Abstract
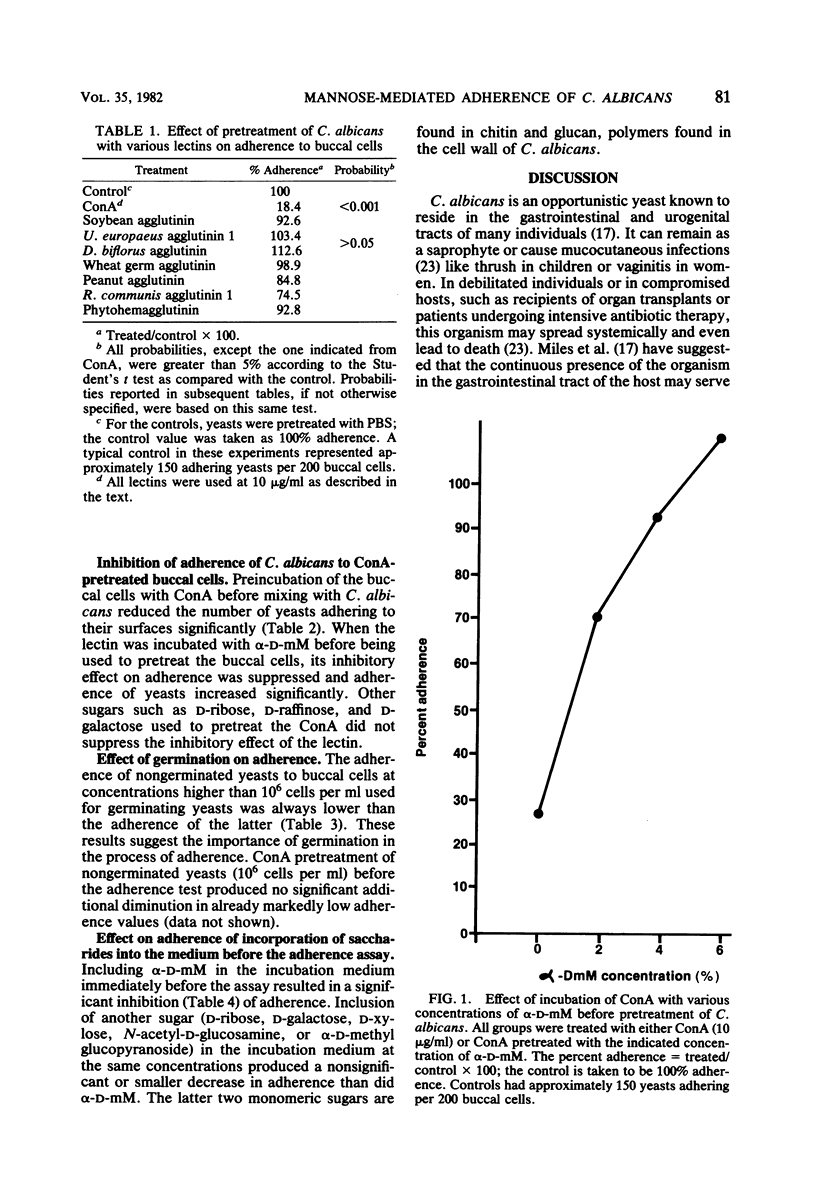
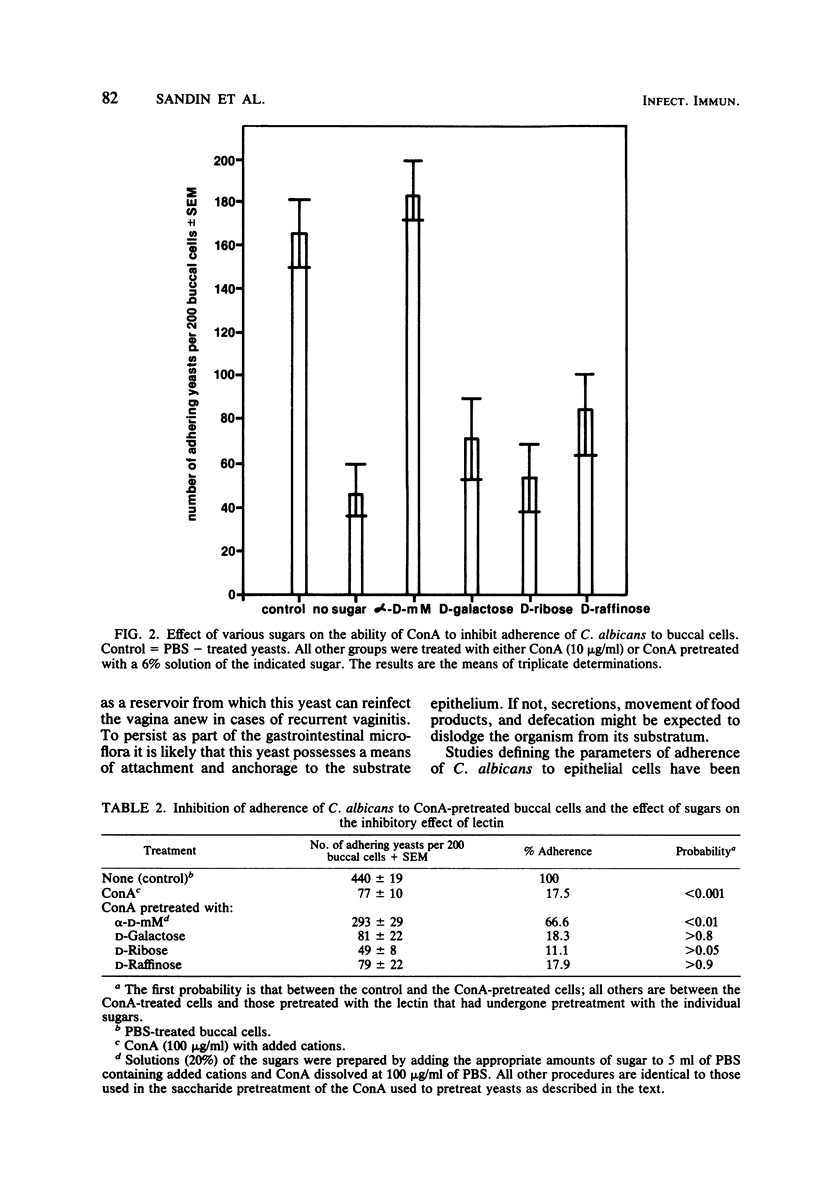
Various lectins and sugars were used to study the possible role of saccharide-containing moieties on the surface of Candida albicans and human buccal cells in the adherence of this yeast to mucosal surfaces. The lectins possessed affinities for several different sugar moieties and were used to pretreat C. albicans or buccal cells before mixing and incubating in the adherence assay. It was found that concanavalin A, a lectin that recognizes mannose and glucose, inhibited adherence of the pretreated yeasts to buccal cells and also inhibited adherence of pretreated buccal cells to nonpretreated yeast cells. Adherence was restored by preincubating the concanavalin A with a mannose derivative, but preincubation of concanavalin A with other sugars did not produce this effect. Lectins that do not recognize mannose had no effect on adherence. The presence of alpha-D-methyl mannopyranoside in the incubation medium during the assay inhibited adherence, whereas other sugars did not. Germinated yeasts adhered to buccal cells more effectively than nongerminated cells and were more susceptible to adherence inhibition by concanavalin A than were nongerminated yeasts. Thus, mannose-containing moieties on the surface of C. albicans and buccal cells could mediate the adherence of this yeast to human epithelium.
Full text
PDF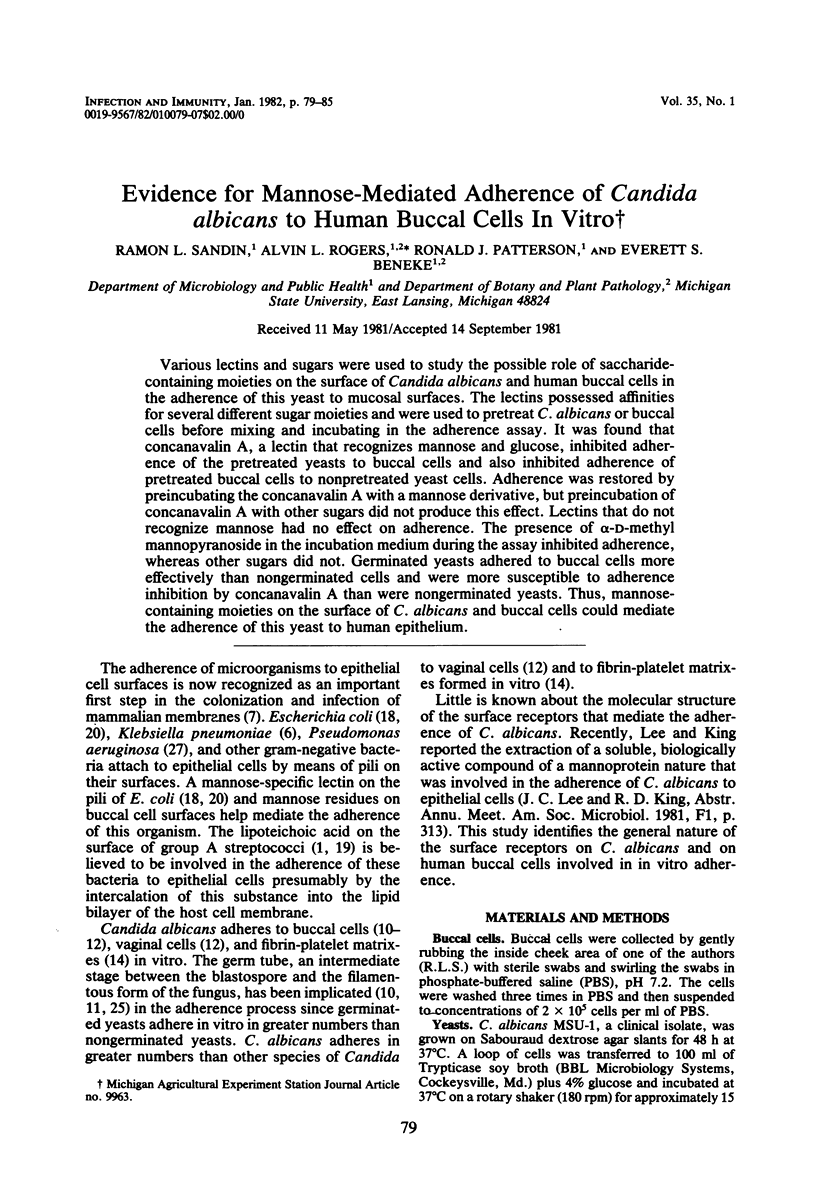




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartelt M. A., Duncan J. L. Adherence of group A streptococci to human epithelial cells. Infect Immun. 1978 Apr;20(1):200–208. doi: 10.1128/iai.20.1.200-208.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassone A., Mattia E., Boldrini L. Agglutination of blastospores of Candida albicans by concanavalin A and its relationship with the distribution of mannan polymers and the ultrastructure of the cell wall. J Gen Microbiol. 1978 Apr;105(2):263–273. doi: 10.1099/00221287-105-2-263. [DOI] [PubMed] [Google Scholar]
- Dazzo F. B., Napoli C. A., Hubbell D. H. Adsorption of bacteria to roots as related to host specificity in the Rhizobium-clover symbiosis. Appl Environ Microbiol. 1976 Jul;32(1):166–171. doi: 10.1128/aem.32.1.166-171.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. G., Richardson M. D., Odds F. C., Holland K. T. Relevance of antigenicity of Candida albicans growth phases to diagnosis of systemic candidiasis. Br Med J. 1973 Oct 13;4(5884):86–87. doi: 10.1136/bmj.4.5884.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fader R. C., Avots-Avotins A. E., Davis C. P. Evidence for pili-mediated adherence of Klebsiella pneumoniae to rat bladder epithelial cells in vitro. Infect Immun. 1979 Aug;25(2):729–737. doi: 10.1128/iai.25.2.729-737.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Houte J. V. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- Goldstein I. J., So L. L. Protein-carbonhydrate interaction. 3. Agar gel-diffusion studies on the interaction of Concanavalin A, a lectin isolated from jack bean, with polysaccharides. Arch Biochem Biophys. 1965 Aug;111(2):407–414. doi: 10.1016/0003-9861(65)90203-1. [DOI] [PubMed] [Google Scholar]
- Kimura L. H., Pearsall N. N. Adherence of Candida albicans to human buccal epithelial cells. Infect Immun. 1978 Jul;21(1):64–68. doi: 10.1128/iai.21.1.64-68.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura L. H., Pearsall N. N. Relationship between germination of Candida albicans and increased adherence to human buccal epithelial cells. Infect Immun. 1980 May;28(2):464–468. doi: 10.1128/iai.28.2.464-468.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King R. D., Lee J. C., Morris A. L. Adherence of Candida albicans and other Candida species to mucosal epithelial cells. Infect Immun. 1980 Feb;27(2):667–674. doi: 10.1128/iai.27.2.667-674.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljemark W. F., Gibbons R. J. Suppression of Candida albicans by human oral streptococci in gnotobiotic mice. Infect Immun. 1973 Nov;8(5):846–849. doi: 10.1128/iai.8.5.846-849.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisch P. A., Calderone R. A. Adherence of Candida albicans to a fibrin-platelet matrix formed in vitro. Infect Immun. 1980 Feb;27(2):650–656. doi: 10.1128/iai.27.2.650-656.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisch P. A., Calderone R. A. Role of surface mannan in the adherence of Candida albicans to fibrin-platelet clots formed in vitro. Infect Immun. 1981 Apr;32(1):92–97. doi: 10.1128/iai.32.1.92-97.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCourtie J., Douglas L. J. Relationship between cell surface composition of Candida albicans and adherence to acrylic after growth on different carbon sources. Infect Immun. 1981 Jun;32(3):1234–1241. doi: 10.1128/iai.32.3.1234-1241.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles M. R., Olsen L., Rogers A. Recurrent vaginal candidiasis. Importance of an intestinal reservoir. JAMA. 1977 Oct 24;238(17):1836–1837. doi: 10.1001/jama.238.17.1836. [DOI] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H., Jefferson W., Campbell G. L. Cell membrane-binding properties of group A streptococcal lipoteichoic acid. J Exp Med. 1975 May 1;141(5):990–1003. doi: 10.1084/jem.141.5.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H. Mannose binding and epithelial cell adherence of Escherichia coli. Infect Immun. 1978 Oct;22(1):247–254. doi: 10.1128/iai.22.1.247-254.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Mirelman D., Sharon N. Adherence of Escherichia coli to human mucosal cells mediated by mannose receptors. Nature. 1977 Feb 17;265(5595):623–625. doi: 10.1038/265623a0. [DOI] [PubMed] [Google Scholar]
- Rauvala H., Carter W. G., Hakomori S. I. Studies on cell adhesion and recognition. I. Extent and specificity of cell adhesion triggered by carbohydrate-reactive proteins (glycosidases and lectins) and by fibronectin. J Cell Biol. 1981 Jan;88(1):127–137. doi: 10.1083/jcb.88.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauvala H., Hakomori S. I. Studies on cell adhesion and recognition. III. The occurrence of alpha-mannosidase at the fibroblast cell surface, and its possible role in cell recognition. J Cell Biol. 1981 Jan;88(1):149–159. doi: 10.1083/jcb.88.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon N., Lis H. Lectins: cell-agglutinating and sugar-specific proteins. Science. 1972 Sep 15;177(4053):949–959. doi: 10.1126/science.177.4053.949. [DOI] [PubMed] [Google Scholar]
- Sobel J. D., Myers P. G., Kaye D., Levison M. E. Adherence of Candida albicans to human vaginal and buccal epithelial cells. J Infect Dis. 1981 Jan;143(1):76–82. doi: 10.1093/infdis/143.1.76. [DOI] [PubMed] [Google Scholar]
- TASCHDJIAN C. L., KOZINN P. J. Laboratory and clinical studies on candidiasis in the newborn infant. J Pediatr. 1957 Apr;50(4):426–433. doi: 10.1016/s0022-3476(57)80252-2. [DOI] [PubMed] [Google Scholar]
- Woods D. E., Straus D. C., Johanson W. G., Jr, Berry V. K., Bass J. A. Role of pili in adherence of Pseudomonas aeruginosa to mammalian buccal epithelial cells. Infect Immun. 1980 Sep;29(3):1146–1151. doi: 10.1128/iai.29.3.1146-1151.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]


