Abstract
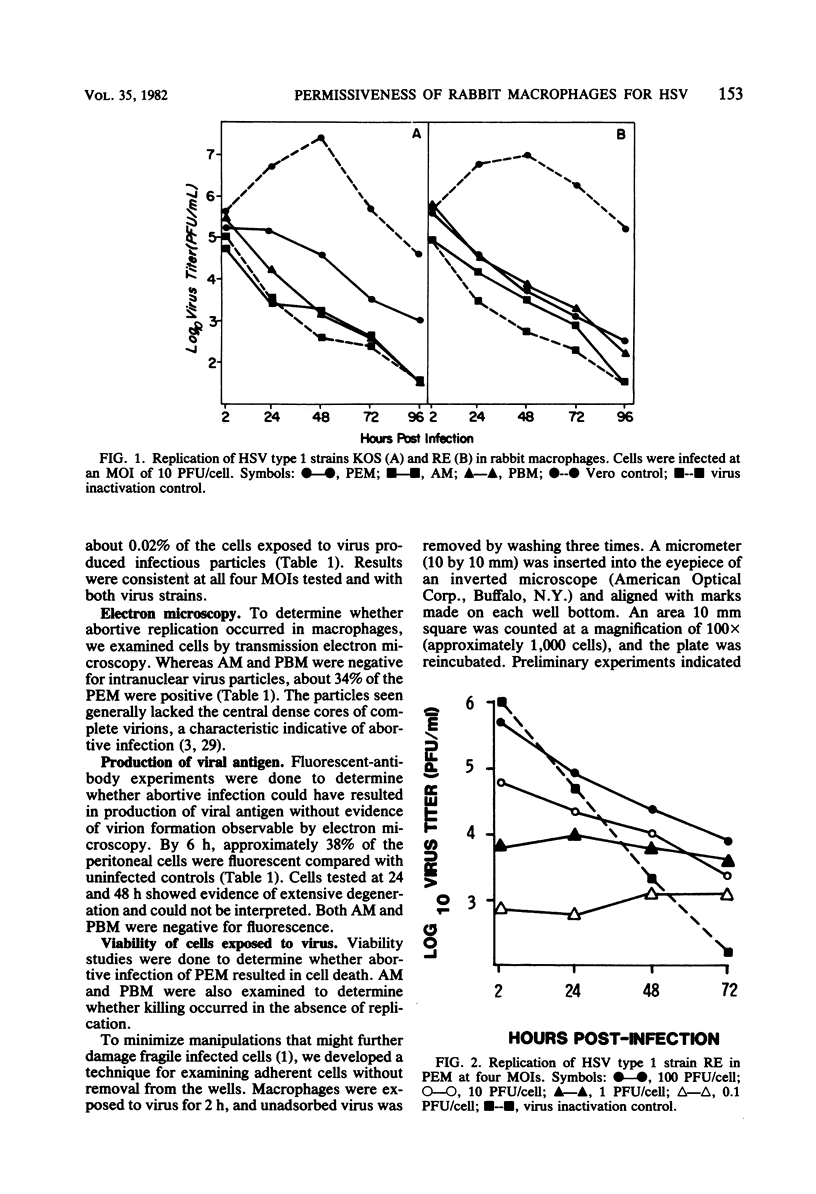
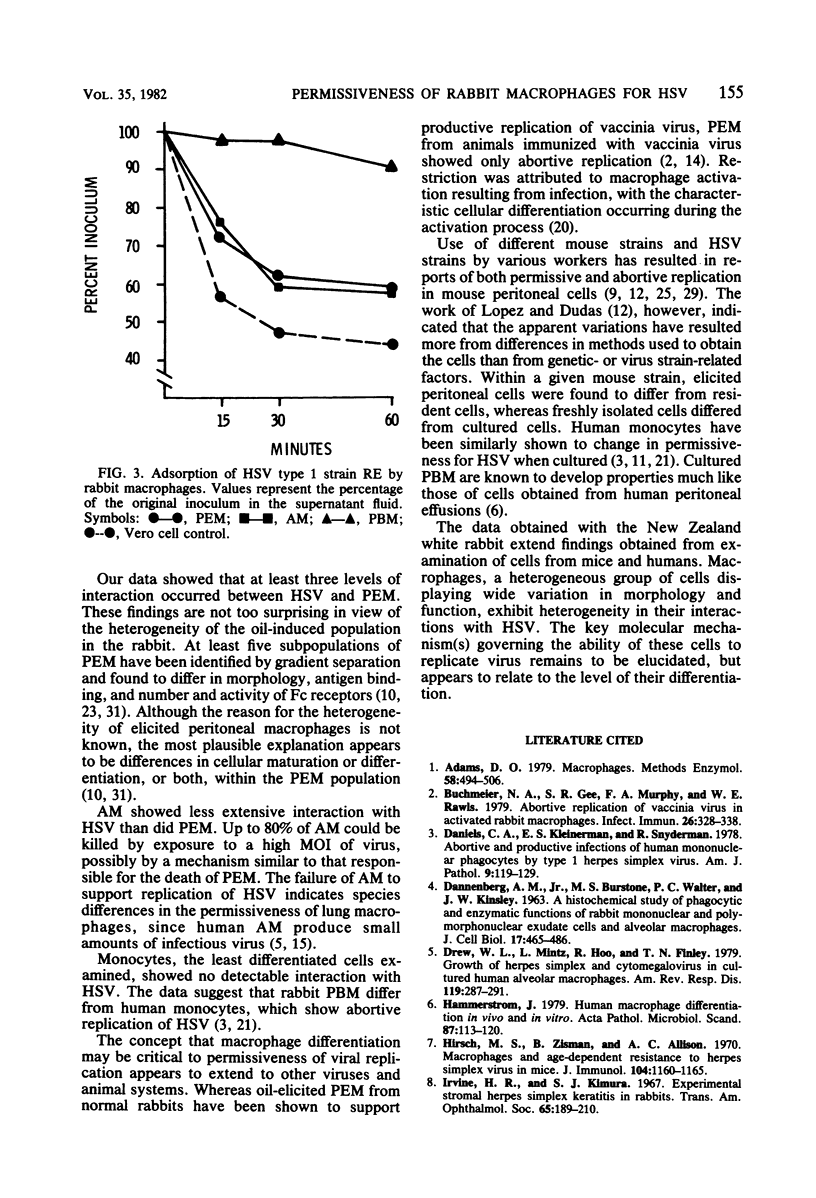
The permissiveness of rabbit monocytes and macrophages for herpes simplex virus was examined. Peripheral blood monocytes, alveolar macrophages, and peritoneal exudate macrophages were studied for their ability to replicate herpes simplex virus strains RE and KOS. Results indicated different degrees of interaction with virus depending on the macrophage type. Only peritoneal exudate macrophages showed evidence of virus replication. Productive infection was limited, with only a small number of cells (0.02%) yielding infectious virus. Higher numbers of cells appeared to be abortively infected. Approximately 40% expressed antigens, whereas virtually all were killed by exposure to virus. Coreless particles were seen by electron microscopy in about one-third. Alveolar macrophages were also killed by virus and showed evidence of virus adsorption, but showed no indication of productive or abortive infection. Monocytes neither adsorbed nor replicated virus, and viability was unaffected. Results suggest that differences in degrees of cellular maturation or differentiation, or both, account for the spectrum of interactions seen between herpes simplex virus and rabbit macrophages.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O. Macrophages. Methods Enzymol. 1979;58:494–505. doi: 10.1016/s0076-6879(79)58164-6. [DOI] [PubMed] [Google Scholar]
- Buchmeier N. A., Gee S. R., Murphy F. A., Rawls W. E. Abortive replication of vaccinia virus in activated rabbit macrophages. Infect Immun. 1979 Oct;26(1):328–338. doi: 10.1128/iai.26.1.328-338.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DANNENBERG A. M., Jr, BURSTONE M. S., WALTER P. C., KINSLEY J. W. A histochemical study of phagocytic and enzymatic functions of rabbit mononuclear and polymorphonuclear exudate cells and alveolar macrophages. I. Survey and quantitation of enzymes, and states of cellular activation. J Cell Biol. 1963 Jun;17:465–486. doi: 10.1083/jcb.17.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels C. A., Kleinerman E. S., Snyderman R. Abortive and productive infections of human mononuclear phagocytes by type I herpes simplex virus. Am J Pathol. 1978 Apr;91(1):119–136. [PMC free article] [PubMed] [Google Scholar]
- Drew W. L., Mintz L., Hoo R., Finley T. N. Growth of herpes simplex and cytomegalovirus in cultured human alveolar macrophages. Am Rev Respir Dis. 1979 Feb;119(2):287–291. doi: 10.1164/arrd.1979.119.2.287. [DOI] [PubMed] [Google Scholar]
- Hammerstrøm J. Human macrophage differentiation in vivo and in vitro. A comparison of human peritoneal macrophages and monocytes. Acta Pathol Microbiol Scand C. 1979 Apr;87C(2):113–120. [PubMed] [Google Scholar]
- Hirsch M. S., Zisman B., Allison A. C. Macrophages and age-dependent resistance to Herpes simplex virus in mice. J Immunol. 1970 May;104(5):1160–1165. [PubMed] [Google Scholar]
- Irvine A. R., Kimura S. J. Experimental stromal herpes simplex keratitis in rabbits. Trans Am Ophthalmol Soc. 1967;65:189–210. [PMC free article] [PubMed] [Google Scholar]
- JOHNSON R. T. THE PATHOGENESIS OF HERPES VIRUS ENCEPHALITIS. II. A CELLULAR BASIS FOR THE DEVELOPMENT OF RESISTANCE WITH AGE. J Exp Med. 1964 Sep 1;120:359–374. doi: 10.1084/jem.120.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kávai M., Laczkó J., Csaba B. Functional heterogeneity of macrophages. Immunology. 1979 Apr;36(4):729–732. [PMC free article] [PubMed] [Google Scholar]
- Linnavuori K., Hovi T. Herpes simplex virus infection in human monocyte cultures: dose-dependent inhibition of monocyte differentiation resulting in abortive infection. J Gen Virol. 1981 Feb;52(Pt 2):381–385. doi: 10.1099/0022-1317-52-2-381. [DOI] [PubMed] [Google Scholar]
- Lopez C., Dudas G. Replication of herpes simplex virus type 1 in macrophages from resistant and susceptible mice. Infect Immun. 1979 Feb;23(2):432–437. doi: 10.1128/iai.23.2.432-437.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MYRVIK Q., LEAKE E. S., FARISS B. Studies on pulmonary alveolar macrophages from the normal rabbit: a technique to procure them in a high state of purity. J Immunol. 1961 Feb;86:128–132. [PubMed] [Google Scholar]
- McGeorge M. B., Morahan P. S. Comparison of various macrophage-inhibitory agents on vaginal and systemic herpes simplex virus type 2 infections. Infect Immun. 1978 Nov;22(2):623–626. doi: 10.1128/iai.22.2.623-626.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren C., Cheng H., Spicer D. L., Tompkins W. A. Lymphocyte and macrophage responses after vaccinia virus infections. Infect Immun. 1976 Oct;14(4):1014–1021. doi: 10.1128/iai.14.4.1014-1021.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz L., Drew W. L., Hoo R., Finley T. N. Age-dependent resistance of human alveolar macrophages to herpes simplex virus. Infect Immun. 1980 May;28(2):417–420. doi: 10.1128/iai.28.2.417-420.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen S. C. Macrophages and age-dependent resistance to hepatitis induced by herpes simplex virus type 2 im mice. Infect Immun. 1978 Jan;19(1):46–50. doi: 10.1128/iai.19.1.46-50.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen S. C. Role of macrophages in natural resistance to virus infections. Microbiol Rev. 1979 Mar;43(1):1–26. doi: 10.1128/mr.43.1.1-26.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols B. A., Bainton D. F., Farquhar M. G. Differentiation of monocytes. Origin, nature, and fate of their azurophil granules. J Cell Biol. 1971 Aug;50(2):498–515. doi: 10.1083/jcb.50.2.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. The concept of the activated macrophage. J Immunol. 1978 Sep;121(3):806–809. [PMC free article] [PubMed] [Google Scholar]
- Plaeger-Marshall S., Smith J. W. Experimental infection of subpopulations of human peripheral blood leukocytes by herpes simplex virus. Proc Soc Exp Biol Med. 1978 Jun;158(2):263–268. doi: 10.3181/00379727-158-40185. [DOI] [PubMed] [Google Scholar]
- Rawls W. E., Laurel D., Melnick J. L., Glicksman J. M., Kaufman R. H. A search for viruses in smegma, premalignant and early malignant cervical tissues. The isolation of Herpesviruses with distinct antigenic properties. Am J Epidemiol. 1968 May;87(3):647–655. doi: 10.1093/oxfordjournals.aje.a120855. [DOI] [PubMed] [Google Scholar]
- Rice S. G., Fishman M. Functional and morphological heterogeneity among rabbit peritoneal macrophages. Cell Immunol. 1974 Mar 30;11(1-3):130–145. doi: 10.1016/0008-8749(74)90014-8. [DOI] [PubMed] [Google Scholar]
- Schröder C. H., Urbaczka G. Excess of interfering over infectious particles in herpes simplex virus passaged at high m.o.i. and their effect on single-cell survival. J Gen Virol. 1978 Dec;41(3):493–501. doi: 10.1099/0022-1317-41-3-493. [DOI] [PubMed] [Google Scholar]
- Sethi K. K., Brandis H. In vitro acquisition of resistance against herpes simplex virus by permissive murine macrophages. Arch Virol. 1979;59(3):157–172. doi: 10.1007/BF01317412. [DOI] [PubMed] [Google Scholar]
- Silverstein S. Macrophages and viral immunity. Semin Hematol. 1970 Apr;7(2):185–214. [PubMed] [Google Scholar]
- Smith J. W., Glorioso J. C. Effect of cross-immunization on monotypic antibody responses to herpes simplex virus types 1 and 2. J Immunol. 1976 Apr;116(4):898–903. [PubMed] [Google Scholar]
- Smith J. W., Rodriguez J. E., McKee A. P. Biological characteristics of cloned populations of herpes simplex virus types 1 and 2. Appl Microbiol. 1971 Feb;21(2):350–357. doi: 10.1128/am.21.2.350-357.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J. G., Cook M. L. Restriction of herpes simplex virus by macrophages. An analysis of the cell-virus interaction. J Exp Med. 1971 Jan 1;133(1):19–38. doi: 10.1084/jem.133.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker W. S. Functional heterogeneity of macrophages in the induction and expression of acquired immunity. J Reticuloendothel Soc. 1976 Jul;20(1):57–65. [PubMed] [Google Scholar]
- Zisman B., Hirsch M. S., Allison A. C. Selective effects of anti-macrophage serum, silica and anti-lymphocyte serum on pathogenesis of herpes virus infection of young adult mice. J Immunol. 1970 May;104(5):1155–1159. [PubMed] [Google Scholar]


