Abstract
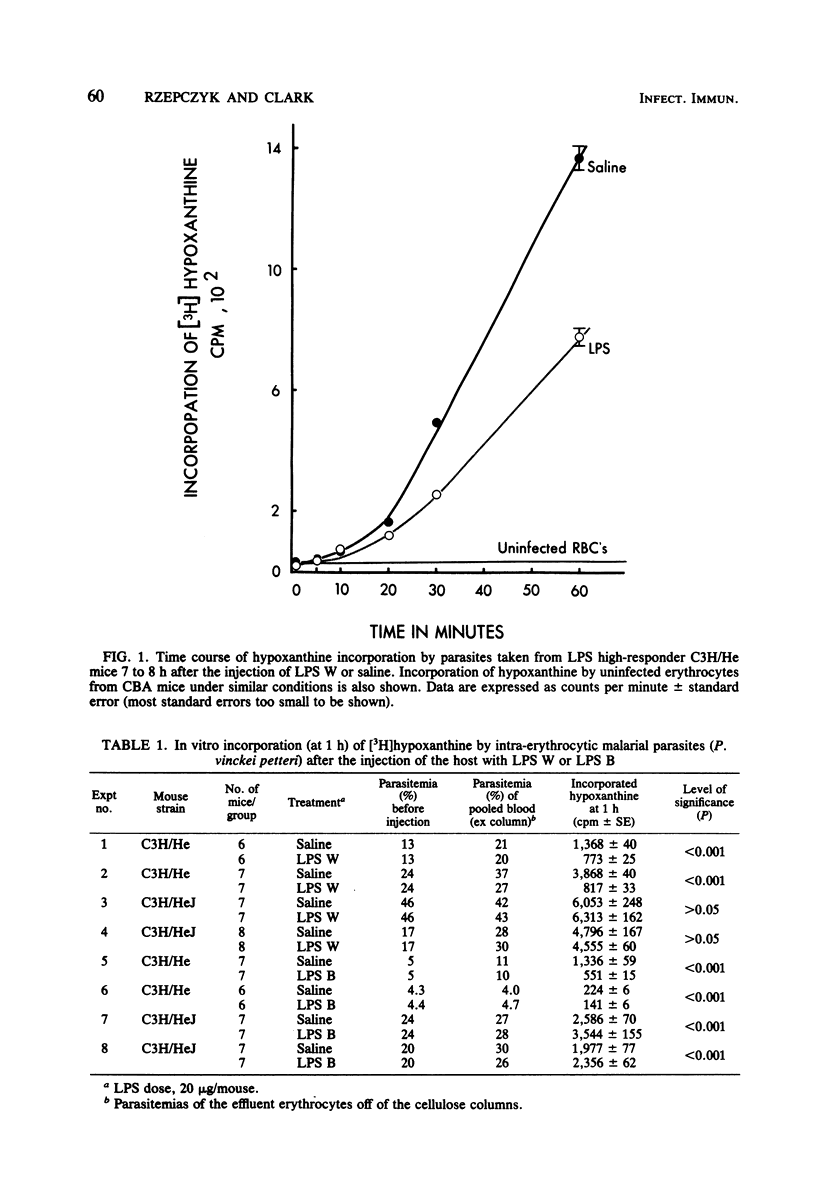
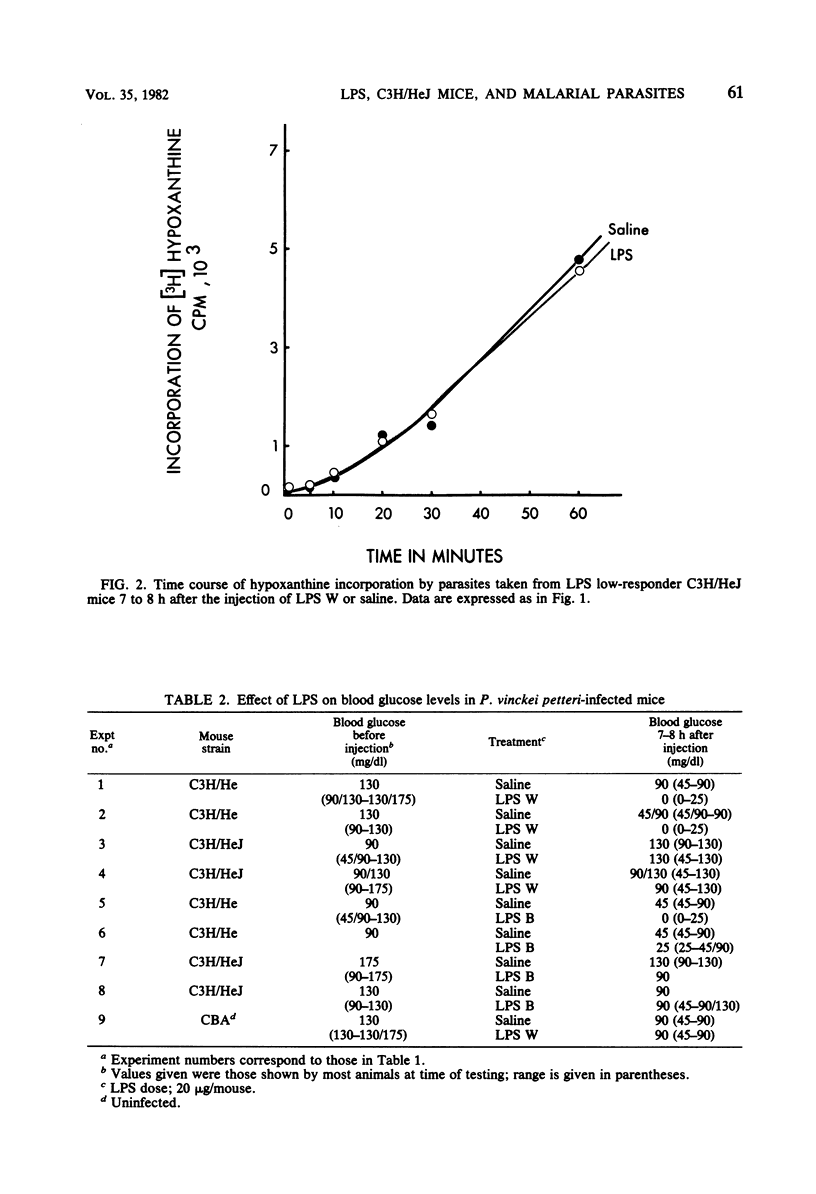
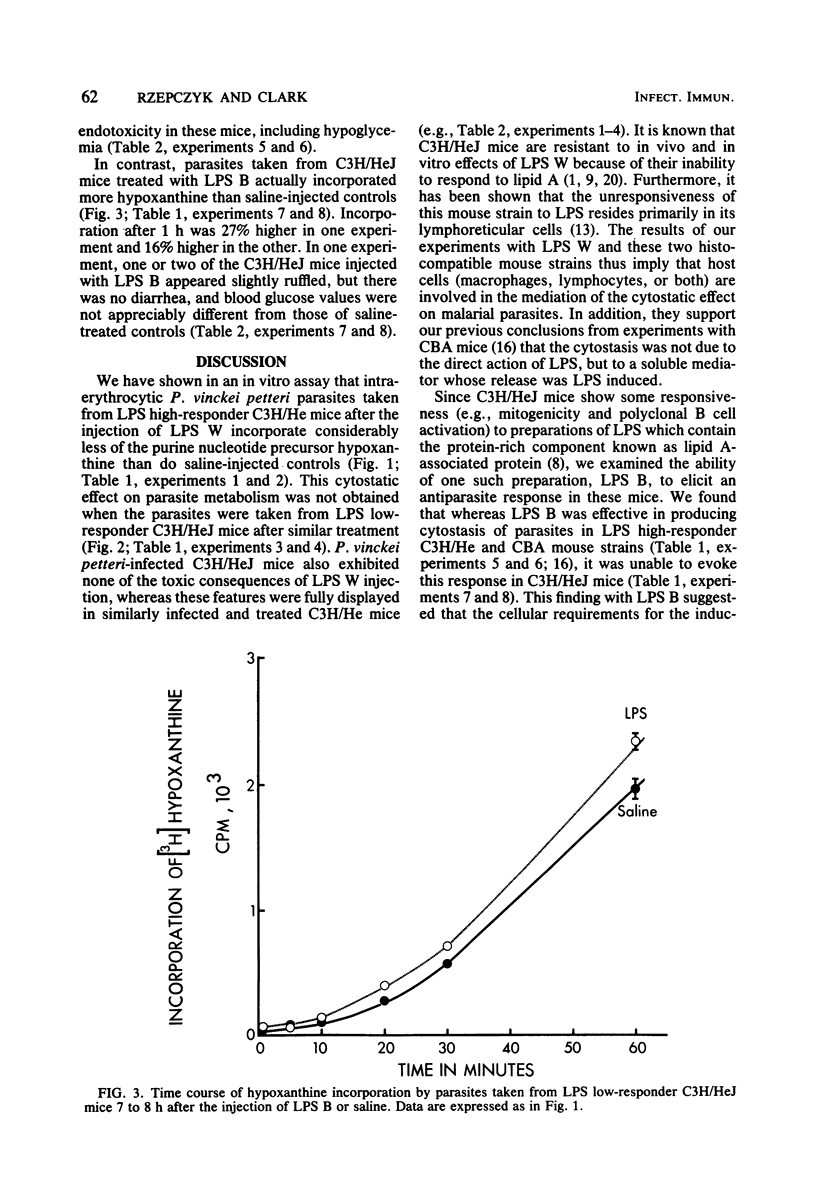
Malarial parasites, Plasmodium vinckei petteri, taken from lipopolysaccharide (LPS) high-responder (C3H/HeJGiFWeHi) mice which had been injected 7 to 8 h previously with either Escherichia coli LPS B or LPS W incorporated the purine nucleotide precursor hypoxanthine more slowly in an in vitro assay than parasites taken from saline-injected controls. In contrast, malarial parasites taken from LPS low-responder C3H/HeJ mice after injection of either LPS B or LPS W did not show reduced levels of hypoxanthine incorporation. These differing results with LPS high- and low-responder mouse strains demonstrated that the cytostatic effect on the parasites seen in the high-responder strain was not due to the direct action of LPS and implied that the cytostasis was mediated via host lymphoreticular cells. Furthermore, the failure of LPS B, a lipid A-associated protein-containing LPS preparation, to elicit a cytostatic effect on P. vinckei petteri in C3H/HeJ mice suggested that the LPS-induced effector mechanisms acting against malarial parasites may be similar to those reported against bacteria and tumors.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apte R. N., Ascher O., Pluznik D. H. Genetic analysis of generation of serum interferon by bacterial lipopolysaccharide. J Immunol. 1977 Dec;119(6):1898–1902. [PubMed] [Google Scholar]
- Chedid L., Parant M., Damais C., Parant F., Juy D., Galelli A. Failure of endotoxin to increase nonspecific resistance to infection of lipopolysaccharide low-responder mice. Infect Immun. 1976 Mar;13(3):722–727. doi: 10.1128/iai.13.3.722-727.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark I. A., Allison A. C., Cox F. E. Protection of mice against Babesia and Plasmodium with BCG. Nature. 1976 Jan 29;259(5541):309–311. doi: 10.1038/259309a0. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Cox F. E., Allison A. C. Protection of mice against Babesia spp. and Plasmodium spp. with killed Corynebacterium parvum. Parasitology. 1977 Feb;74(1):9–18. doi: 10.1017/s003118200004748x. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Virelizier J. L., Carswell E. A., Wood P. R. Possible importance of macrophage-derived mediators in acute malaria. Infect Immun. 1981 Jun;32(3):1058–1066. doi: 10.1128/iai.32.3.1058-1066.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galelli A., le Garrec Y., Chedid L. Transfer by bone marrow cells of increased natural resistance to Klebsiella pneumoniae induced by lipopolysaccharide in genetically deficient C3H/He mice. Infect Immun. 1979 Feb;23(2):232–238. doi: 10.1128/iai.23.2.232-238.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman M. G., Parks D. E., Weigle W. O. Immunologic responsiveness of the C3H/HeJ mouse: differential ability of butanol-extracted lipopolysaccharide (LPS) to evoke LPS-mediated effects. J Exp Med. 1978 Mar 1;147(3):800–813. doi: 10.1084/jem.147.3.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor R. R., Sheagren J. N., Wolff S. M. Endotoxin-induced modification of Plasmodium berghei infection in mice. J Immunol. 1969 Jan;102(1):131–139. [PubMed] [Google Scholar]
- Martin L. K., Einheber A., Sadun E. H., Wren R. E. Effect of bacterial endotoxin on the course of Plasmodium berghei infection. Exp Parasitol. 1967 Apr;20(2):186–199. doi: 10.1016/0014-4894(67)90038-0. [DOI] [PubMed] [Google Scholar]
- Michalek S. M., Moore R. N., McGhee J. R., Rosenstreich D. L., Mergenhagen S. E. The primary role of lymphoreticular cells in the mediation of host responses to bacterial endotoxim. J Infect Dis. 1980 Jan;141(1):55–63. doi: 10.1093/infdis/141.1.55. [DOI] [PubMed] [Google Scholar]
- Männel D. N., Meltzer M. S., Mergenhagen S. E. Generation and characterization of a lipopolysaccharide-induced and serum-derived cytotoxic factor for tumor cells. Infect Immun. 1980 Apr;28(1):204–211. doi: 10.1128/iai.28.1.204-211.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Männel D. N., Moore R. N., Mergenhagen S. E. Macrophages as a source of tumoricidal activity (tumor-necrotizing factor). Infect Immun. 1980 Nov;30(2):523–530. doi: 10.1128/iai.30.2.523-530.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parant M. A., Parant F. J., Chedid L. A. Enhancement of resistance to infections by endotoxin-induced serum factor from Mycobacterium bovis BCG-infected mice. Infect Immun. 1980 Jun;28(3):654–659. doi: 10.1128/iai.28.3.654-659.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn T. C., Wyler D. J. Resolution of acute malaria (Plasmodium berghei in the rat): reversibility and spleen dependence. Am J Trop Med Hyg. 1980 Jan;29(1):1–4. doi: 10.4269/ajtmh.1980.29.1. [DOI] [PubMed] [Google Scholar]
- Rzepczyk C. M., Clark I. A. Demonstration of a lipopolysaccharide-induced cytostatic effect on malarial parasites. Infect Immun. 1981 Aug;33(2):343–347. doi: 10.1128/iai.33.2.343-347.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah R. G., Green S., Moore M. A. Colony stimulating and inhibiting activities in mouse serum after Corynebacterium parvum-endotoxin treatment. J Reticuloendothel Soc. 1978 Jan;23(1):29–41. [PubMed] [Google Scholar]
- Sherman I. W. Biochemistry of Plasmodium (malarial parasites). Microbiol Rev. 1979 Dec;43(4):453–495. doi: 10.1128/mr.43.4.453-495.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl L. M., Rosenstreich D. L., Glode L. M., Sandberg A. L., Mergenhagen S. E. Defective prostaglandin synthesis by C3H/HeJ mouse macrophages stimulated with endotoxin preparations. Infect Immun. 1979 Jan;23(1):8–13. doi: 10.1128/iai.23.1.8-13.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]


