Abstract
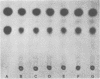
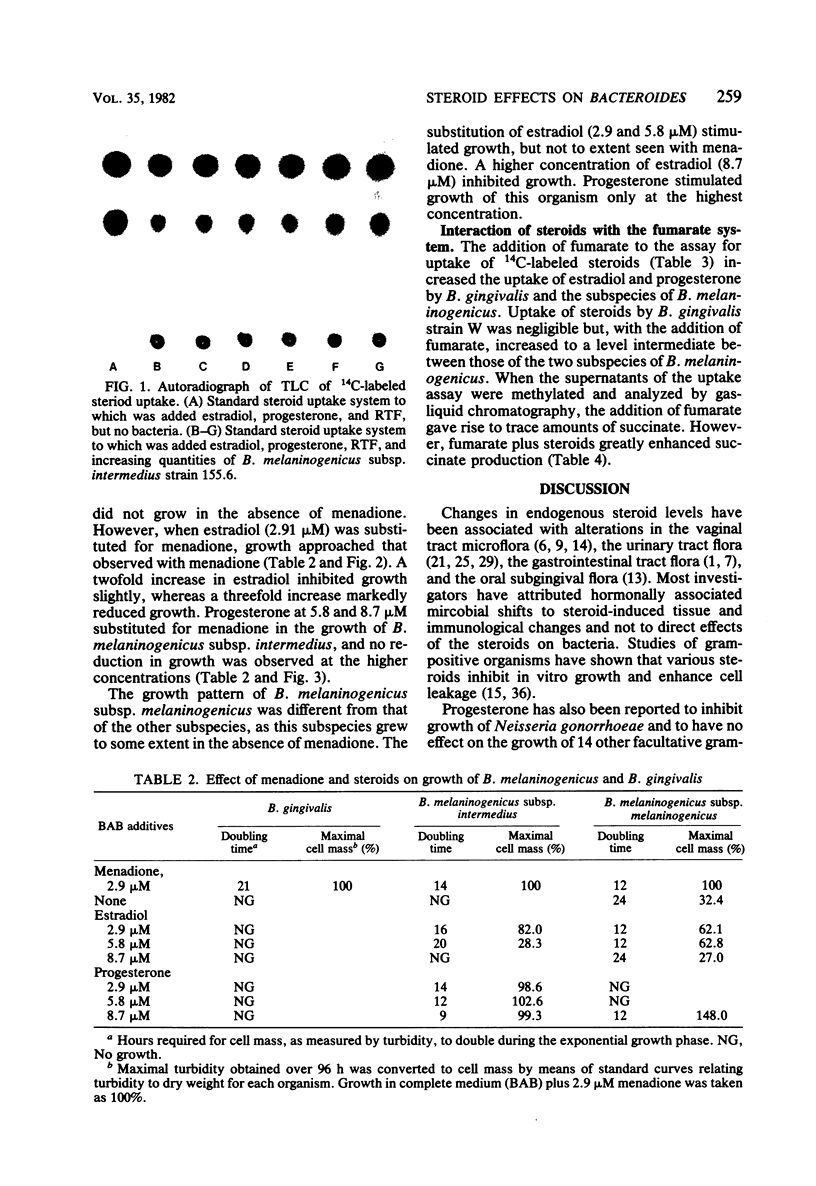
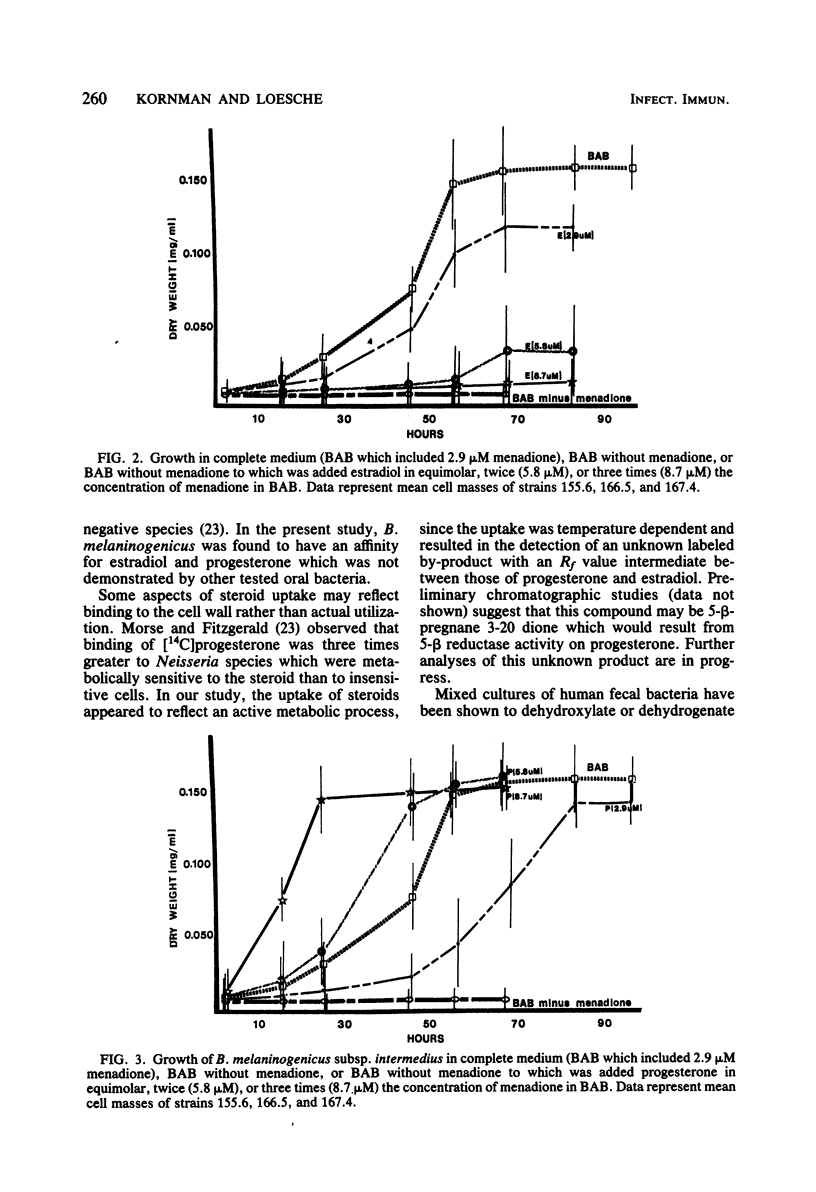
Bacteroides melaninogenicus subsp. intermedius increases in the subgingival microflora during pregnancy. These studies evaluated direct interactions between hormonal steroids and oral Bacteroides species. Resting cell suspensions of pure cultures of plaque organisms were incubated anaerobically with [14C]estradiol and [14C]progesterone. Uptake of labeled compound per microgram of bacterial protein was determined by thin-layer chromatography and liquid scintillation counting. B. melaninogenicus subsp. intermedius and B. melaninogenicus subsp. melaninogenicus took up 2.6 x 10(-4) to 5.4 x 10(-4) mumol of estradiol or progesterone per microgram of cell protein. Minimal steroid uptake was observed with B. gingivalis and five other organisms. Uptake of steroids by B. melaninogenicus subsp. intermedius was temperature dependent and resulted in a labeled product as detected on thin-layer chromatography. Growth curves indicated that intermedius and melaninogenicus subspecies of B. melaninogenicus but not B. gingivalis could substitute progesterone or estradiol for vitamin K, an essential growth factor. Growth of B. melaninogenicus subsp. intermedius in steroids was concentration dependent. Addition of fumarate to resting cells of B. melaninogenicus subspecies as well as B. gingivalis increased steroid uptake by 70 to 500% and resulted in the gas-liquid chromatographic detection of succinate. Cultures given fumarate alone or steroids alone produced no succinate. Steroids appeared to directly interact with the fumarate reductase system and foster the growth of B. melaninogenicus subsp. intermedius. This interaction may be of ecological significance.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adlercreutz H., Martin F., Järvenpä P., Fotsis T. Steroid absorption and enterohepatic recycling. Contraception. 1979 Sep;20(3):201–223. doi: 10.1016/0010-7824(79)90094-5. [DOI] [PubMed] [Google Scholar]
- Bokkenheuser V. D., Winter J., Kelly W. G. Metabolism of biliary steroids by human fecal flora. Am J Clin Nutr. 1978 Oct;31(10 Suppl):S221–S226. doi: 10.1093/ajcn/31.10.S221. [DOI] [PubMed] [Google Scholar]
- CASAS-CAMPILLO C., BALANDRANO D., GALARZA A. Steroids. 159. Antimicrobial properties of 21,21-dimethoxy progesterone and other progesterone analogues. J Bacteriol. 1961 Mar;81:366–375. doi: 10.1128/jb.81.3.366-375.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimasoni G. The crevicular fluid. Monogr Oral Sci. 1974;3(0):1–122. [PubMed] [Google Scholar]
- GIBBONS R. J., MACDONALD J. B. Hemin and vitamin K compounds as required factors for the cultivation of certain strains of Bacteroides melaninogenicus. J Bacteriol. 1960 Aug;80:164–170. doi: 10.1128/jb.80.2.164-170.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goplerud C. P., Ohm M. J., Galask R. P. Aerobic and anaerobic flora of the cervix during pregnancy and the puerperium. Am J Obstet Gynecol. 1976 Dec 1;126(7):858–868. doi: 10.1016/0002-9378(76)90674-8. [DOI] [PubMed] [Google Scholar]
- Järvenpä P., Kosunen T., Fotsis T., Adlercreutz H. In vitro metabolism of estrogens by isolated intestinal micro-organisms and by human faecal microflora. J Steroid Biochem. 1980 Mar;13(3):345–349. doi: 10.1016/0022-4731(80)90014-x. [DOI] [PubMed] [Google Scholar]
- Kelly W. G., De Leon O., Winter J., Bokkenheuser V. D. Exchange of hydrogen at C21 during dehydroxylation of deoxycorticosterone by mixed cultures of human fecal flora. J Steroid Biochem. 1977 Jan;8(1):73–75. doi: 10.1016/0022-4731(77)90220-5. [DOI] [PubMed] [Google Scholar]
- Kornman K. S., Loesche W. J. The subgingival microbial flora during pregnancy. J Periodontal Res. 1980 Mar;15(2):111–122. doi: 10.1111/j.1600-0765.1980.tb00265.x. [DOI] [PubMed] [Google Scholar]
- LEV M. The growth-promoting activity of compounds of the vitamin K group and analogues for a rumen strain of Fusiformis nigrescens. J Gen Microbiol. 1959 Jun;20(3):697–703. doi: 10.1099/00221287-20-3-697. [DOI] [PubMed] [Google Scholar]
- Larsen B., Markovetz A. J., Galask R. P. Quantitative alterations in the genital microflora of female rats in relation to the estrous cycle. J Infect Dis. 1976 Nov;134(5):486–489. doi: 10.1093/infdis/134.5.486. [DOI] [PubMed] [Google Scholar]
- Loesche W. J. Chemotherapy of dental plaque infections. Oral Sci Rev. 1976;9:65–107. [PubMed] [Google Scholar]
- Loesche W. J., Hockett R. N., Syed S. A. The predominant cultivable flora of tooth surface plaque removed from institutionalized subjects. Arch Oral Biol. 1972 Sep;17(9):1311–1325. doi: 10.1016/0003-9969(72)90164-1. [DOI] [PubMed] [Google Scholar]
- Loesche W. J., Syed S. A. Bacteriology of human experimental gingivitis: effect of plaque and gingivitis score. Infect Immun. 1978 Sep;21(3):830–839. doi: 10.1128/iai.21.3.830-839.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald I. A., Webb G. R., Mahony D. E. Fecal hydroxysteroid dehydrogenase activities in vegetarian Seventh-Day Adventists, control subjects, and bowel cancer patients. Am J Clin Nutr. 1978 Oct;31(10 Suppl):S233–S238. doi: 10.1093/ajcn/31.10.S233. [DOI] [PubMed] [Google Scholar]
- Marrie T. J., Swantee C. A., Hartlen M. Aerobic and anaerobic urethral flora of healthy females in various physiological age groups and of females with urinary tract infections. J Clin Microbiol. 1980 Jun;11(6):654–659. doi: 10.1128/jcm.11.6.654-659.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall S., Linfoot J. Influence of hormones on urinary tract infection. Therapeutic implications. Urology. 1977 Jun;9(6):675–679. doi: 10.1016/0090-4295(77)90321-1. [DOI] [PubMed] [Google Scholar]
- Morse S. A., Fitzgerald T. J. Effect of progesterone on Neisseria gonorrhoeae. Infect Immun. 1974 Dec;10(6):1370–1377. doi: 10.1128/iai.10.6.1370-1377.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrod J. A., Hylemon P. B. Partial purification and characterization of NAD-dependent 7alpha-hydroxysteroid dehydrogenase from Bacteroides thetaiotaomicron. Biochim Biophys Acta. 1977 Feb 23;486(2):351–358. doi: 10.1016/0005-2760(77)90031-5. [DOI] [PubMed] [Google Scholar]
- Silk M., Perez-Varela M. R. Effects of oral contraceptives on urinary bacterial growth rate. Invest Urol. 1970 Sep;8(2):239–241. [PubMed] [Google Scholar]
- Slots J. Microflora in the healthy gingival sulcus in man. Scand J Dent Res. 1977 May;85(4):247–254. doi: 10.1111/j.1600-0722.1977.tb00560.x. [DOI] [PubMed] [Google Scholar]
- Slots J., Möenbo D., Langebaek J., Frandsen A. Microbiota of gingivitis in man. Scand J Dent Res. 1978 May;86(3):174–181. doi: 10.1111/j.1600-0722.1978.tb01929.x. [DOI] [PubMed] [Google Scholar]
- Slots J. The predominant cultivable microflora of advanced periodontitis. Scand J Dent Res. 1977 Jan-Feb;85(2):114–121. doi: 10.1111/j.1600-0722.1977.tb00541.x. [DOI] [PubMed] [Google Scholar]
- Syed S. A. Characteristics of Bacteroides asaccharolyticus from dental plaques of beagle dogs. J Clin Microbiol. 1980 May;11(5):522–526. doi: 10.1128/jcm.11.5.522-526.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed S. A., Svanberg M., Svanberg G. The predominant cultivable dental plaque flora of beagle dogs with gingivitis. J Periodontal Res. 1980 Mar;15(2):123–136. doi: 10.1111/j.1600-0765.1980.tb00266.x. [DOI] [PubMed] [Google Scholar]
- Tanner A. C., Haffer C., Bratthall G. T., Visconti R. A., Socransky S. S. A study of the bacteria associated with advancing periodontitis in man. J Clin Periodontol. 1979 Oct;6(5):278–307. doi: 10.1111/j.1600-051x.1979.tb01931.x. [DOI] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udenfriend S., Stein S., Böhlen P., Dairman W., Leimgruber W., Weigele M. Fluorescamine: a reagent for assay of amino acids, peptides, proteins, and primary amines in the picomole range. Science. 1972 Nov 24;178(4063):871–872. doi: 10.1126/science.178.4063.871. [DOI] [PubMed] [Google Scholar]
- Yotis W. W. In vivo and in vitro action of norethindrone on staphylococci. J Bacteriol. 1967 Nov;94(5):1353–1358. doi: 10.1128/jb.94.5.1353-1358.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Palenstein Helderman W. H. Total viable count and differential count of vibrio (campylobacter) sputorum, fusobacterium nucleatum, selenomonas sputigena, bacteroides ochraceus and veillonella in the inflamed and non inflamed human gingival crevice. J Periodontal Res. 1975 Nov;10(5):294–305. doi: 10.1111/j.1600-0765.1975.tb00037.x. [DOI] [PubMed] [Google Scholar]