Abstract
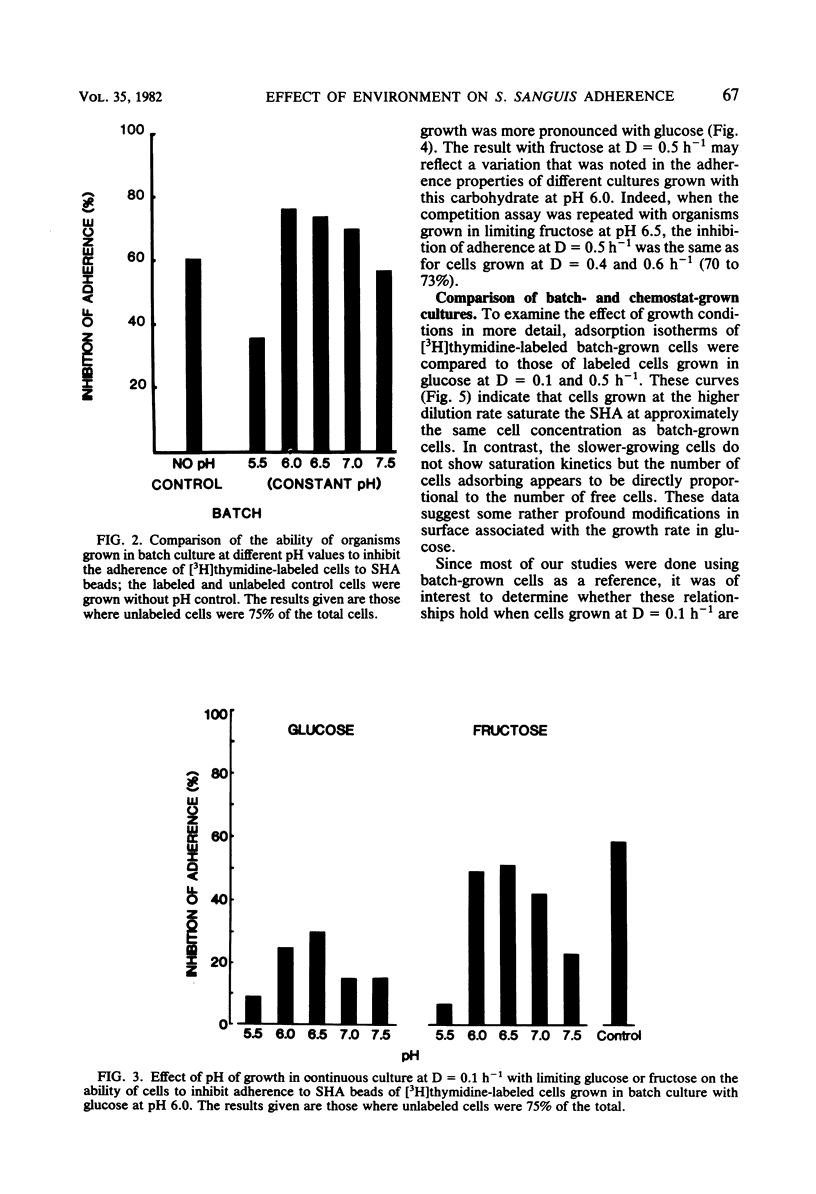
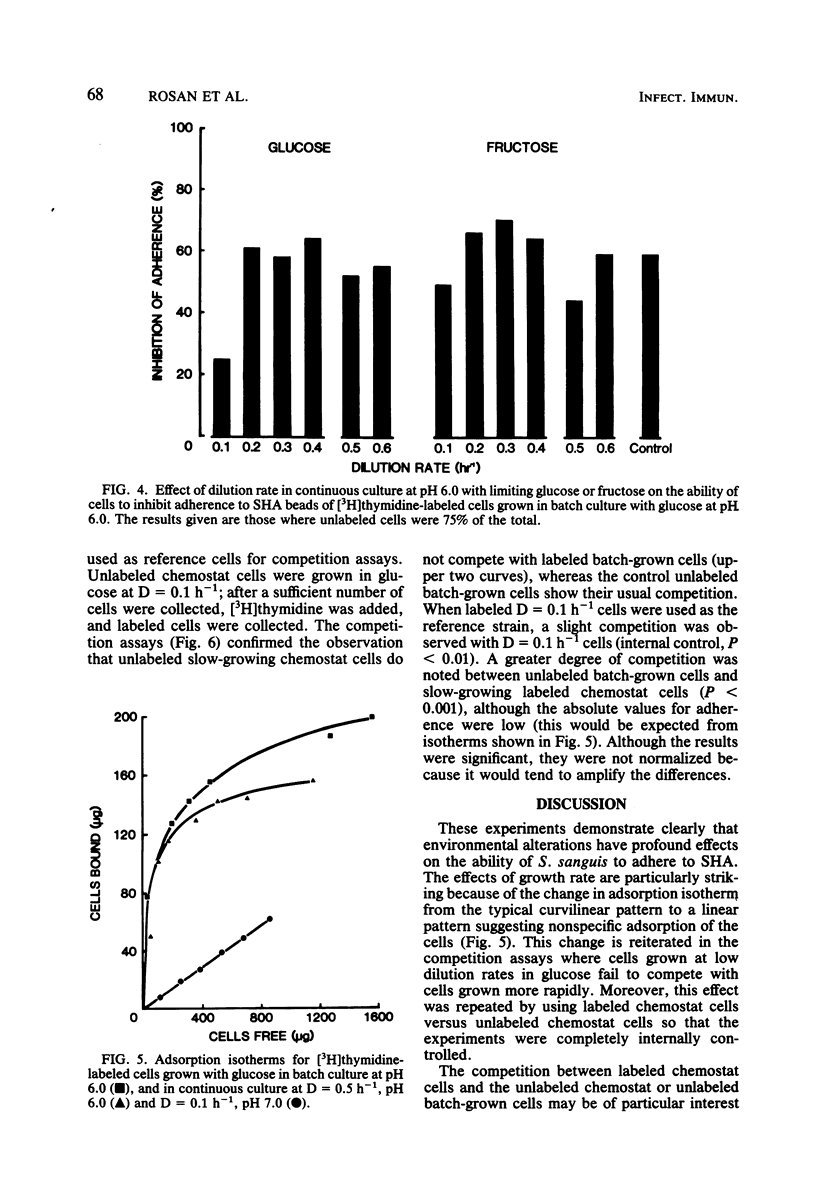
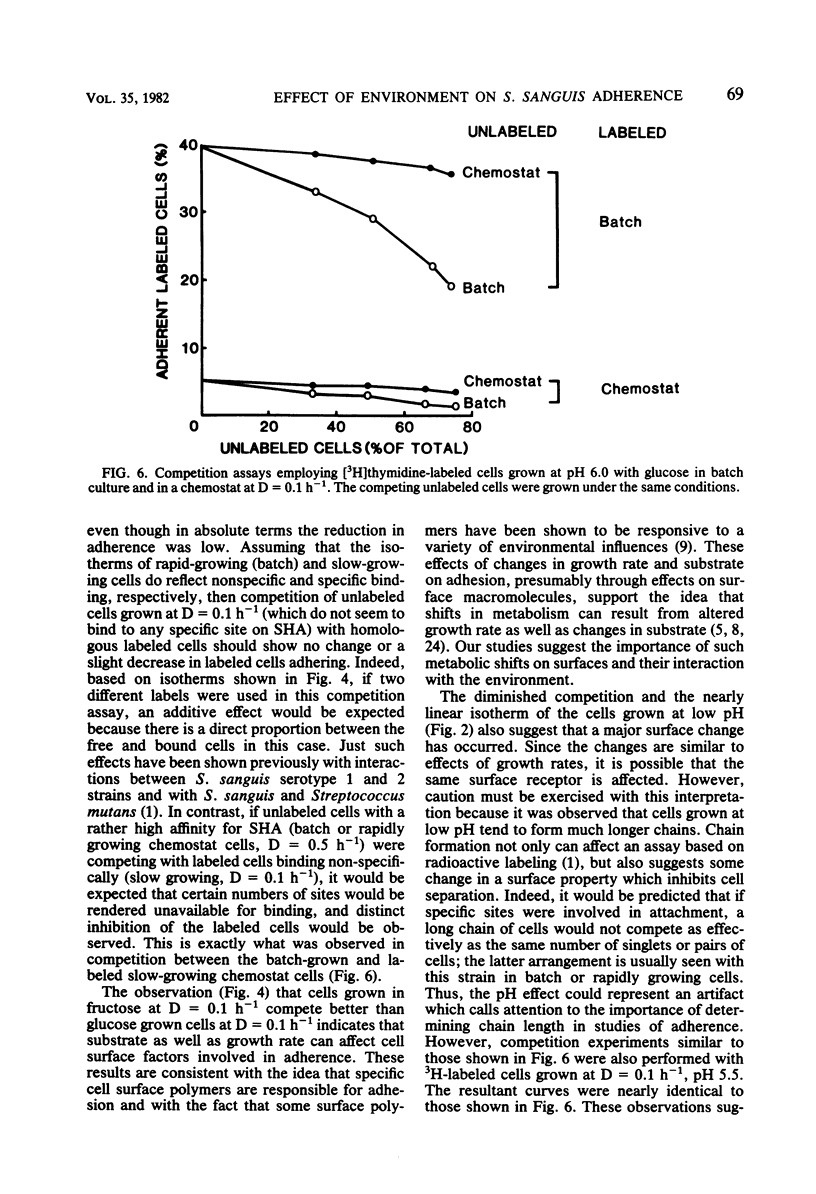
Streptococcus sanguis is a major component of early dental plaque. The ability of S. sanguis to adhere to salivary pellicle appears to involve specific bacterial surface receptors. The nature of these receptors is still not known; however, the component molecules may be subject to environmental control as has been shown for teichoic acids and certain proteins. To study these environmental effects, a chemostat was employed to vary the growth conditions of Streptococcus sanguis strain G9B. This strain has been used extensively to study the adhesion of [3H]thymidine-labeled batch-grown cells to saliva-coated hydroxyapatite beads. The effects of dilution rate, pH, and carbon source on adhesion were studied with a competition assay in which the labeled batch cells were used as a reference standard. In this assay, cells from the chemostat were harvested and compared for their ability to inhibit adhesion of labeled cells relative to unlabeled control batch-grown cells. Subsequent studies used chemostat grown cells labeled with [3H]thymidine as a reference standard so that results were internally controlled and reflected only the particular alteration in environment which was studied. These results indicated that when glucose was used as a growth-limiting substrate, cells grown at relatively high dilution rates (D = 0.5 h−1; mean generation time = 1.4 h) behaved similarly to batch-grown cells and appeared to compete for the same binding sites. Cells grown at D = 0.1 h−1 (mean generation time = 7 h) no longer competed with either batch-grown cells or chemostat cells grown at D = 0.5 h−1. Moreover, adsorption isotherms of such slow-growing cells (D = 0.1 h−1) suggested that binding was no longer specific. When fructose was used as the growth-limiting carbohydrate, cells grown at D = 0.1 h−1 did not show this loss of specificity and competed nearly as well as control batch-grown glucose cells. However, the effect of pH appeared to be independent of carbohydrate source, because cells grown in either glucose or fructose at pH 5.5 at D = 0.1 h−1 lost the ability to compete with reference batch or chemostat cells grown at D = 0.5 h−1. This effect was very sharp, since cells grown in the pH range from 6 to 7.5 at D = 0.5 h−1 competed nearly as well as control cells. A similar effect of pH was found for batch cultures grown with excess glucose. These studies reinforce the idea that the environment can profoundly affect the bacterial surface and consequently the ability of the organism to adhere, a property which appears to be a primary event in some infectious diseases and in dental plaque formation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelbaum B., Golub E., Holt S. C., Rosan B. In vitro studies of dental plaque formation: adsorption of oral streptococci to hydroxyaptite. Infect Immun. 1979 Aug;25(2):717–728. doi: 10.1128/iai.25.2.717-728.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J., Grahnén H., Jonsson G. Lactobacilli and streptococci in the mouth of children. Caries Res. 1975;9(5):333–339. doi: 10.1159/000260166. [DOI] [PubMed] [Google Scholar]
- Carlsson J., Grahnén H., Jonsson G., Wikner S. Establishment of Streptococcus sanguis in the mouths of infants. Arch Oral Biol. 1970 Dec;15(12):1143–1148. doi: 10.1016/0003-9969(70)90005-1. [DOI] [PubMed] [Google Scholar]
- Clark W. B., Bammann L. L., Gibbons R. J. Comparative estimates of bacterial affinities and adsorption sites on hydroxyapatite surfaces. Infect Immun. 1978 Mar;19(3):846–853. doi: 10.1128/iai.19.3.846-853.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellwood D. C., Hunter J. R., Longyear V. M. Growth of Streptococcus mutans in a chemostat. Arch Oral Biol. 1974 Aug;19(8):659–664. doi: 10.1016/0003-9969(74)90134-4. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Moreno E. C., Spinell D. M. Model delineating the effects of a salivary pellicle on the adsorption of Streptococcus miteor onto hydroxyapatite. Infect Immun. 1976 Oct;14(4):1109–1112. doi: 10.1128/iai.14.4.1109-1112.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. On the formation of dental plaques. J Periodontol. 1973 Jun;44(6):347–360. doi: 10.1902/jop.1973.44.6.347. [DOI] [PubMed] [Google Scholar]
- Hamilton I. R., Phipps P. J., Ellwood D. C. Effect of growth rate and glucose concentration on the biochemical properties of Streptococcus mutans Ingbritt in continuous culture. Infect Immun. 1979 Dec;26(3):861–869. doi: 10.1128/iai.26.3.861-869.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy L., Jacques N. A., Forester H., Campbell L. K., Knox K. W., Wicken A. J. Effect of fructose and other carbohydrates on the surface properties, lipoteichoic acid production, and extracellular proteins of Streptococcus mutans Ingbritt grown in continuous culture. Infect Immun. 1981 Jan;31(1):78–87. doi: 10.1128/iai.31.1.78-87.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques N. A., Hardy L., Campbell L. K., Knox K. W., Evans J. D., Wicken A. J. Effect of carbohydrate source and growth conditions on the production of lipoteichoic acid by Streptococcus mutans Ingbritt. Infect Immun. 1979 Dec;26(3):1079–1087. doi: 10.1128/iai.26.3.1079-1087.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques N. A., Hardy L., Knox K. W., Wicken A. J. Effect of growth conditions on the formation of extracellular lipoteichoic acid by Streptococcus mutans BHT. Infect Immun. 1979 Jul;25(1):75–84. doi: 10.1128/iai.25.1.75-84.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. J. A special relationship between spherical and filamentous microorganisms in mature human dental plaque. Arch Oral Biol. 1972 Mar;17(3):613–616. doi: 10.1016/0003-9969(72)90081-7. [DOI] [PubMed] [Google Scholar]
- Knox K. W., Jacques N. A., Campbell L. K., Wicken A. J., Hurst S. F., Bleiweis A. S. Phenotypic stability of the cell wall of Streptococcus mutans Ingbritt grown under various conditions. Infect Immun. 1979 Dec;26(3):1071–1078. doi: 10.1128/iai.26.3.1071-1078.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancy P., Jr, Appelbaum B., Holt S. C., Rosan B. Quantitative in vitro assay for "corncob" formation. Infect Immun. 1980 Aug;29(2):663–670. doi: 10.1128/iai.29.2.663-670.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Listgarten M. A., Mayo H., Amsterdam M. Ultrastructure of the attachment device between coccal and filamentous microorganisms in "corn cob" formations of dental plaque. Arch Oral Biol. 1973 May;18(5):651–656. doi: 10.1016/0003-9969(73)90105-2. [DOI] [PubMed] [Google Scholar]
- McIntire F. C., Vatter A. E., Baros J., Arnold J. Mechanism of coaggregation between Actinomyces viscosus T14V and Streptococcus sanguis 34. Infect Immun. 1978 Sep;21(3):978–988. doi: 10.1128/iai.21.3.978-988.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouton C., Reynolds H. S., Genco R. J. Characterization of tufted streptococci isolated from the "corn cob" configuration of human dental plaque. Infect Immun. 1980 Jan;27(1):235–245. doi: 10.1128/iai.27.1.235-245.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz H. L. Microbial population shifts in developing human dental plaque. Arch Oral Biol. 1967 Dec;12(12):1561–1568. doi: 10.1016/0003-9969(67)90190-2. [DOI] [PubMed] [Google Scholar]
- Rosan B. Antigens of Streptococcus sanguis. Infect Immun. 1973 Feb;7(2):205–211. doi: 10.1128/iai.7.2.205-211.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rølla G., Oppermann R. V., Bowen W. H., Ciardi J. E., Knox K. W. High amounts of lipoteichoic acid in sucrose-induced plaque in vivo. Caries Res. 1980;14(4):235–238. doi: 10.1159/000260459. [DOI] [PubMed] [Google Scholar]
- Slee A. M., Tanzer J. M. Phosphoenolpyruvate-dependent sucrose phosphotransferase activity in Streptococcus mutans NCTC 10449. Infect Immun. 1979 Jun;24(3):821–828. doi: 10.1128/iai.24.3.821-828.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Houte J., Gibbons R. J., Pulkkinen A. J. Adherence as an ecological determinant for streptococci in the human mouth. Arch Oral Biol. 1971 Oct;16(10):1131–1141. doi: 10.1016/0003-9969(71)90042-2. [DOI] [PubMed] [Google Scholar]


