflatoxigenic variant strains of Aspergillus parasiticus. Appl. Environ. Microbiol. 1996;62:3399–3404. doi: 10.1128/aem.62.9.3399-3404.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kale S., Bennett J.W. Strain Instability in Filamentous Fungi. In: Bhatnagar D., Lillehoj E.B., Arora D.K., editors. Handbook of Applied Mycology, Mycotoxins in Ecological Systems. Vol. 5. Tylor and Francis; New York, NY, USA: 1991. pp. 311–332. [Google Scholar]
- 196.Yabe K., Nakamura H., Ando Y., Terakado N., Nakajima H., Hamasaki T. Isolation and characterization of Aspergillus parasiticus mutants with impaired aflatoxin production by a novel tip culture method. Appl. Environ. Microbiol. 1988;54:2096–2100. doi: 10.1128/aem.54.8.2096-2100.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Demain A.L. Induction of secondary metabolism. Int. Microbiol. 1998;1:259–264. [PubMed] [Google Scholar]
- 198.Payne G.A., Brown M.P. Genetics and physiology of aflatoxin biosynthesis. Annu. Rev. Phytopathol. 1998;36:329–362. doi: 10.1146/annurev.phyto.36.1.329. [DOI] [PubMed] [Google Scholar]
- 199.Guo B.Z., Holbrook C.C., Yu J., Lee R.D., Lynch R.E. Application of Technology of Gene Expression in Response to Drought Stress and Elimination of Preharvest Aflatoxin Contamination. In: Abbas H.K., editor. Aflatoxin and Food Safety. CRC Press; Boca Raton, FL, USA: 2005. pp. 313–331. [Google Scholar]
- 200.Feng G.H., Leonard T.J. Culture conditions control expression of the genes for aflatoxin and sterigmatocystin biosynthesis in Aspergillus parasiticus and A. nidulans. Appl. Environ. Microbiol. 1998;64:2275–2277. doi: 10.1128/aem.64.6.2275-2277.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Cuero R., Ouellet T., Yu J., Mogongwa N. Metal ion enhancement of fungal growth, gene expression and aflatoxin synthesis in Aspergillus flavus: RT-PCR characterization. J. Appl. Microbiol. 2003;94:953–961. doi: 10.1046/j.1365-2672.2003.01870.x. [DOI] [PubMed] [Google Scholar]
- 202.Adye J., Mateles R.I. Incorporation of labelled compounds into aflatoxins. Biochim. Biophys. Acta. 1964;86:418–420. doi: 10.1016/0304-4165(64)90077-7. [DOI] [PubMed] [Google Scholar]
- 203.Bennett J.W., Rubin P.L., Lee L.S., Chen P.N. Influence of trace elements and nitrogen sources on versicolorin production by a mutant strain of Aspergillus parasiticus. Mycopathologia. 1979;69:161–166. doi: 10.1007/BF00452829. [DOI] [PubMed] [Google Scholar]
- 204.Luchese R.H., Harrigan W.F. Biosynthesis of aflatoxin-the role of nutritional factors. J. Appl. Bacteriol. 1993;74:5–14. doi: 10.1111/j.1365-2672.1993.tb02989.x. [DOI] [PubMed] [Google Scholar]
- 205.Buchanan R.L., Lewis D.F. Regulation of aflatoxin biosynthesis: Effect of glucose on activities of various glycolytic enzymes. Appl. Environ. Microbiol. 1984;48:306–310. doi: 10.1128/aem.48.2.306-310.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Woloshuk C.P., Cavaletto J.R., Cleveland T.E. Inducers of aflatoxin biosynthesis from colonized maize kernels are generated by an amylase activity from Aspergillus flavus. Phytopathol. 1997;87:164–169. doi: 10.1094/PHYTO.1997.87.2.164. [DOI] [PubMed] [Google Scholar]
- 207.Fanelli C., Fabbri A.A., Brasini S., de Luca C., Passi S. Effect of different inhibitors of sterol biosynthesis on both fungal growth and aflatoxin production. Nat. Toxins. 1995;3:109–113. doi: 10.1002/nt.2620030209. [DOI] [PubMed] [Google Scholar]
- 208.Fanelli C., Fabbri A.A. Relationship between lipids and aflatoxin biosynthesis. Mycopathologia. 1989;107:115–120. doi: 10.1007/BF00707547. [DOI] [PubMed] [Google Scholar]
- 209.Fanelli C., Fabbri A.A., Finotti E., Passi S. Stimulation of aflatoxin biosynthesis by lipophilic epoxides. J. Gen. Microbiol. 1983;129:1721–1723. doi: 10.1099/00221287-129-6-1721. [DOI] [PubMed] [Google Scholar]
- 210.Yu J., Mohawed S.M., Bhatnagar D., Cleveland T.E. Substrate-induced lipase gene expression and aflatoxin production in Aspergillus parasiticus and Aspergillus flavus. J. Appl. Microbiol. 2003;95:1334–1342. doi: 10.1046/j.1365-2672.2003.02096.x. [DOI] [PubMed] [Google Scholar]
- 211.Davis N.D., Diener U.L., Agnihotri V.P. Production of aflatoxins B1 and G1 in chemically defined medium. Mycopathol. Mycol. Appl. 1967;31:251–256. doi: 10.1007/BF02053422. [DOI] [PubMed] [Google Scholar]
- 212.Reddy T.V., Viswanathan L., Venkitasubramanian T.A. High aflatoxin production on a chemically defined medium. Appl. Microbiol. 1971;22:393–396. doi: 10.1128/am.22.3.393-396.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Reddy T.V., Viswanathan L., Venkitasubramanian T.A. Factors affecting aflatoxin production by Aspergillus parasiticus in a chemically defined medium. J. Gen. Microbiol. 1979;114:409–413. doi: 10.1099/00221287-114-2-409. [DOI] [PubMed] [Google Scholar]
- 214.Kachholz T., Demain A.L. Nitrate repression of averufin and aflatoxin biosynthesis Aspergillus parasiticus. J. Nat. Prod. 1983;46:499–506. doi: 10.1021/np50028a013. [DOI] [Google Scholar]
- 215.Niehaus W.G.J., Jiang W.P. Nitrate induces enzymes of the mannitol cycle and suppresses versicolorin synthesis in Aspergillus parasiticus. Mycopathologia. 1989;107:131–137. doi: 10.1007/BF00707550. [DOI] [PubMed] [Google Scholar]
- 216.Chang P.K., Ehrlich K.C., Linz J.E., Bhatnagar D., Cleveland T.E., Bennett J.W. Characterization of the Aspergillus parasiticus niaD and niiA gene cluster. Curr. Genet. 1996;30:68–75. doi: 10.1007/s002940050102. [DOI] [PubMed] [Google Scholar]
- 217.Chang P.K., Yu J., Bhatnagar D., Cleveland T.E. Characterization of the Aspergillus parasiticus major nitrogen regulatory gene, areA. Biochim. Biophys. Acta. 2000;1491:263–266. doi: 10.1016/S0167-4781(00)00004-X. [DOI] [PubMed] [Google Scholar]
- 218.Schroeder H.W., Hein H., Jr. Effect of diurnal temperature cycles on the production of aflatoxin. Appl. Microbiol. 1968;16:988–990. doi: 10.1128/am.16.7.988-990.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Diener U.L., Davis N.D. Limiting temperature and relative humidity for aflatoxin production by Aspergillus flavus in stored peanuts. J. Am. Oil Chem. Soc. 1970;47:347–351. doi: 10.1007/BF02639000. [DOI] [PubMed] [Google Scholar]
- 220.Roy A.K., Chourasia H.K. Effect of temperature on aflatoxin production in Mucuna pruriens seeds. Appl. Environ. Microbiol. 1989;55:531–532. doi: 10.1128/aem.55.2.531-532.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.OBrian G.R., Georgianna D.R., Wilkinson J.R., Yu J., Abbas H.K., Bhatnagar D., Cleveland T.E., Nierman W.C., Payne G.A. The effect of elevated temperature on gene transcription and aflatoxin biosynthesis. Mycologia. 2007;99:232–239. doi: 10.3852/mycologia.99.2.232. [DOI] [PubMed] [Google Scholar]
- 222.Yu J., Fedorova N.D., Montalbano B.G., Bhatnagar D., Cleveland T.E., Bennett J.W., Nierman W.C. Tight control of mycotoxin biosynthesis gene expression in Aspergillus flavus by temperature as revealed by RNA-Seq. FEMS Microbiol. Lett. 2011;322:145–149. doi: 10.1111/j.1574-6968.2011.02345.x. [DOI] [PubMed] [Google Scholar]
- 223.Sanders T.H., Blankenship P.D., Cole R.J., Hill R.A. Effect of soil temperature and drought on peanut pod and stem temperatures relative to Aspergillus flavus invasion and aflatoxin contamination. Mycopathologia. 1984;86:51–54. doi: 10.1007/BF00437229. [DOI] [PubMed] [Google Scholar]
- 224.Cotty P.J., Jaime-Garcia R. Influences of climate on aflatoxin producing fungi and aflatoxin contamination. Int. J. Food Microbiol. 2007;119:109–115. doi: 10.1016/j.ijfoodmicro.2007.07.060. [DOI] [PubMed] [Google Scholar]
- 225.Keller N.P., Nesbitt C., Sarr B., Phillips T.D., Burow G.B. pH regulation of sterigmatocystin and aflatoxin biosynthesis in Aspergillus spp. Phytopathol. 1997;87:643–648. doi: 10.1094/PHYTO.1997.87.6.643. [DOI] [PubMed] [Google Scholar]
- 226.Tilburn J., Sarkar S., Widdick D.A., Espeso E.A., Orejas M., Mungroo J., Penalva M.A., Arst H.N., Jr. The Aspergillus PacC zinc finger transcription factor mediates regulation of both acid- and alkaline expressed genes by ambient pH. EMBO J. 1995;14:779–790. doi: 10.1002/j.1460-2075.1995.tb07056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Espeso E.A., Tilburn J., Arst H.N., Jr., Penalva M.A. pH regulation is a major determinant in expression of a fungal penicillin biosynthetic gene. EMBO J. 1993;12:3947–3956. doi: 10.1002/j.1460-2075.1993.tb06072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Espeso E.A., Arst H.N., Jr. On the mechanism by which alkaline pH prevents expression of an acid-expressed gene. Mol. Cell Biol. 2000;20:3355–3363. doi: 10.1128/MCB.20.10.3355-3363.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Hicks J.K., Yu J.H., Keller N.P., Adams T.H. Aspergillus sporulation and mycotoxin production both require inactivation of the FadA G alpha protein-dependent signaling pathway. EMBO J. 1997;16:4916–4923. doi: 10.1093/emboj/16.16.4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Calvo A.M., Wilson R.A., Bok J.W., Keller N.P. Relationship between secondary metabolism and fungal development. Microbiol. Mol. Biol. Rev. 2002;66:447–459. doi: 10.1128/MMBR.66.3.447-459.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Reib J. Development of Aspergillus parasiticus and formation of aflatoxin B1 under the influence of conidiogenesis affecting compounds. Arch. Microbiol. 1982;133:236–238. doi: 10.1007/BF00415008. [DOI] [PubMed] [Google Scholar]
- 232.Torres J., Guarro J., Suarez G., Suñe N., Calvo M.A., Ramírez C. Morphological changes in strains of Aspergillus flavus Link ex Fries and Aspergillus parasiticus Speare related with aflatoxin production. Mycopathologia. 1980;72:171–180. doi: 10.1007/BF00572660. [DOI] [PubMed] [Google Scholar]
- 233.Guzman-de-Pena D., Aguirre J., Ruiz-Herrera J. Correlation between the regulation of sterigmatocystin biosynthesis and asexual and sexual sporulation in Emericella nidulans. Antonie Leeuwenhoek. 1998;73:199–205. doi: 10.1023/A:1000820221945. [DOI] [PubMed] [Google Scholar]
- 234.Yu J.H., Keller N. Regulation of secondary metabolism in filamentous fungi. Annu. Rev. Phytopathol. 2005;43:437–458. doi: 10.1146/annurev.phyto.43.040204.140214. [DOI] [PubMed] [Google Scholar]
- 235.Shimizu K., Keller N.P. Genetic involvement of a cAMP-dependent protein kinase in a G protein signaling pathway regulating morphological and chemical transitions in Aspergillus nidulans. Genetics. 2001;157:591–600. doi: 10.1093/genetics/157.2.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Jayashree T., Praveen Rao J., Subramanyam C. Regulation of aflatoxin production by Ca(2+)/calmodulin-dependent protein phosphorylation and dephosphorylation. FEMS Microbiol. Lett. 2000;183:215–219. doi: 10.1111/j.1574-6968.2000.tb08960.x. [DOI] [PubMed] [Google Scholar]
- 237.Mahoney N., Molyneux R.J. Phytochemical inhibition of aflatoxigenicity in Aspergillus flavus by constituents of walnut (Juglans regia) J. Agric. Food Chem. 2004;52:1882–1889. doi: 10.1021/jf030812p. [DOI] [PubMed] [Google Scholar]
- 238.Kim J.H., Campbell B.C., Molyneux R., Mahoney N., Chan K.L., Yu J., Wilkinson J.R., Cary J., Bhatnagar D., Cleveland T.E. Gene targets for fungal and mycotoxin control. Mycotoxin Res. 2006;22:3–8. doi: 10.1007/BF02954550. [DOI] [PubMed] [Google Scholar]
- 239.Reverberi M., Zjalic S., Racelli A., Fabbri A.A., Fanelli C. Oxidant/antioxidant balance in Aspergillus parasiticus affects aflatoxin biosynthesis. Mycotoxin Res. 2006;22:39–47. doi: 10.1007/BF02954556. [DOI] [PubMed] [Google Scholar]
- 240.Jayashree T., Subramanyam C. Oxidative stress as a prerequisite for aflatoxin production by Aspergillus parasiticus. Free Radic. Biol. Med. 2000;29:981–985. doi: 10.1016/S0891-5849(00)00398-1. [DOI] [PubMed] [Google Scholar]
- 241.Reverberi M., Fabbri A.A., Zjalic S., Ricelli A., Punelli F., Fanelli C. Antioxidant enzymes stimulation in Aspergillus parasiticus by Lentinula edodes inhibits aflatoxin production. Appl. Microbiol. Biotechnol. 2005;69:207–215. doi: 10.1007/s00253-005-1979-1. [DOI] [PubMed] [Google Scholar]
- 242.Zeringue H.J., Bhatnagar D. Neem and Control of Aflatoxin Contamination. In: Singh R.P., Chari M.S., Raheja A.K., Kraus W., editors. Neem and Environment. Vol. 2. Science Publishers, Inc.; Enfield, NH, USA: 1993. pp. 713–727. [Google Scholar]
- 243.Zeringue H.J., Jr., Brown R.L., Neucere J.N., Cleveland T.E. Relationships between C6-C12 alkanal and alkenal volatile contents and resistance of maize genotypes to Aspergillus flavus and aflatoxin production. J. Agric. Food Chem. 1996;44:403–407. doi: 10.1021/jf950313r. [DOI] [Google Scholar]
- 244.Zeringue H.J., Jr. Effects of methyl jasmonate on phytoalexin production and aflatoxin control in the developing cotton boll. Biochem. Syst. Ecol. 2002;30:497–503. doi: 10.1016/S0305-1978(01)00125-9. [DOI] [Google Scholar]
- 245.Greene-McDowelle D.M., Ingber B., Wright M.S., Zeringue H.J., Jr., Bhatnagar D., Cleveland T.E. The effects of selected cotton-leaf volatiles on growth, development and aflatoxin production of Aspergillus parasiticus. Toxicon. 1999;37:883–893. doi: 10.1016/S0041-0101(98)00209-8. [DOI] [PubMed] [Google Scholar]
- 246.Wright M.S., Greene-McDowelle D.M., Zeringue H.J., Bhatnagar D., Cleveland T.E. Effects of volatile aldehydes from Aspergillus-resistant varieties of corn on Aspergillus parasiticus growth and aflatoxin biosynthesis. Toxicon. 2000;38:1215–1223. doi: 10.1016/S0041-0101(99)00221-4. [DOI] [PubMed] [Google Scholar]
- 247.Wilson R.A., Gardner H.W., Keller N.P. Differentiation of aflatoxin-producing and non-producing strains of Aspergillus flavus group. Lett. Appl. Microbiol. 2001;33:291–295. doi: 10.1046/j.1472-765X.2001.00998.x. [DOI] [PubMed] [Google Scholar]
- 248.Xiulan S., Xiaolian Z., Jian T., Zhou J., Chu F.S. Preparation of gold-labeled antibody probe and its use in immunochromatography assay for detection of aflatoxin B1. Int. J. Food Microbiol. 2005;99:185–194. doi: 10.1016/j.ijfoodmicro.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 249.Chu F.S., Fan T.S., Zhang G.S., Xu Y.C., Faust S., McMahon P.L. Improved enzyme-linked immunosorbent assay for aflatoxin B1 in agricultural commodities. J. Assoc. Off. Anal. Chem. 1987;70:854–857. [PubMed] [Google Scholar]
- 250.Malloy C.D., Marr J.S. Mycotoxins and public health: A review. J. Public Health Manag. Pract. 1997;3:61–69. doi: 10.1097/00124784-199705000-00013. [DOI] [PubMed] [Google Scholar]
- 251.Yu J., Cleveland T.E. Aspergillus flavus Genomics for Discovering Genes Involved in Aflatoxin Biosynthesis. In: Rimando A.M., Baerson S.R., editors. Polyketides Biosynthesis, Biological Activity, and Genetic Engineering. Vol. 955. American Chemical Society; Washington, DC, USA: 2007. pp. 246–260. [Google Scholar]
- 252.Yu J., Cleveland T.E., Nierman W.C., Bennett J.W. Aspergillus flavus genomics: Gateway to human and animal health, food safety, and crop resistance to diseases. Rev. Iberoam. Micol. 2005;22:194–202. doi: 10.1016/S1130-1406(05)70043-7. [DOI] [PubMed] [Google Scholar]
- 253.Cleveland T.E., Yu J., Bhatnagar D., Chen Z.-Y., Brown R., Chang P.K., Cary J.W. Progress in Elucidating the Molecular Basis of the Host Plant-Aspergillus flavus Interaction: A Basis for Devising Strategies to Reduce Aflatoxin Contamination in Crops. In: Abbas H.K., editor. Aflatoxin and Food Safety. CRC Press; Boca Raton, FL, USA: 2005. pp. 167–193. [Google Scholar]
- 254.Cleveland T.E., Cary J.W., Brown R.L., Bhatnagar D., Yu J., Chang P.K., Chaln C.A., Rajasekaran K. Use of biotechnology to eliminate aflatoxin in preharvest crops. Bull. Inst. Compr. Agric. Sci. Kinki Univ. 1997;5:75–90. [Google Scholar]
- 255.Lillehoj E.B., Wall J.H. Decontamination of Aflatoxin-Contaminated Maize Grain; Proceedings of US Universities-CIMMYT Maize Aflatoxin Workshop; El Batan, Mexico. April 1987; pp. 260–279. [Google Scholar]
- 256.Cotty P.J., Bayman D.S., Egel D.S., Elias K.S. Agriculture, Aflatoxins and Aspergillus. In: Powell K., editor. The Genus Aspergillus. Plenum Press; New York, NY, USA: 1994. pp. 1–27. [Google Scholar]
- 257.Tubajika K.M., Damann K.E. Sources of resistance to aflatoxin production in maize. J. Agric. Food Chem. 2001;49:2652–2656. doi: 10.1021/jf001333i. [DOI] [PubMed] [Google Scholar]
- 258.Brown R.L., Chen Z.Y., Cleveland T.E., Russin J.S. Advances in the development of host resistance in corn to aflatoxin contamination by Aspergillus flavus. Phytopathology. 1999;89:113–117. doi: 10.1094/PHYTO.1999.89.2.113. [DOI] [PubMed] [Google Scholar]
- 259.Chen Z.Y., Brown R.L., Damann K.E., Cleveland T.E. PR10 expression in maize and its effect on host resistance against Aspergillus flavus infection and aflatoxin production. Mol. Plant Pathol. 2010;11:69–81. doi: 10.1111/j.1364-3703.2009.00574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]