Abstract
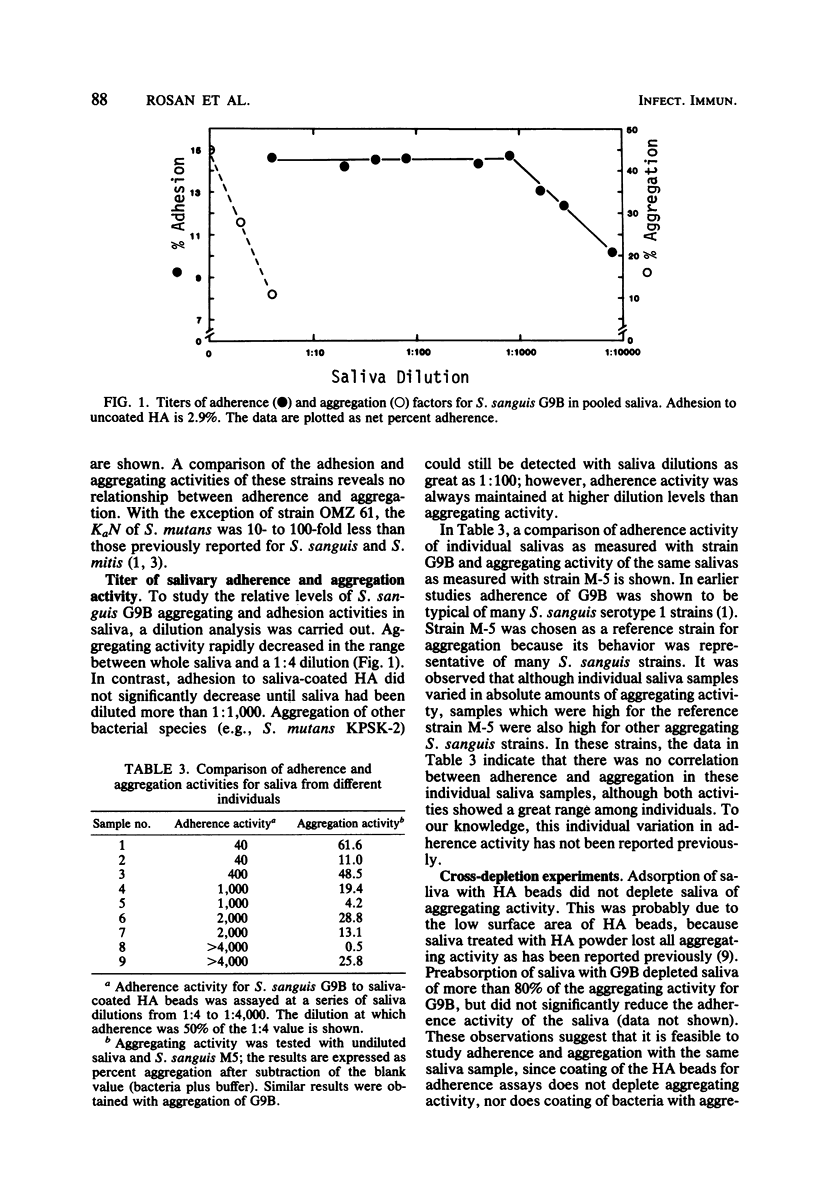
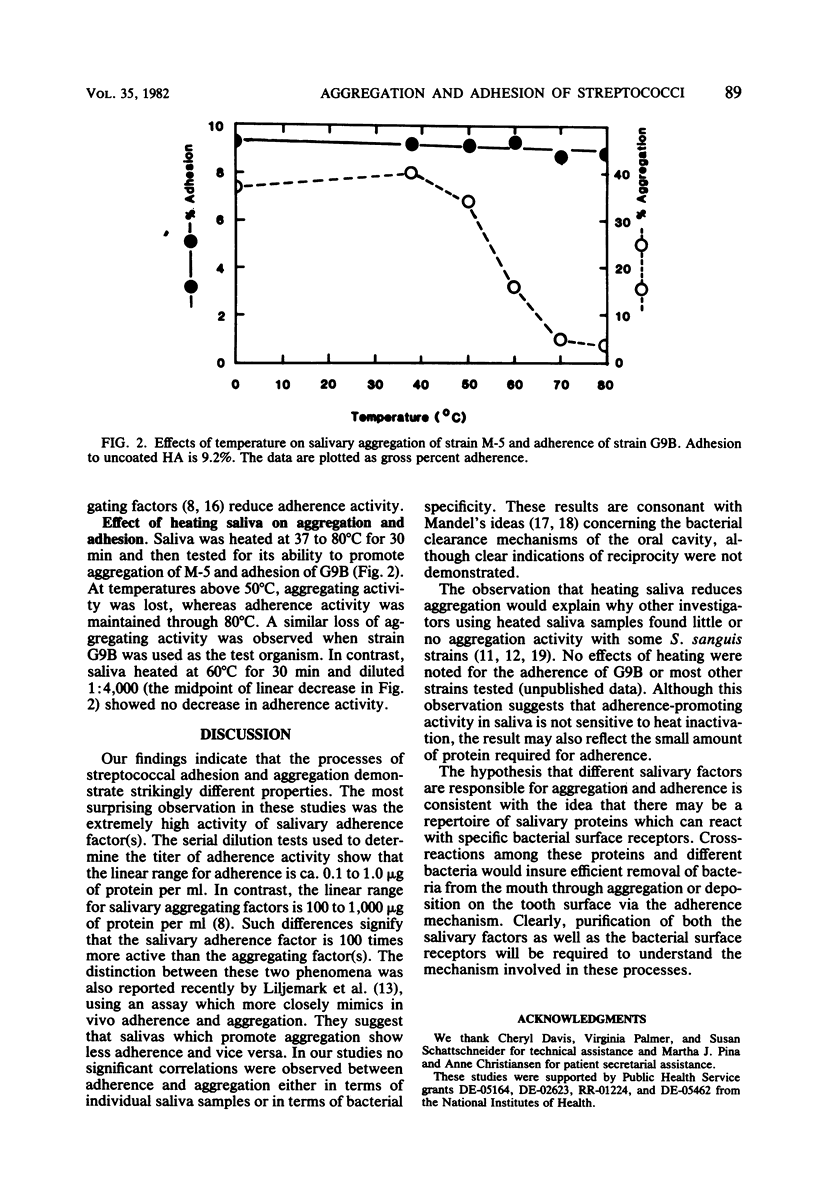
Comparison of saliva-mediated aggregation of Streptococcus sanguis, Streptococcus mitis, and Streptococcus mutans and adhesion of these organisms to saliva-coated hydroxyapatite showed that there was no relationship between these two activities. Adsorption of salivary aggregating activity to bacteria appears to have little effect on the ability of the residual saliva to support adherence; conversely, adsorption of salivary adherence factors to hydroxyapatite does not affect aggregation. Although heating saliva significantly reduces bacterial aggregation, it has little or no effect on adherence. A comparison of aggregation and adhesion with serial dilutions of saliva demonstrated that adhesion could still be detected at 100 to 500-fold-lower concentrations of salivary protein that bacterial aggregation. These findings support the concept that aggregation and adherence involve two distinct mechanisms of microbial clearance in the oral cavity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelbaum B., Golub E., Holt S. C., Rosan B. In vitro studies of dental plaque formation: adsorption of oral streptococci to hydroxyaptite. Infect Immun. 1979 Aug;25(2):717–728. doi: 10.1128/iai.25.2.717-728.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J. A numerical taxonomic study of human oral streptococci. Odontol Revy. 1968;19(2):137–160. [PubMed] [Google Scholar]
- Clark W. B., Bammann L. L., Gibbons R. J. Comparative estimates of bacterial affinities and adsorption sites on hydroxyapatite surfaces. Infect Immun. 1978 Mar;19(3):846–853. doi: 10.1128/iai.19.3.846-853.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facklam R. R. Physiological differentiation of viridans streptococci. J Clin Microbiol. 1977 Feb;5(2):184–201. doi: 10.1128/jcm.5.2.184-201.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Houte J. V. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Moreno E. C., Spinell D. M. Model delineating the effects of a salivary pellicle on the adsorption of Streptococcus miteor onto hydroxyapatite. Infect Immun. 1976 Oct;14(4):1109–1112. doi: 10.1128/iai.14.4.1109-1112.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub E. E., Thaler M., Davis C., Malamud D. Bacterial aggregating activity in human saliva: simultaneous determination of free and bound cells. Infect Immun. 1979 Dec;26(3):1028–1034. doi: 10.1128/iai.26.3.1028-1034.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay D. I., Gibbons R. J., Spinell D. M. Characteristics of some high molecular weight constituents with bacterial aggregating activity from whole saliva and dental plaque. Caries Res. 1971;5(2):111–123. doi: 10.1159/000259739. [DOI] [PubMed] [Google Scholar]
- Hillman J. D., Van Houte J., Gibbons R. J. Sorption of bacteria to human enamel powder. Arch Oral Biol. 1970 Sep;15(9):899–903. doi: 10.1016/0003-9969(70)90163-9. [DOI] [PubMed] [Google Scholar]
- Levine M. J., Herzberg M. C., Levine M. S., Ellison S. A., Stinson M. W., Li H. C., van Dyke T. Specificity of salivary-bacterial interactions: role of terminal sialic acid residues in the interaction of salivary glycoproteins with Streptococcus sanguis and Streptococcus mutans. Infect Immun. 1978 Jan;19(1):107–115. doi: 10.1128/iai.19.1.107-115.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljemark W. F., Bloomquist C. G., Germaine G. R. Effect of bacterial aggregation on the adherence of oral streptococci to hydroxyapatite. Infect Immun. 1981 Mar;31(3):935–941. doi: 10.1128/iai.31.3.935-941.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljemark W. F., Bloomquist C. G., Ofstehage J. C. Aggregation and adherence of Streptococcus sanguis: role of human salivary immunoglobulin A. Infect Immun. 1979 Dec;26(3):1104–1110. doi: 10.1128/iai.26.3.1104-1110.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljemark W. F., Schauer S. V. Competitive binding among oral strptococci to hydroxyapatite. J Dent Res. 1977 Feb;56(2):157–165. doi: 10.1177/00220345770560021001. [DOI] [PubMed] [Google Scholar]
- Magnusson I., Ericson T. Effect of salivary agglutinins of reactions between hydroxyapatite and a serotype c strain of Streptococcus mutans. Caries Res. 1976;10(4):273–286. doi: 10.1159/000260208. [DOI] [PubMed] [Google Scholar]
- Malamud D., Appelbaum B., Kline R., Golub E. E. Bacterial aggregating activity in human saliva: comparisons of bacterial species and strains. Infect Immun. 1981 Mar;31(3):1003–1006. doi: 10.1128/iai.31.3.1003-1006.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel I. D. Nonimmunologic aspects of caries resistance. J Dent Res. 1976 Apr;55(Spec No):C22–C31. doi: 10.1177/002203457605500316011. [DOI] [PubMed] [Google Scholar]
- McBride B. C., Gisslow M. T. Role of sialic acid in saliva-induced aggregation of Streptococcus sanguis. Infect Immun. 1977 Oct;18(1):35–40. doi: 10.1128/iai.18.1.35-40.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaughey C., Field B. D., Stowell E. C. Effects of salivary proteins on the adsorption of cariogenic streptococci by hydroxyapatite. J Dent Res. 1971 Jul-Aug;50(4):917–922. doi: 10.1177/00220345710500042201. [DOI] [PubMed] [Google Scholar]
- Orstavik D., Kraus F. W., Henshaw L. C. In vitro attachment of streptococci to the tooth surface. Infect Immun. 1974 May;9(5):794–800. doi: 10.1128/iai.9.5.794-800.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosan B. Absence of glycerol teichoic acids in certain oral streptococci. Science. 1978 Sep 8;201(4359):918–920. doi: 10.1126/science.684416. [DOI] [PubMed] [Google Scholar]
- Rosan B. Antigens of Streptococcus sanguis. Infect Immun. 1973 Feb;7(2):205–211. doi: 10.1128/iai.7.2.205-211.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]


