Abstract
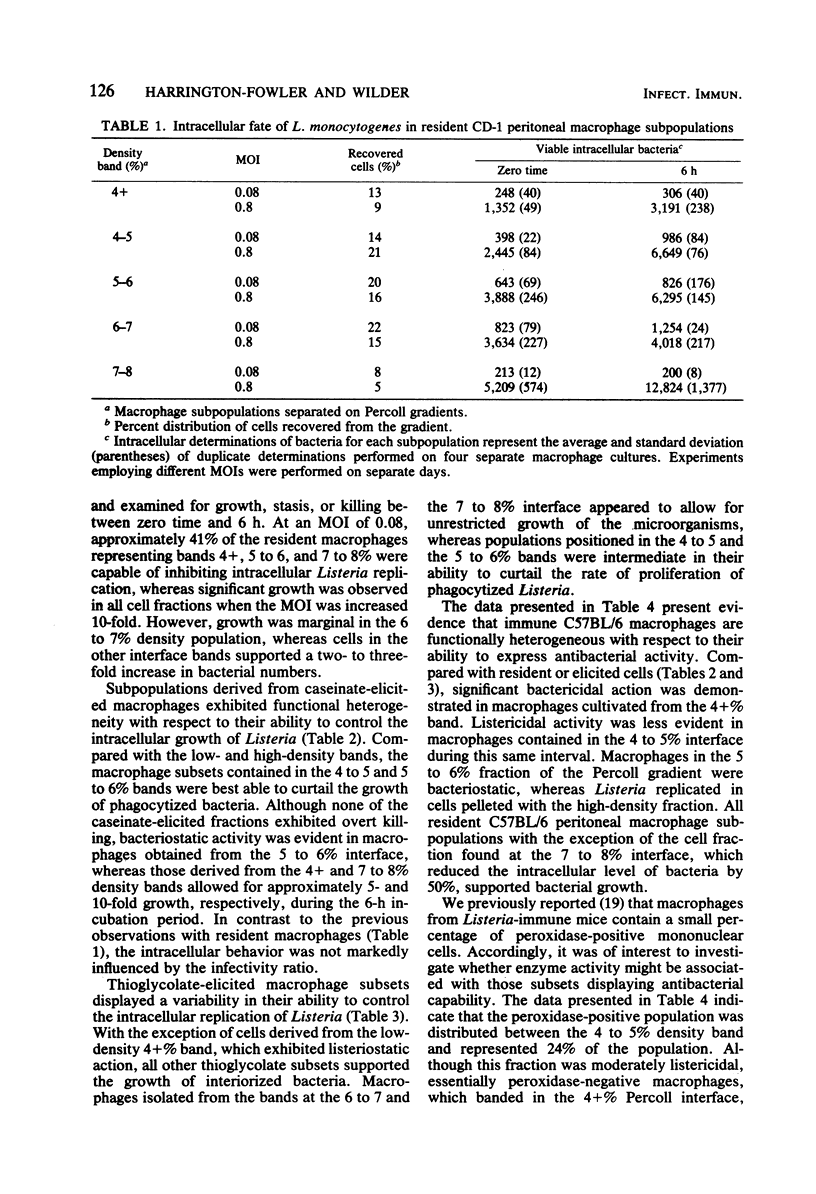
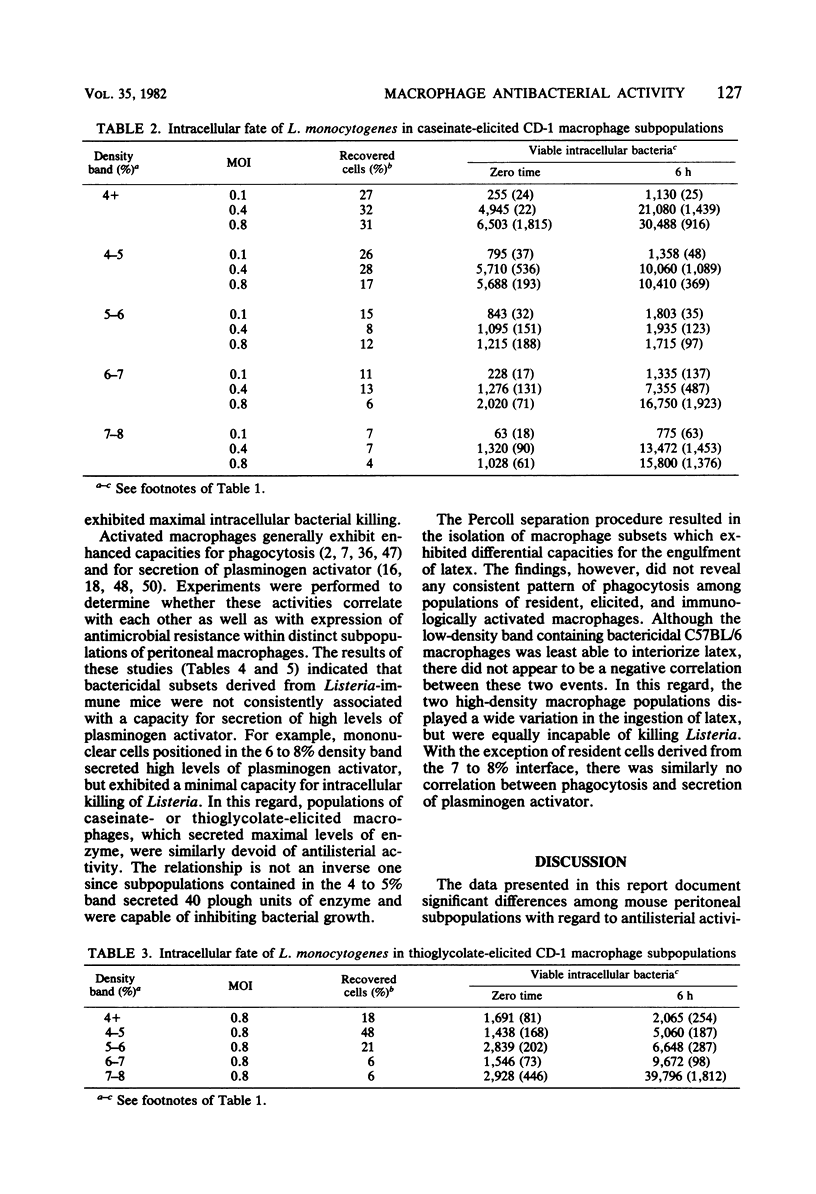
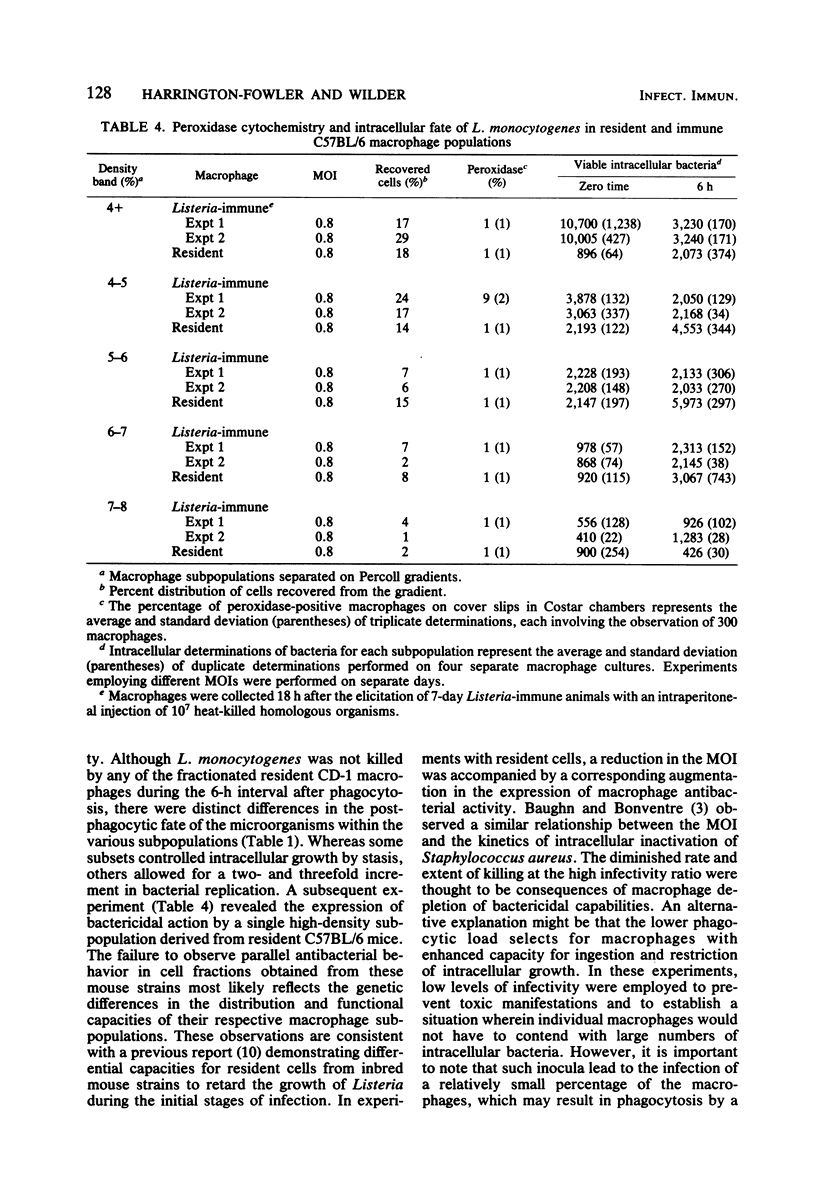
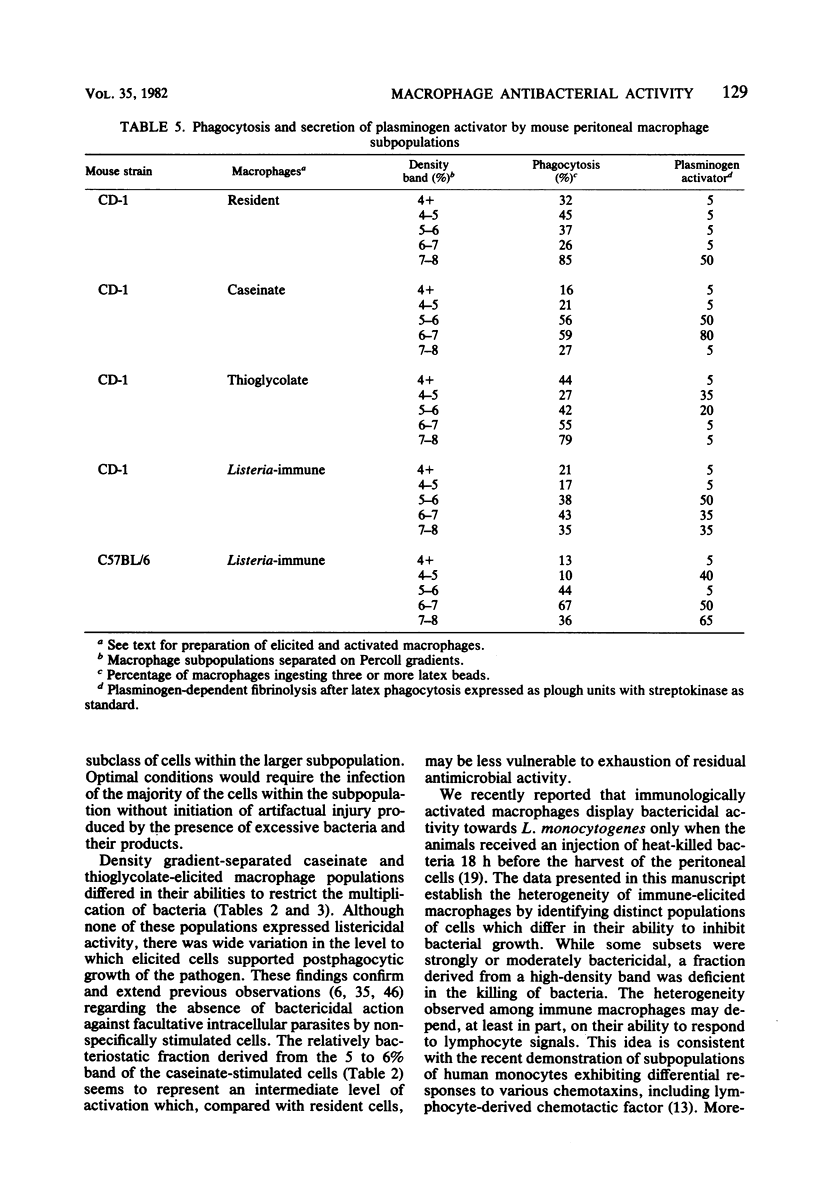
Peritoneal macrophages derived from CD-1 and C57BL/6 mice were separated into distinct groups based on their buoyant densities on discontinuous gradients of Percoll and assayed for antibacterial activity against Listeria monocytogenes. Subpopulations of peritoneal macrophages derived from Listeria-immune mice present a wide variation in their ability to control intracellular infection. Distinct subsets were found which exhibited bacteriostatic and listericidal activity. The fractionation procedure yielded a population of peroxidase-positive macrophages which were devoid of antilisterial action. Subpopulations of resident and elicited macrophages were also functionally heterogeneous in their ability to restrict intracellular growth of bacterial. In some experiments, subclasses were examined for secretion of plasminogen activator and phagocytosis of latex particles. These activities varied considerably with the status of activation of the macrophages, but failed to correlate with antimicrobial activity within given subpopulations.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baughin R. E., Bonventre P. F. Cell-mediated immune phenomena induced by lymphokines from splenic lymphocytes of mice with chronic staphylococcal infection. Infect Immun. 1975 Feb;11(2):313–319. doi: 10.1128/iai.11.2.313-319.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughn R., Bonventre P. F. Phagocytosis and intracellular killing of Staphylococcus aureus by normal mouse peritoneal macrophages. Infect Immun. 1975 Aug;12(2):346–352. doi: 10.1128/iai.12.2.346-352.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beller D. I., Kiely J. M., Unanue E. R. Regulation of macrophage populations. I. Preferential induction of Ia-rich peritoneal exudates by immunologic stimuli. J Immunol. 1980 Mar;124(3):1426–1432. [PubMed] [Google Scholar]
- Beller D. I., Unanue E. R. IA antigens and antigen-presenting function of thymic macrophages. J Immunol. 1980 Mar;124(3):1433–1440. [PubMed] [Google Scholar]
- Bennedsen J., Riisgaard S., Rhodes J. M., Larsen S. O. In vitro studies on normal, stimulated and immunologically activated mouse macrophages. III. Intracellular multiplication of Listeria monocytogenes. Acta Pathol Microbiol Scand C. 1977 Aug;85C(4):246–252. doi: 10.1111/j.1699-0463.1977.tb03638.x. [DOI] [PubMed] [Google Scholar]
- Blanden R. V., Mackaness G. B., Collins F. M. Mechanisms of acquired resistance in mouse typhoid. J Exp Med. 1966 Oct 1;124(4):585–600. doi: 10.1084/jem.124.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle M. D., Ormerod M. G. The destruction of allogeneic tumour cells by peritoneal macrophages from immune mice: purification of lytic effector cells. Cell Immunol. 1975 May;17(1):247–258. doi: 10.1016/s0008-8749(75)80024-4. [DOI] [PubMed] [Google Scholar]
- Campbell M. W., Sholley M. M., Miller G. A. Macrophage heterogeneity in tumor resistance: cytostatic and cytotoxic activity of Corynebacterium parvum-activated and proteose peptone-elicited rat macrophages against Moloney sarcoma tumor cells. Cell Immunol. 1980 Mar 1;50(1):153–168. doi: 10.1016/0008-8749(80)90014-3. [DOI] [PubMed] [Google Scholar]
- Cheers C., McKenzie I. F., Pavlov H., Waid C., York J. Resistance and susceptibility of mice to bacterial infection: course of listeriosis in resistant or susceptible mice. Infect Immun. 1978 Mar;19(3):763–770. doi: 10.1128/iai.19.3.763-770.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowing C., Pincus S. H., Sachs D. H., Dickler H. B. A subpopulation of adherent accessory cells bearing both I-A and I-E or C subregion antigens is required for antigen-specific murine T lymphocyte proliferation. J Immunol. 1978 Nov;121(5):1680–1686. [PubMed] [Google Scholar]
- Cowing C., Schwartz B. D., Dickler H. B. Macrophage Ia antigens. I. macrophage populations differ in their expression of Ia antigens. J Immunol. 1978 Feb;120(2):378–384. [PubMed] [Google Scholar]
- Falk W., Leonard E. J. Human monocyte chemotaxis: migrating cells are a subpopulation with multiple chemotaxin specificities on each cell. Infect Immun. 1980 Sep;29(3):953–959. doi: 10.1128/iai.29.3.953-959.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldyne M. E., Stobo J. D. Synthesis of prostaglandins by subpopulations of human peripheral blood monocytes. Prostaglandins. 1979 Nov;18(5):687–695. doi: 10.1016/0090-6980(79)90089-3. [DOI] [PubMed] [Google Scholar]
- Gordon S., Unkeless J. C., Cohn Z. A. Induction of macrophage plasminogen activator by endotoxin stimulation and phagocytosis: evidence for a two-stage process. J Exp Med. 1974 Oct 1;140(4):995–1010. doi: 10.1084/jem.140.4.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granelli-Piperno A., Reich E. A study of proteases and protease-inhibitor complexes in biological fluids. J Exp Med. 1978 Jul 1;148(1):223–234. doi: 10.1084/jem.148.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greineder D. K., Connorton K. J., David J. R. Plasminogen activator production by human monocytes. I. Enhancement by activated lymphocytes and lymphocyte products. J Immunol. 1979 Dec;123(6):2808–2813. [PubMed] [Google Scholar]
- Harrington-Fowler L., Henson P. M., Wilder M. S. Fate of Listeria monocytogenes in resident and activated macrophages. Infect Immun. 1981 Jul;33(1):11–16. doi: 10.1128/iai.33.1.11-16.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKIN C. R., ROWLEY D., AUZINS I. THE BASIS FOR IMMUNITY TO MOUSE TYPHOID. I. THE CARRIER STATE. Aust J Exp Biol Med Sci. 1964 Apr;42:215–228. doi: 10.1038/icb.1964.23. [DOI] [PubMed] [Google Scholar]
- JENKIN C., BENACERRAF B. In vitro studies on the interaction between mouse peritoneal macrophages and strains of Salmonella and Escherichia coli. J Exp Med. 1960 Aug 1;112:403–417. doi: 10.1084/jem.112.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAPLOW L. S. SIMPLIFIED MYELOPEROXIDASE STAIN USING BENZIDINE DIHYDROCHLORIDE. Blood. 1965 Aug;26:215–219. [PubMed] [Google Scholar]
- Kress Y., Bloom B. R., Wittner M., Rowen A., Tanowitz H. Resistance of Trypanosoma cruzi to killing by macrophages. Nature. 1975 Oct 2;257(5525):394–396. doi: 10.1038/257394a0. [DOI] [PubMed] [Google Scholar]
- Lee K. C., Berry D. Functional heterogeneity in macrophages activated by Corynebacterium parvum: characterization of subpopulations with different activities in promoting immune responses and suppressing tumor cell growth. J Immunol. 1977 May;118(5):1530–1540. [PubMed] [Google Scholar]
- Lee K. C., Kay J., Wong M. Separation of functionally distinct subpopulations of Corynebacterium parvum-activated macrophages with predominantly stimulatory or suppressive effect on the cell-mediated cytotoxic T cell response. Cell Immunol. 1979 Jan;42(1):28–41. doi: 10.1016/0008-8749(79)90218-1. [DOI] [PubMed] [Google Scholar]
- Lee K. C., Wong M., McIntyre D. Characterization of macrophage subpopulations responsive to activation by endotoxin and lymphokines. J Immunol. 1981 Jun;126(6):2474–2479. [PubMed] [Google Scholar]
- MACKANESS G. B. The phagocytosis and inactivation of staphylococci by macrophages of normal rabbits. J Exp Med. 1960 Jul 1;112:35–53. doi: 10.1084/jem.112.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntrye J., Rowley D., Jenkin C. R. The functional heterogeneity of macrophages at the single cell level. Aust J Exp Biol Med Sci. 1967 Dec;45(6):675–680. doi: 10.1038/icb.1967.67. [DOI] [PubMed] [Google Scholar]
- Miller G. A., Campbell M. W., Hudson J. L. Separation of rat peritoneal macrophages into functionally distinct subclasses by centrifugal elutriation. J Reticuloendothel Soc. 1980 Feb;27(2):167–174. [PubMed] [Google Scholar]
- Nathan C. F., Asofsky R., Terry W. D. Characterization of the nonphagocytic adherent cell from the peritoneal cavity of normal and BCG-treated mice. J Immunol. 1977 May;118(5):1612–1621. [PubMed] [Google Scholar]
- Nathan C. F., Hill V. M., Terry W. D. Isolation of a subpopulation of adherent peritoneal cells with anti-tumour activity. Nature. 1976 Mar 11;260(5547):146–148. doi: 10.1038/260146a0. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Terry W. D. Decreased phagocytosis by peritoneal macrophages from BCG-treated mice: induction of the phagocytic defect in normal macrophages with BCG in vitro. Cell Immunol. 1977 Mar 15;29(2):295–311. doi: 10.1016/0008-8749(77)90324-0. [DOI] [PubMed] [Google Scholar]
- Nogueira N., Gordon S., Cohn Z. Trypanosoma cruzi: modification of macrophage function during infection. J Exp Med. 1977 Jul 1;146(1):157–171. doi: 10.1084/jem.146.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. Cellular kinetics associated with the development of acquired cellular resistance. J Exp Med. 1969 Aug 1;130(2):299–314. doi: 10.1084/jem.130.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dorisio M. S., Vandenbark G. R., LoBuglio A. F. Human monocyte killing of Staphylococcus aureus: modulation by agonists of cyclic adenosine 3',5'-monophosphate and cyclic guanosine 3',5'-monophosphate. Infect Immun. 1979 Nov;26(2):604–610. doi: 10.1128/iai.26.2.604-610.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picker L. J., Raff H. V., Goldyne M. E., Stobo J. D. Metabolic heterogeneity among human monocytes and its modulation by PGE2. J Immunol. 1980 Jun;124(6):2557–2562. [PubMed] [Google Scholar]
- Rice S. G., Fishman M. Functional and morphological heterogeneity among rabbit peritoneal macrophages. Cell Immunol. 1974 Mar 30;11(1-3):130–145. doi: 10.1016/0008-8749(74)90014-8. [DOI] [PubMed] [Google Scholar]
- Ruco L. P., Meltzer M. S. Macrophage activation for tumor cytotoxicity: increased lymphokine responsiveness of peritoneal macrophages during acute inflammation. J Immunol. 1978 Mar;120(3):1054–1062. [PubMed] [Google Scholar]
- Salvin S. B., Cheng S. L. Lymphoid Cells in Delayed Hypersensitivity II. In Vitro Phagocytosis and Cellular Immunity. Infect Immun. 1971 Apr;3(4):548–552. doi: 10.1128/iai.3.4.548-552.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz R. M., Pavlidis N. A., Stoychkov J. N., Chirigos M. A. Prevention of macrophage tumoricidal activity by agents known to increase cellular cyclic AMP. Cell Immunol. 1979 Jan;42(1):71–78. doi: 10.1016/0008-8749(79)90222-3. [DOI] [PubMed] [Google Scholar]
- Schumacher G. F., Schill W. B. Radial diffusion in gel for micro determination of enzymes. II. Plasminogen activator, elastase, and nonspecific proteases. Anal Biochem. 1972 Jul;48(1):9–26. doi: 10.1016/0003-2697(72)90165-0. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H., Yano A., Paul W. E. Interaction between antigen-presenting cells and primed T lymphocytes: an assessment of Ir gene expression in the antigen-presenting cell. Immunol Rev. 1978;40:153–180. doi: 10.1111/j.1600-065x.1978.tb00405.x. [DOI] [PubMed] [Google Scholar]
- Serio C., Gandour D. M., Walker W. S. Macrophage functional heterogeneity: evidence for different antibody-dependent effector cell activities and expression of Fc-receptors among macrophage subpopulations. J Reticuloendothel Soc. 1979 Feb;25(2):197–206. [PubMed] [Google Scholar]
- Simon H. B., Sheagren J. N. Cellular immunity in vitro. I. Immunologically mediated enhancement of macrophage bactericidal capacity. J Exp Med. 1971 Jun 1;133(6):1377–1389. doi: 10.1084/jem.133.6.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs M., Kühner A. V., Glass E. A., David J. R., Karnovsky M. L. Metabolic and functonal studies on activated mouse macrophages. J Exp Med. 1973 Feb 1;137(2):537–542. doi: 10.1084/jem.137.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unkeless J. C., Gordon S., Reich E. Secretion of plasminogen activator by stimulated macrophages. J Exp Med. 1974 Apr 1;139(4):834–850. doi: 10.1084/jem.139.4.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassalli J. D., Reich E. Macrophage plasminogen activator: induction by products of activated lymphoid cells. J Exp Med. 1977 Feb 1;145(2):429–437. doi: 10.1084/jem.145.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker W. S. Functional heterogeneity of macrophages in the induction and expression of acquired immunity. J Reticuloendothel Soc. 1976 Jul;20(1):57–65. [PubMed] [Google Scholar]
- Walker W. S. Functional heterogeneity of macrophages: subclasses of peritoneal macrophages with different antigen-binding activities and immune complex receptors. Immunology. 1974 May;26(5):1025–1037. [PMC free article] [PubMed] [Google Scholar]
- Wing E. J., Gardner I. D., Ryning F. W., Remington J. S. Dissociation of effector functions in populations of activated macrophages. Nature. 1977 Aug 18;268(5621):642–644. doi: 10.1038/268642a0. [DOI] [PubMed] [Google Scholar]
- Zembala M., Asherson G. L. The rapid purification of peritoneal exudate macrophages by ficoll (polysucrose) density gradient centrifugation. Immunology. 1970 Oct;19(4):677–681. [PMC free article] [PubMed] [Google Scholar]
- van Furth R., Hirsch J. G., Fedorko M. E. Morphology and peroxidase cytochemistry of mouse promonocytes, monocytes, and macrophages. J Exp Med. 1970 Oct 1;132(4):794–812. doi: 10.1084/jem.132.4.794. [DOI] [PMC free article] [PubMed] [Google Scholar]


