Abstract
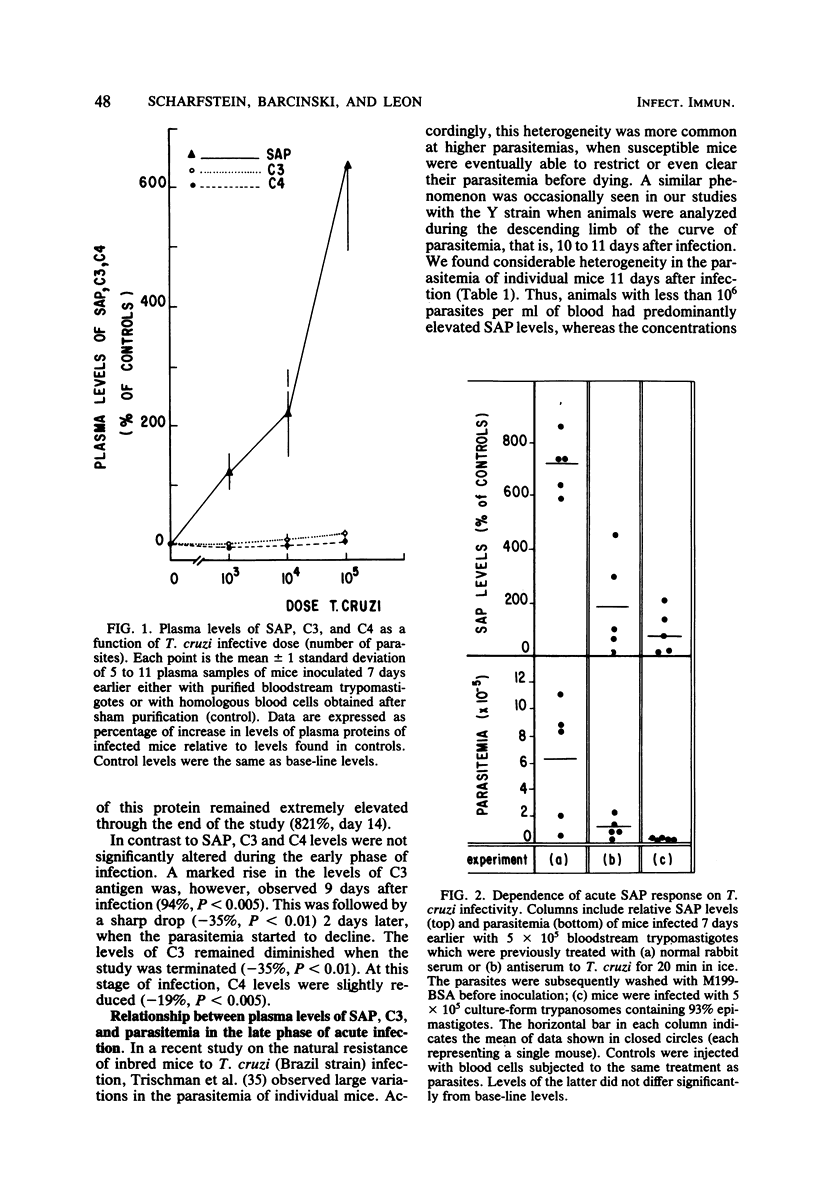
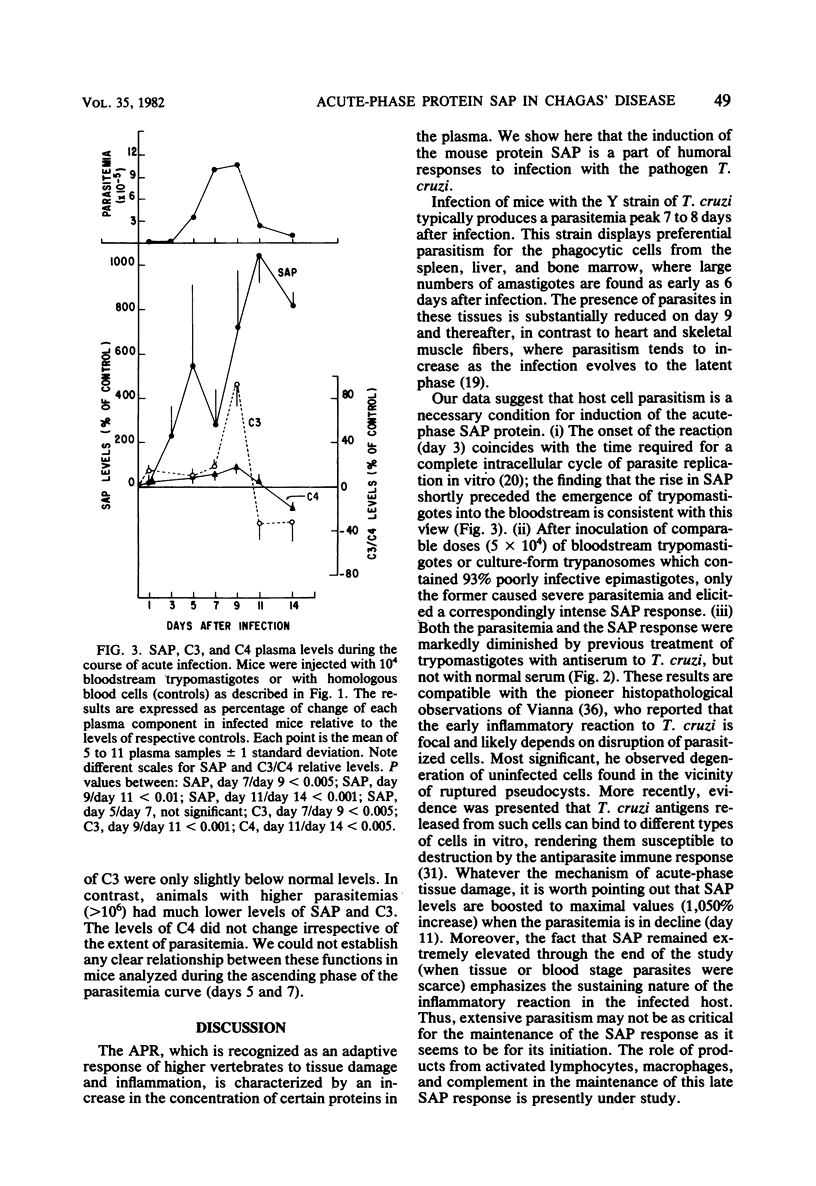
Serum amyloid P protein (SAP), which shares several structural properties with C-reactive protein, has been recently identified as an acute-phase reactant in mice. In this study, the systemic inflammatory response of mice to infection with Trypanosoma cruzi was characterized with respect to induction of SAP as well as to stage-specific alterations on complement C3 and C4 levels. The SAP response depended on the dose and infectivity of the parasites. Kinetic data indicated a close temporal relationship between the onset of parasitemia and induction of SAP. The levels of SAP were maximally enhanced (1,050%) by the time parasitemia started to regress, and the response remained elevated as the infection entered the latency phase. The decline in parasitemia was paralleled by a significant reduction in C3 levels. A reciprocal relationship between the extent of parasitemia and SAP/C3 levels became apparent when these parameters were compared in individual inbred mice during the time of decreasing parasitemia.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benson M. D., Aldo-Benson M. Effect of purified protein SAA on immune response in vitro: mechanisms of suppression. J Immunol. 1979 May;122(5):2077–2082. [PubMed] [Google Scholar]
- Brener Z. Biology of Trypanosoma cruzi. Annu Rev Microbiol. 1973;27:347–382. doi: 10.1146/annurev.mi.27.100173.002023. [DOI] [PubMed] [Google Scholar]
- Budzko D. B., Pizzimenti M. C., Kierszenbaum F. Effects of complement depletion in experimental chagas disease: immune lysis of virulent blood forms of Trypanosoma cruzi. Infect Immun. 1975 Jan;11(1):86–91. doi: 10.1128/iai.11.1.86-91.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess D. E., Hanson W. L. Trypanosoma cruzi: the T-cell dependence of the primary immune response and the effects of depletion of T cells and Ig-bearing cells on immunological memory. Cell Immunol. 1980 Jun;52(1):176–186. doi: 10.1016/0008-8749(80)90410-4. [DOI] [PubMed] [Google Scholar]
- Corsini A. C., Costa M. G., Oliveira O. L., Camargo I. J., Rangel H. A. A fraction (FAd) from Trypanosoma cruzi epimastigotes depresses the immune response in mice. Immunology. 1980 Aug;40(4):505–511. [PMC free article] [PubMed] [Google Scholar]
- Croft S. M., Mortensen R. F., Gewurz H. Binding of C-reactive protein to antigen-induced but not mitogen-induced T lymphoblasts. Science. 1976 Aug 20;193(4254):685–687. doi: 10.1126/science.1085034. [DOI] [PubMed] [Google Scholar]
- Cunningham D. S., Craig W. H., Kuhn R. E. Reduction of complement levels in mice infected with Trypanosoma cruzi. J Parasitol. 1978 Dec;64(6):1044–1049. [PubMed] [Google Scholar]
- Cunningham D. S., Kuhn R. E., Rowland E. C. Suppression of humoral responses during Trypanosoma cruzi infections in mice. Infect Immun. 1978 Oct;22(1):155–160. doi: 10.1128/iai.22.1.155-160.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham D. S., Kuhn R. E. Trypanosoma cruzi-induced suppression of the primary immune response in murine cell cultures to T-cell-dependent and -independent antigens. J Parasitol. 1980 Feb;66(1):16–27. [PubMed] [Google Scholar]
- Cunningham D. S., Kuhn R. E. Trypanosoma cruzi-induced suppressor substance. I. cellular involvement and partial characterization. J Immunol. 1980 May;124(5):2122–2129. [PubMed] [Google Scholar]
- Ferreira A., Nussenzweig V. Genetic linkage between serum levels of the third component of complement and the H-2 complex. J Exp Med. 1975 Feb 1;141(2):513–517. doi: 10.1084/jem.141.2.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotschlich E. C., Edelman G. M. Binding properties and specificity of C-reactive protein. Proc Natl Acad Sci U S A. 1967 Mar;57(3):706–712. doi: 10.1073/pnas.57.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J., Bagai R. C. Immune deficiency states associated with malignant disease in man. Med Clin North Am. 1972 Mar;56(2):501–514. doi: 10.1016/s0025-7125(16)32410-5. [DOI] [PubMed] [Google Scholar]
- Levin M., Pras M., Franklin E. C. Immunologic studies of the major nonimmunoglobulin protein of amyloid. I. Identification and partial characterization of a related serum component. J Exp Med. 1973 Aug 1;138(2):373–380. doi: 10.1084/jem.138.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo R. C., Brener Z. Tissue tropism of different Trypanosoma cruzi strains. J Parasitol. 1978 Jun;64(3):475–482. [PubMed] [Google Scholar]
- Mortensen R. F., Braun D., Gewurz H. Effects of C-reactive protein on lymphocyte functions. III. Inhibition of antigen-induced lymphocyte stimulation and lymphokine production. Cell Immunol. 1977 Jan;28(1):59–68. doi: 10.1016/s0008-8749(77)80006-3. [DOI] [PubMed] [Google Scholar]
- Mortensen R. F. C-Reactive protein (CRP)-mediated inhibition of the induction of in vitro antibody formation. I. T-cell dependence of the inhibition. Cell Immunol. 1979 May;44(2):270–282. doi: 10.1016/0008-8749(79)90005-4. [DOI] [PubMed] [Google Scholar]
- Mortensen R. F., Gewurz H. Effects of C-reactive protein on the lymphoid system. II. Inhibition of mixed lymphocyte reactivity and generation of cytotoxic lymphocytes. J Immunol. 1976 May;116(5):1244–1250. [PubMed] [Google Scholar]
- Nogueira N., Cohn Z. A. Trypanosoma cruzi: in vitro induction of macrophage microbicidal activity. J Exp Med. 1978 Jul 1;148(1):288–300. doi: 10.1084/jem.148.1.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira N., Gordon S., Cohn Z. Trypanosoma cruzi: modification of macrophage function during infection. J Exp Med. 1977 Jul 1;146(1):157–171. doi: 10.1084/jem.146.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmand A. P., Friedenson B., Gewurz H., Painter R. H., Hofmann T., Shelton E. Characterization of C-reactive protein and the complement subcomponent C1t as homologous proteins displaying cyclic pentameric symmetry (pentraxins). Proc Natl Acad Sci U S A. 1977 Feb;74(2):739–743. doi: 10.1073/pnas.74.2.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepys M. B., Baltz M., Gomer K., Davies A. J., Doenhoff M. Serum amyloid P-component is an acute-phase reactant in the mouse. Nature. 1979 Mar 15;278(5701):259–261. doi: 10.1038/278259a0. [DOI] [PubMed] [Google Scholar]
- Pepys M. B., Dash A. C. Isolation of amyloid P component (protein AP) from normal serum as a calcium-dependent binding protein. Lancet. 1977 May 14;1(8020):1029–1031. doi: 10.1016/s0140-6736(77)91260-0. [DOI] [PubMed] [Google Scholar]
- Pepys M. B., Dyck R. F., de Beer F. C., Skinner M., Cohen A. S. Binding of serum amyloid P-component (SAP) by amyloid fibrils. Clin Exp Immunol. 1979 Nov;38(2):284–293. [PMC free article] [PubMed] [Google Scholar]
- Playfair J. H. Effective and ineffective immune responses to parasites: evidence from experimental models. Curr Top Microbiol Immunol. 1978;80:37–64. doi: 10.1007/978-3-642-66956-9_2. [DOI] [PubMed] [Google Scholar]
- Ribeiro Dos Santos R., Hudson L. Trypanosoma cruzi: immunological consequences of parasite modification of host cells. Clin Exp Immunol. 1980 Apr;40(1):36–41. [PMC free article] [PubMed] [Google Scholar]
- Selinger M. J., McAdam K. P., Kaplan M. M., Sipe J. D., Vogel S. N., Rosenstreich D. L. Monokine-induced synthesis of serum amyloid A protein by hepatocytes. Nature. 1980 Jun 12;285(5765):498–500. doi: 10.1038/285498a0. [DOI] [PubMed] [Google Scholar]
- Shreffler D C, Owen R D. A Serologically Detected Variant in Mouse Serum: Inheritance and Association with the Histocompatibility-2 Locus. Genetics. 1963 Jan;48(1):9–25. doi: 10.1093/genetics/48.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trischmann T., Tanowitz H., Wittner M., Bloom B. Trypanosoma cruzi: role of the immune response in the natural resistance of inbred strains of mice. Exp Parasitol. 1978 Aug;45(2):160–168. doi: 10.1016/0014-4894(78)90055-3. [DOI] [PubMed] [Google Scholar]


