Abstract
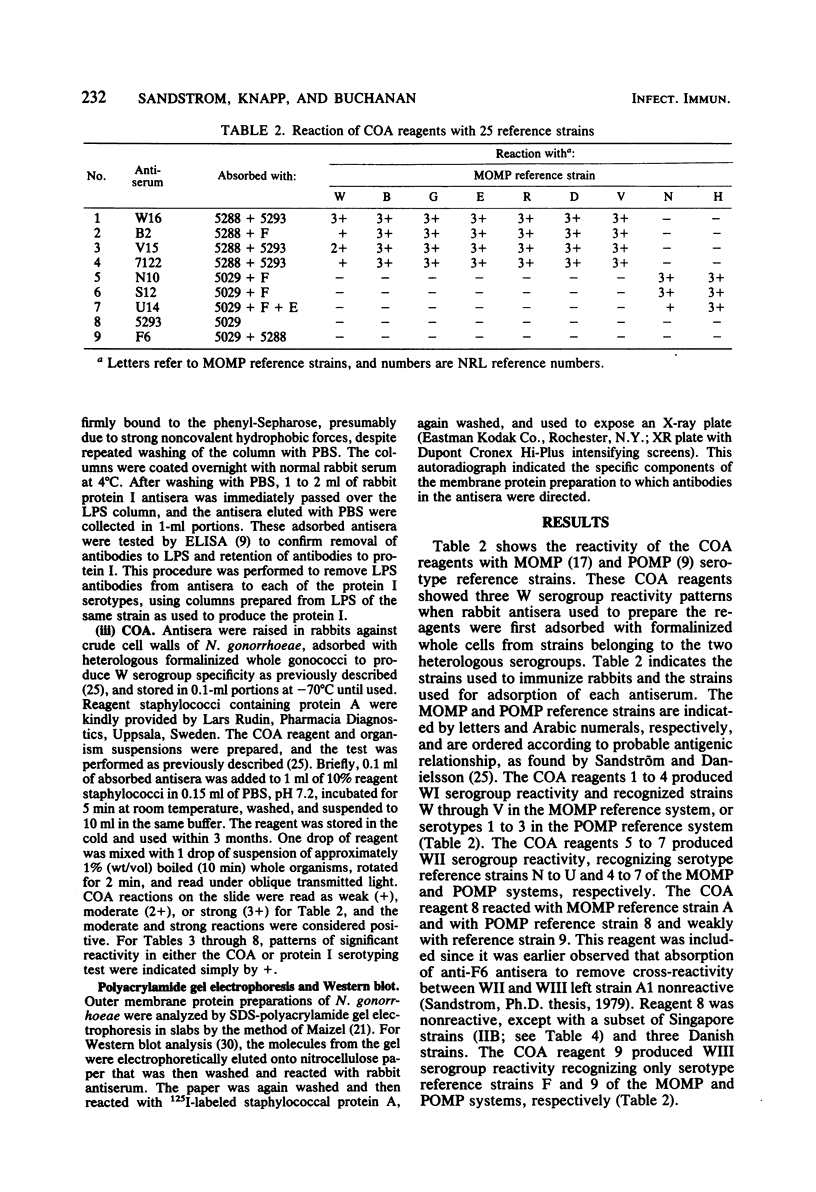
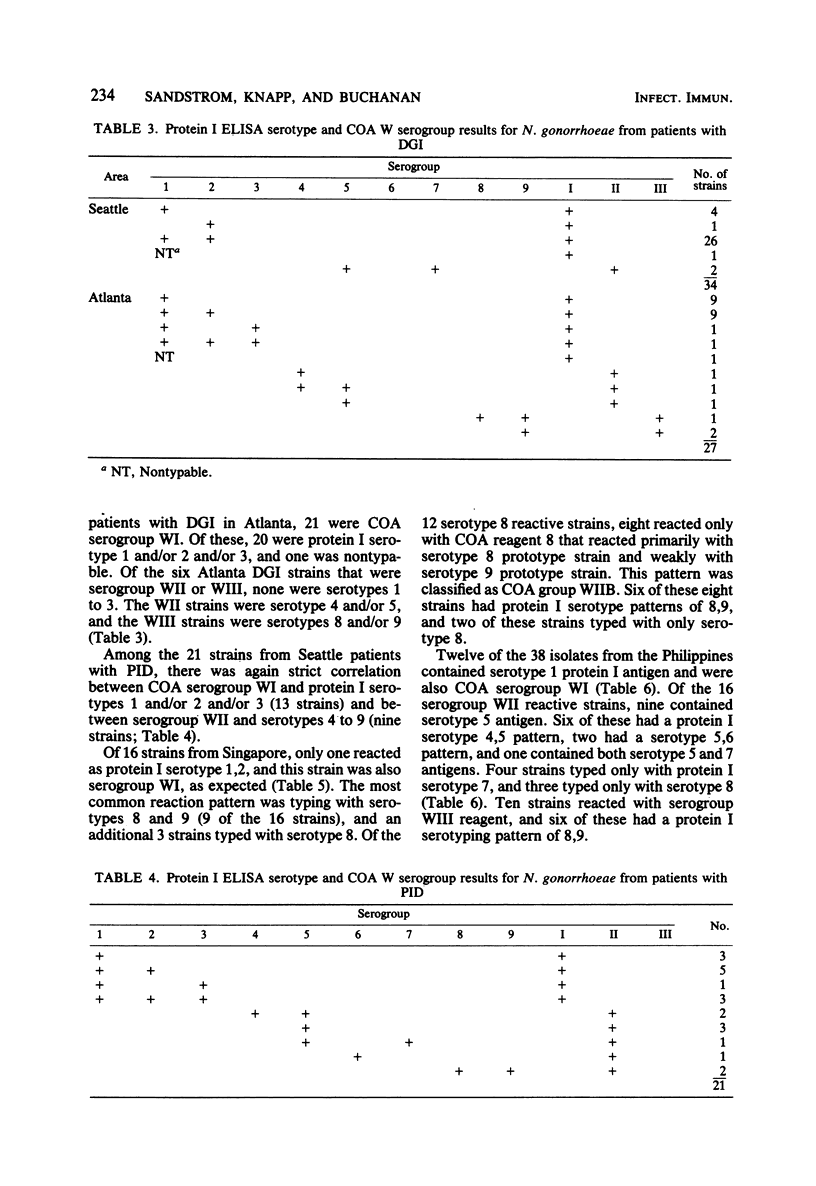
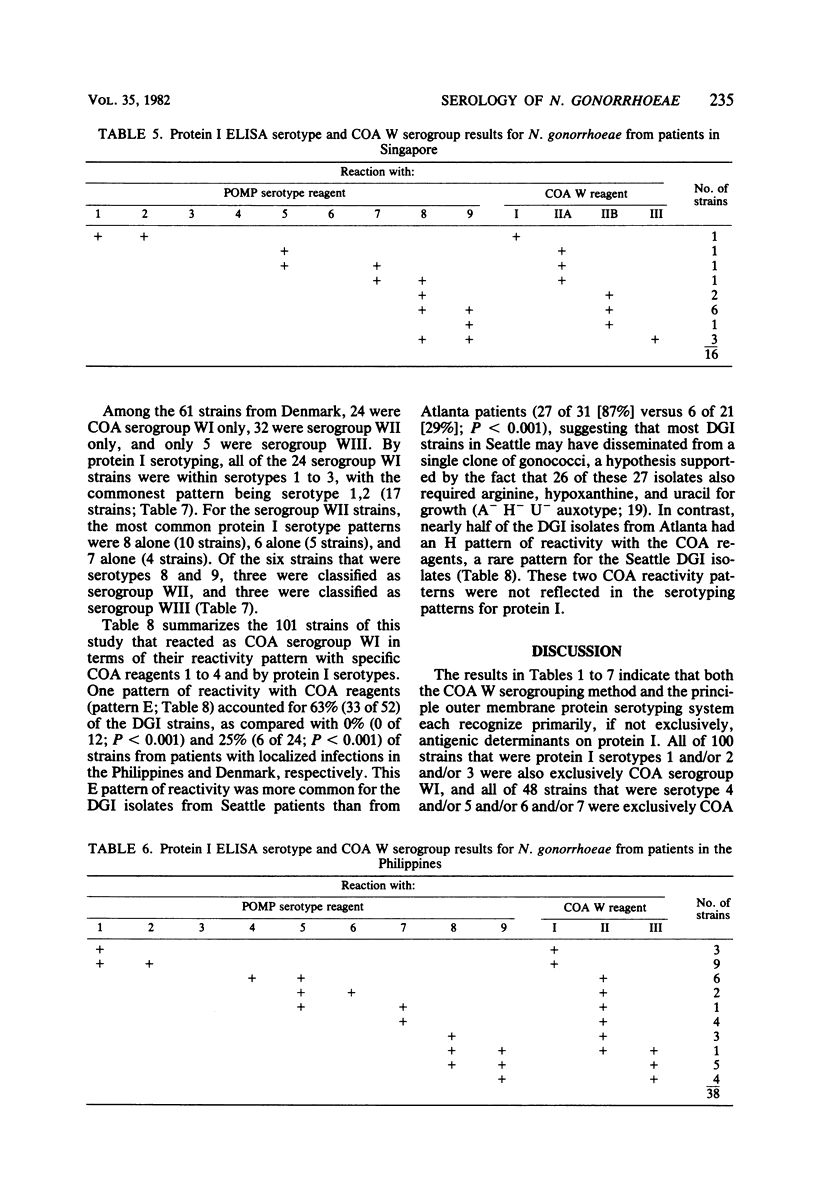
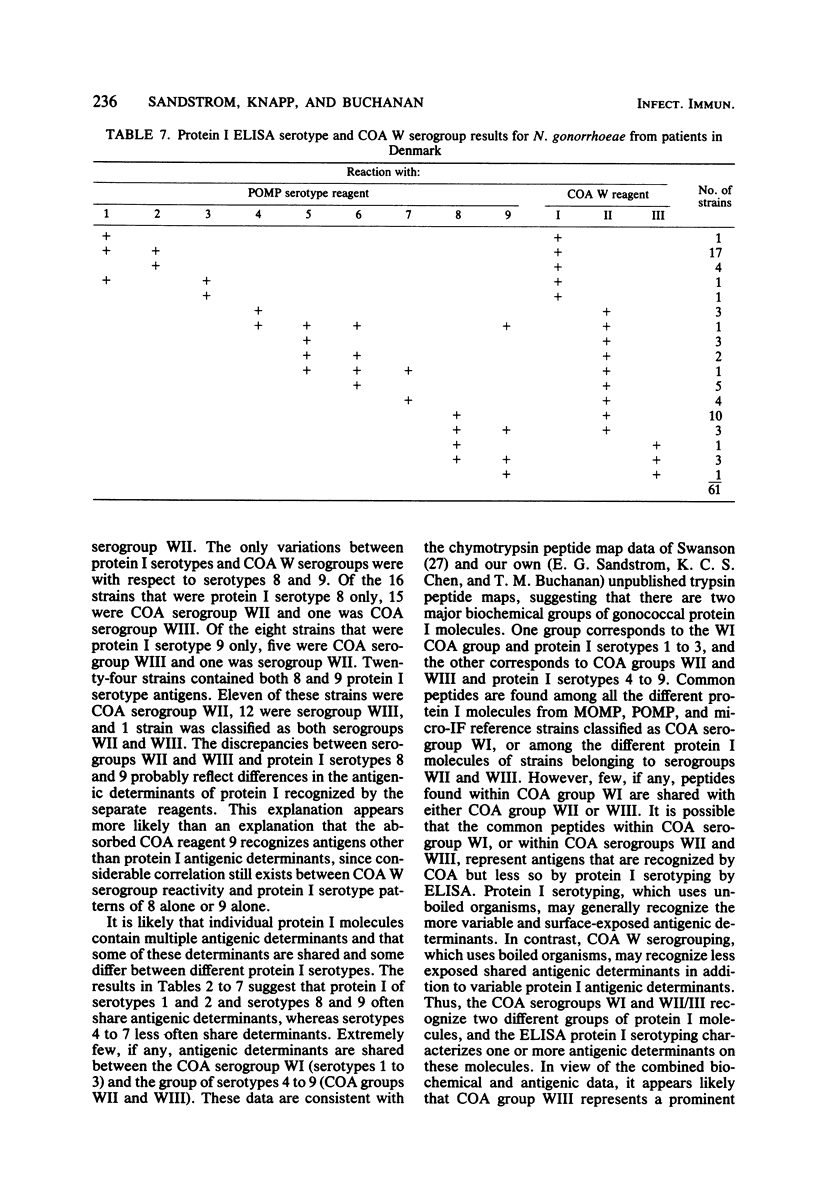
A total of 224 strains were serogrouped by coagglutination (COA) and serotyped by protein I enzyme-linked immunosorbent assay (ELISA). Of these strains, 61 were from patients with disseminated gonococcal infection, 21 were from patients with pelvic inflammatory disease, and 115 were from patients with uncomplicated gonococcal infection in Singapore, the Philippines, and Denmark. Twenty-seven were laboratory reference strains. Of the patient strains, 102 belonged to COA serogroup WI, and all of the 100 strains that typed with protein I serotypes 1, 2, or 3 were in this group. Most of the strains of gonococci from the 61 patients with disseminated gonococcal infection were within this group (COA WI, 53 or 87%; protein I serotypes 1, 2, or 3, 51 or 84%). All 46 strains that were protein I serotypes 4 through 7 were also COA serogroup WII. Protein I serotypes 8 and 9 accounted for 49 (25%) of the 197 patient strains. Twenty-eight of these strains typed as COA serogroup WII, 20 typed as serogroup WIII, and 1 typed as serogroups WII and WIII. COA W serogrouping and protein I ELISA both appeared to detect antigens on the protein I molecule of the outer membrane of Neisseria gonorrhoeae. Protein I serotyping, which uses unboiled organisms, may generally recognize more variable and surface-exposed antigenic determinants. In contrast, COA W serogrouping, which uses boiled organisms, may recognize less exposed shared antigenic determinants in addition to variable protein I antigenic determinants. Both methods may prove useful for further studies of the epidemiology and pathogenesis of gonorrhea.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apicella M. A., Gagliardi N. C. Antigenic heterogeneity of the non-serogroup antigen structure of Neisseria gonorrhoeae lipopolysaccharides. Infect Immun. 1979 Dec;26(3):870–874. doi: 10.1128/iai.26.3.870-874.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apicella M. A. Serogrouping of Neisseria gonorrhoeae: identification of four immunologically distinct acidic polysaccharides. J Infect Dis. 1976 Oct;134(4):377–383. doi: 10.1093/infdis/134.4.377. [DOI] [PubMed] [Google Scholar]
- Arko R. J. An immunologic model in laboratory animals for the study of Neisseria gonorrhoeae. J Infect Dis. 1974 Apr;129(4):451–455. doi: 10.1093/infdis/129.4.451. [DOI] [PubMed] [Google Scholar]
- Arko R. J., Duncan W. P., Brown W. J., Peacock W. L., Tomizawa T. Immunity in infection with Neisseria gonorrhoeae: duration and serological response in the chimpanzee. J Infect Dis. 1976 Apr;133(4):441–447. [PubMed] [Google Scholar]
- Arko R. J., Kraus S. J., Brown W. J., Buchanan T. M., Kuhn U. S. Neisseria gonorrhoeae: effects of systemic immunization on resistance of chimpanzees to urethral infection. J Infect Dis. 1974 Aug;130(2):160–164. doi: 10.1093/infdis/130.2.160. [DOI] [PubMed] [Google Scholar]
- Buchanan T. M. Antigen-specific serotyping of Neisseria gonorrhoeae. I. Use of an enzyme-linked immunosorbent assay to quantitate pilus antigens on gonococci. J Infect Dis. 1978 Sep;138(3):319–315. doi: 10.1093/infdis/138.3.319. [DOI] [PubMed] [Google Scholar]
- Buchanan T. M., Arko R. J. Immunity to gonococcal infection induced by vaccination with isolated outer membranes of Neisseria gonorrhoeae in guinea pigs. J Infect Dis. 1977 Jun;135(6):879–887. doi: 10.1093/infdis/135.6.879. [DOI] [PubMed] [Google Scholar]
- Buchanan T. M., Eschenbach D. A., Knapp J. S., Holmes K. K. Gonococcal salpingitis is less likely to recur with Neisseria gonorrhoeae of the same principal outer membrane protein antigenic type. Am J Obstet Gynecol. 1980 Dec 1;138(7 Pt 2):978–980. doi: 10.1016/0002-9378(80)91091-1. [DOI] [PubMed] [Google Scholar]
- Buchanan T. M., Hildebrandt J. F. Antigen-specific serotyping of Neisseria gonorrhoeae: characterization based upon principal outer membrane protein. Infect Immun. 1981 Jun;32(3):985–994. doi: 10.1128/iai.32.3.985-994.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan T. M., Pearce W. A. Pili as a mediator of the attachment of gonococci to human erythrocytes. Infect Immun. 1976 May;13(5):1483–1489. doi: 10.1128/iai.13.5.1483-1489.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan T. M., Pearce W. A., Schoolnik G. K., Arko R. J. Protection against infection with Neisseria gonorrhoeae by immunization with outer membrane protein complex and purified pili. J Infect Dis. 1977 Aug;136 (Suppl):S132–S137. doi: 10.1093/infdis/136.supplement.s132. [DOI] [PubMed] [Google Scholar]
- Danielsson D., Sandström E. Serology of Neisseria gonorrhoeae. Demonstration by co-agglutination and immunoelectrophoresis of antigenic differences associated with colour/opacity colonial variants. Acta Pathol Microbiol Scand B. 1980 Feb;88(1):39–46. doi: 10.1111/j.1699-0463.1980.tb02601.x. [DOI] [PubMed] [Google Scholar]
- Eschenbach D. A., Buchanan T. M., Pollock H. M., Forsyth P. S., Alexander E. R., Lin J. S., Wang S. P., Wentworth B. B., MacCormack W. M., Holmes K. K. Polymicrobial etiology of acute pelvic inflammatory disease. N Engl J Med. 1975 Jul 24;293(4):166–171. doi: 10.1056/NEJM197507242930403. [DOI] [PubMed] [Google Scholar]
- Hermodson M. A., Chen K. C., Buchanan T. M. Neisseria pili proteins: amino-terminal amino acid sequences and identification of an unusual amino acid. Biochemistry. 1978 Feb 7;17(3):442–445. doi: 10.1021/bi00596a010. [DOI] [PubMed] [Google Scholar]
- Hildebrandt J. F., Mayer L. W., Wang S. P., Buchanan T. M. Neisseria gonorrhoeae acquire a new principal outer-membrane protein when transformed to resistance to serum bactericidal activity. Infect Immun. 1978 Apr;20(1):267–272. doi: 10.1128/iai.20.1.267-272.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston K. H., Holmes K. K., Gotschlich E. C. The serological classification of Neisseria gonorrhoeae. I. Isolation of the outer membrane complex responsible for serotypic specificity. J Exp Med. 1976 Apr 1;143(4):741–758. doi: 10.1084/jem.143.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLOGG D. S., Jr, PEACOCK W. L., Jr, DEACON W. E., BROWN L., PIRKLE D. I. NEISSERIA GONORRHOEAE. I. VIRULENCE GENETICALLY LINKED TO CLONAL VARIATION. J Bacteriol. 1963 Jun;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp J. S., Holmes K. K. Disseminated gonococcal infections caused by Neisseria gonorrhoeae with unique nutritional requirements. J Infect Dis. 1975 Aug;132(2):204–208. doi: 10.1093/infdis/132.2.204. [DOI] [PubMed] [Google Scholar]
- Maeland J. A. Serological cross-reactions of aqueous ether extracted endotoxin from Neisseria gonorrhoeae strains. Acta Pathol Microbiol Scand. 1969;77(3):505–517. doi: 10.1111/j.1699-0463.1969.tb04257.x. [DOI] [PubMed] [Google Scholar]
- Pearce W. A., Buchanan T. M. Attachment role of gonococcal pili. Optimum conditions and quantitation of adherence of isolated pili to human cells in vitro. J Clin Invest. 1978 Apr;61(4):931–943. doi: 10.1172/JCI109018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandström E., Danielsson D. Serology of Neisseria gonorrhoeae. Classification by co-agglutination. Acta Pathol Microbiol Scand B. 1980 Feb;88(1):27–38. doi: 10.1111/j.1699-0463.1980.tb02600.x. [DOI] [PubMed] [Google Scholar]
- Schoolnik G. K., Buchanan T. M., Holmes K. K. Gonococci causing disseminated gonococcal infection are resistant to the bactericidal action of normal human sera. J Clin Invest. 1976 Nov;58(5):1163–1173. doi: 10.1172/JCI108569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XVIII. 125I-labeled peptide mapping of the major protein of the gonococcal cell wall outer membrane. Infect Immun. 1979 Mar;23(3):799–810. doi: 10.1128/iai.23.3.799-810.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S. E., Reynolds G., Short H. B., Thornsberry C., Biddle J. W., Jacobs N. F., Rein M. F., Zaidi A., Young F. E., Shulman J. A. Auxotypes and antibiotic susceptibility patterns of Neisseria gonorrhoeae from disseminated and local infections. Sex Transm Dis. 1978 Oct-Dec;5(4):127–131. doi: 10.1097/00007435-197810000-00001. [DOI] [PubMed] [Google Scholar]
- Wang S. P., Holmes K. K., Knapp J. S., Ott S., Kyzer D. D. Immunologic classification of Neisseria gonorrhoeae with micro-immunofluorescence. J Immunol. 1977 Sep;119(3):795–803. [PubMed] [Google Scholar]