Abstract
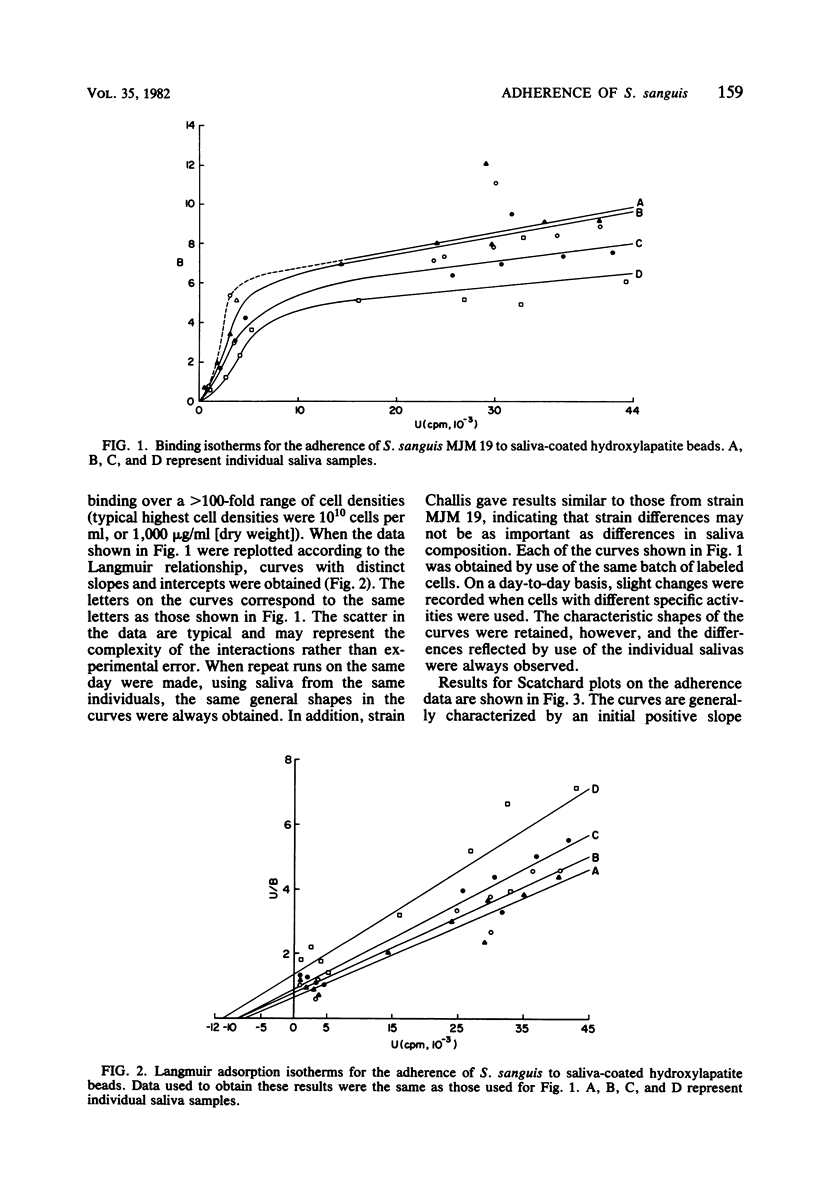
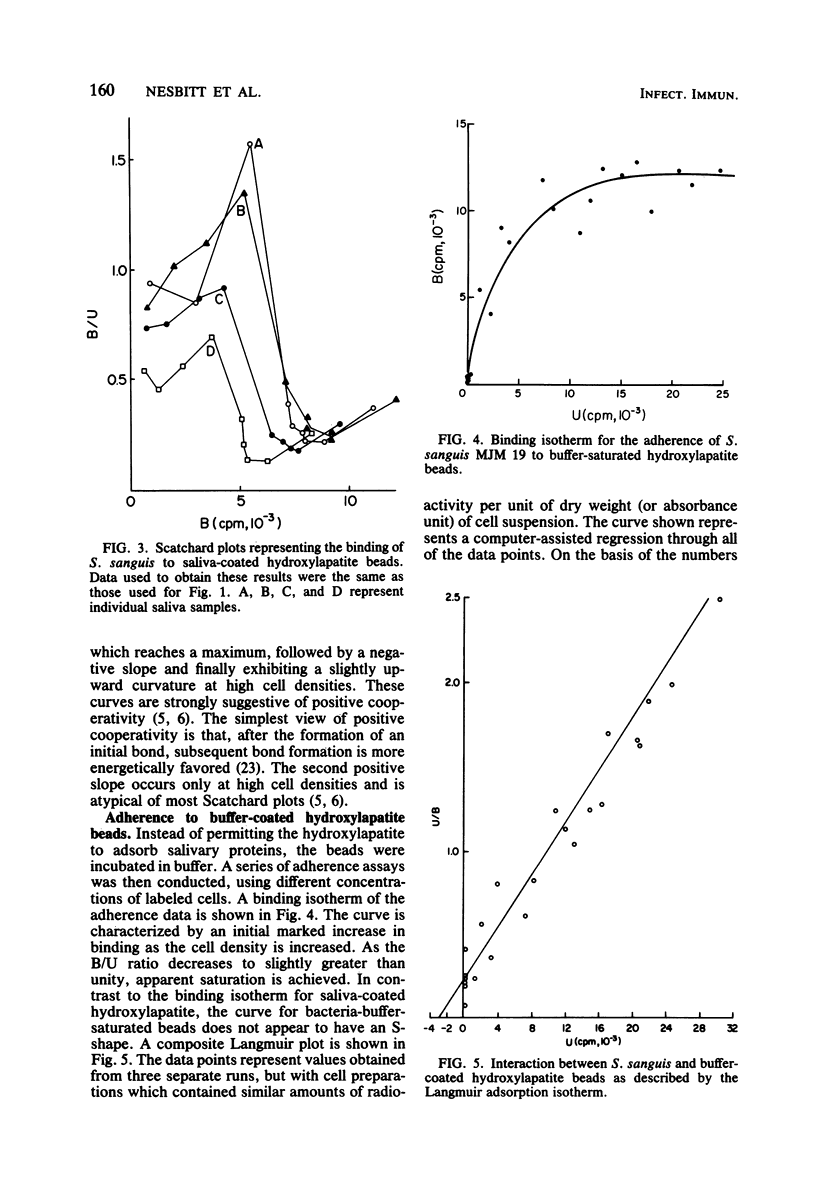
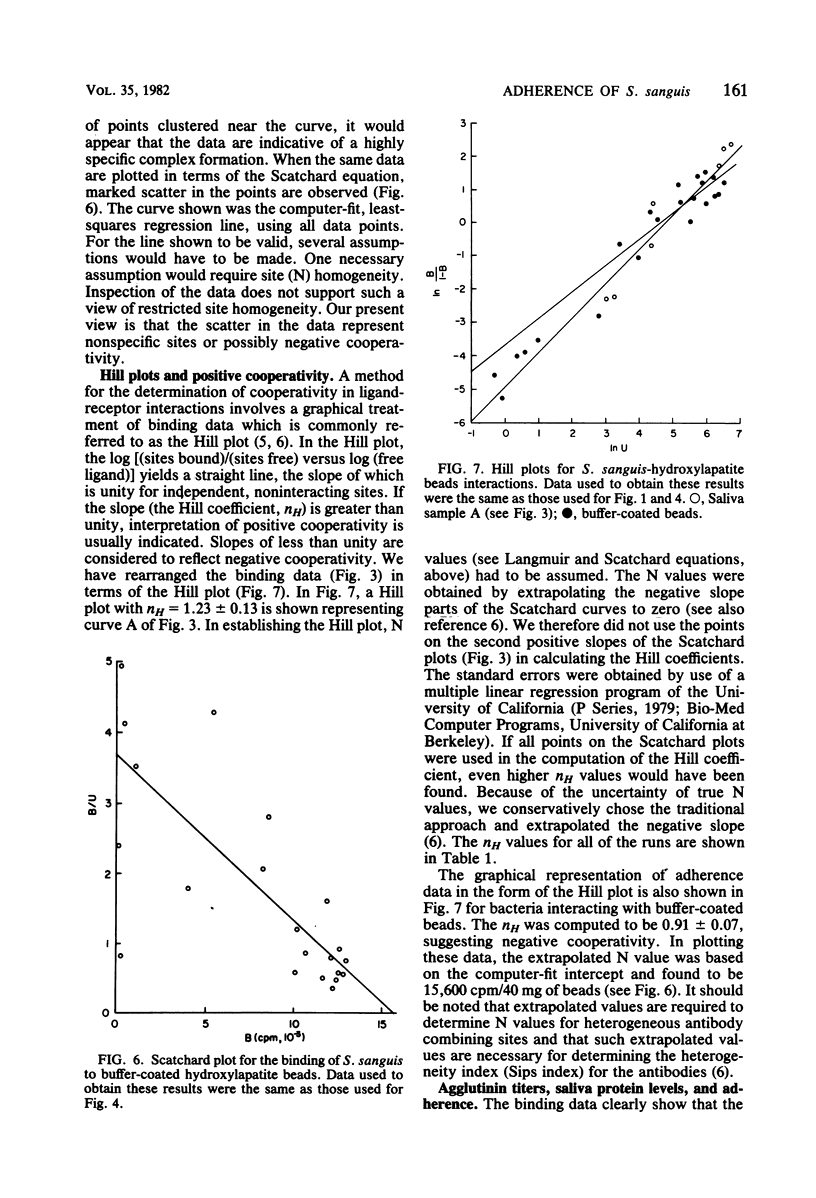
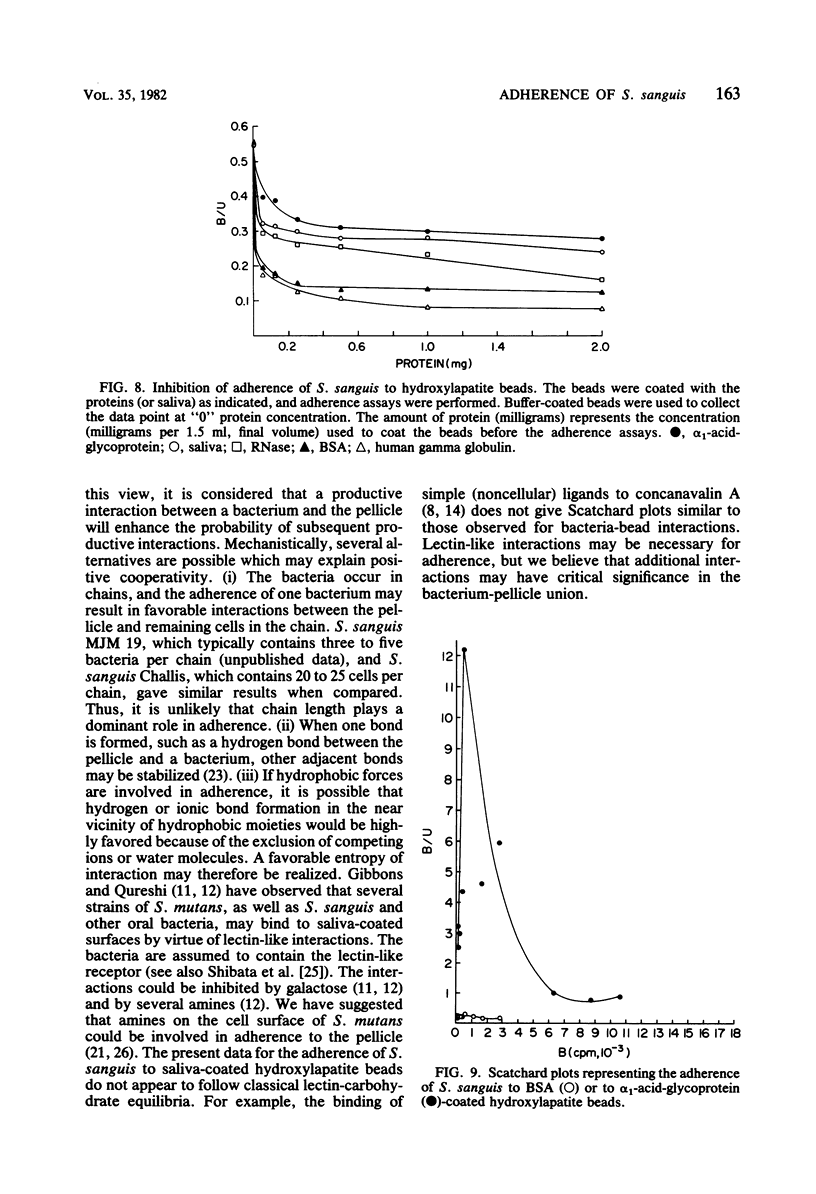
The adherence of Streptococcus sanguis to hydroxylapatite beads has been analyzed by binding isotherms, Langmuir isotherms, and Scatchard plots. For saliva-coated beads, the Scatchard curves contained components with both positive and negative slopes. The results are interpreted as evidence for positive cooperativity in the binding process. Although all Scatchard curves were similar in shape, distinct differences were observed between saliva samples from different individuals. Salivary agglutinins against whole S. sanguis cells did not appear to influence the shapes of the curves or the extent of adherence. In addition, different strains of S. sanguis yielded similar Scatchard plots. When the binding of S. sanguis to buffer-coated hydroxylapatite beads was analyzed by Scatchard plots or binding isotherms, curves were generated which suggested that either direct ligand-ligand or nonspecific interactions were occurring. Hill plots of the adherence data yielded curves with slopes greater than unity for saliva-coated beads, providing additional support for the view that the interactions between S. sanguis and the pellicle involve cooperative phenomena. In contrast, a Hill plot for the binding data of S. sanguis to buffer-coated hydroxylapatite beads gave a curve with a slope of 0.91 ± 0.07, suggesting negative cooperativity or limited specificity. When adherence data were plotted by the Langmuir method, curves were obtained which could not discriminate between the binding of the bacteria to the hydroxylapatite beads coated with either saliva or buffer. It was also observed that several different proteins and whole saliva tended to inhibit adherence. Scatchard plots, however, describing the binding of S. sanguis to the proteincoated beads were unique and revealed possible specific and nonspecific interactions. Scatchard analyses of binding data may be useful in understanding the mechanism(s) of adherence of streptococci to smooth surfaces.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelbaum B., Golub E., Holt S. C., Rosan B. In vitro studies of dental plaque formation: adsorption of oral streptococci to hydroxyaptite. Infect Immun. 1979 Aug;25(2):717–728. doi: 10.1128/iai.25.2.717-728.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold R. R., Mestecky J., McGhee J. R. Naturally occurring secretory immunoglobulin A antibodies to Streptococcus mutans in human colostrum and saliva. Infect Immun. 1976 Aug;14(2):355–362. doi: 10.1128/iai.14.2.355-362.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J. Presence of various types of non-haemolytic streptococci in dental plaque and in other sites of the oral cavity in man. Odontol Revy. 1967;18(1):55–74. [PubMed] [Google Scholar]
- Clark W. B., Bammann L. L., Gibbons R. J. Comparative estimates of bacterial affinities and adsorption sites on hydroxyapatite surfaces. Infect Immun. 1978 Mar;19(3):846–853. doi: 10.1128/iai.19.3.846-853.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlquist F. W. The meaning of Scatchard and Hill plots. Methods Enzymol. 1978;48:270–299. doi: 10.1016/s0076-6879(78)48015-2. [DOI] [PubMed] [Google Scholar]
- Doyle R. J., Matthews T. H., Streips U. N. Chemical basis for selectivity of metal ions by the Bacillus subtilis cell wall. J Bacteriol. 1980 Jul;143(1):471–480. doi: 10.1128/jb.143.1.471-480.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle R. J., Stroupe S. D. Perturbation of the calcium-binding site in concanavalin A by a saccharide ligand. Carbohydr Res. 1978 Dec;67(2):545–548. doi: 10.1016/s0008-6215(00)84147-6. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Houte J. V. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Moreno E. C., Spinell D. M. Model delineating the effects of a salivary pellicle on the adsorption of Streptococcus miteor onto hydroxyapatite. Infect Immun. 1976 Oct;14(4):1109–1112. doi: 10.1128/iai.14.4.1109-1112.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Qureshi J. V. Inhibition of adsorption of Streptococcus mutans strains to saliva-treated hydroxyapatite by galactose and certain amines. Infect Immun. 1979 Dec;26(3):1214–1217. doi: 10.1128/iai.26.3.1214-1217.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Qureshi J. V. Selective binding of blood group-reactive salivary mucins by Streptococcus mutans and other oral organisms. Infect Immun. 1978 Dec;22(3):665–671. doi: 10.1128/iai.22.3.665-671.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. J., Hayes C. E. The lectins: carbohydrate-binding proteins of plants and animals. Adv Carbohydr Chem Biochem. 1978;35:127–340. doi: 10.1016/s0065-2318(08)60220-6. [DOI] [PubMed] [Google Scholar]
- Hamada S., Slade H. D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980 Jun;44(2):331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian M., Roland K., Mestecky J. Interference of secretory immunoglobulin A with sorption of oral bacteria to hydroxyapatite. Infect Immun. 1981 Mar;31(3):942–951. doi: 10.1128/iai.31.3.942-951.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liljemark W. F., Schauer S. V. Studies on the bacterial components which bind Streptococcus sanguis and Streptococcus mutans to hydroxyapatite. Arch Oral Biol. 1975 Sep;20(9):609–615. doi: 10.1016/0003-9969(75)90082-5. [DOI] [PubMed] [Google Scholar]
- Nesbitt W. E., Staat R. H., Rosan B., Taylor K. G., Doyle R. J. Association of protein with the cell wall of Streptococcus mutans. Infect Immun. 1980 Apr;28(1):118–126. doi: 10.1128/iai.28.1.118-126.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orstavik D., Kraus F. W., Henshaw L. C. In vitro attachment of streptococci to the tooth surface. Infect Immun. 1974 May;9(5):794–800. doi: 10.1128/iai.9.5.794-800.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBINSON D. R., JENCKS W. P. THE EFFECT OF COMPOUNDS OF THE UREA-GUANIDINIUM CLASS ON THE ACTIVITY COEFFICIENT OF ACETYLTETRAGLYCINE ETHYL ESTER AND RELATED COMPOUNDS. J Am Chem Soc. 1965 Jun 5;87:2462–2470. doi: 10.1021/ja01089a028. [DOI] [PubMed] [Google Scholar]
- Ryan M. F., Westphal U. Steroid-protein interactions. XXIV. Progesterone-binding affinity of isoelectric variants of human 1 -acid glycoprotein. J Biol Chem. 1972 Jun 25;247(12):4050–4056. [PubMed] [Google Scholar]
- Shibata S., Nagata K., Nakamura R., Tsunemitsu A., Misaki A. Interaction of parotid saliva basic glycoprotein with Streptococcus sanguis ATCC 10557. J Periodontol. 1980 Sep;51(9):499–504. doi: 10.1902/jop.1980.51.9.499. [DOI] [PubMed] [Google Scholar]
- Staat R. H., Langley S. D., Doyle R. J. Streptococcus mutans adherence: presumptive evidence for protein-mediated attachment followed by glucan-dependent cellular accumulation. Infect Immun. 1980 Feb;27(2):675–681. doi: 10.1128/iai.27.2.675-681.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler T. T., Clark W. B., Birdsell D. C. Adherence of Actinomyces viscosus T14V and T14AV to hydroxyapatite surfaces in vitro and human teeth in vivo. Infect Immun. 1979 Sep;25(3):1066–1074. doi: 10.1128/iai.25.3.1066-1074.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]


