Abstract
We present a building block approach toward functionalized CB[7] derivatives by the condensation of methylene bridged glycoluril hexamer 1 and glycoluril bis(cyclic ethers) 2 and 12. The CB[7] derivatives Me2CB[7] and CyCB[7] are highly soluble in water (264 mM and 181 mM, respectively). As a result of the high intrinsic solubility of Me2CB[7], it is able to solubilize the insoluble benzimidazole drug albendazole. The reaction of hexamer 1 with glycoluril derivative 12 which bears a primary alkyl chloride group gives CB[7] derivative 18 in 16% isolated yield. Compound 18 reacts with NaN3 to yield azide-substituted CB[7] 19 in 81% yield which subsequently undergoes click reaction with propargylammonium chloride (21) to yield CB[7] derivative 20 in 95% yield which bears a covalently attached triazolyl ammonium group along its equator. The results of NMR spectroscopy (1H, variable temperature, and DOSY) and electrospray mass spectrometry establish that 20 undergoes self-assembly to form the cyclic tetrameric assembly (204) in aqueous solution. CB[7] derivatives bearing reactive functional groups (e.g. N3, Cl) are now available for incorporation into more complex functional systems.
Introduction
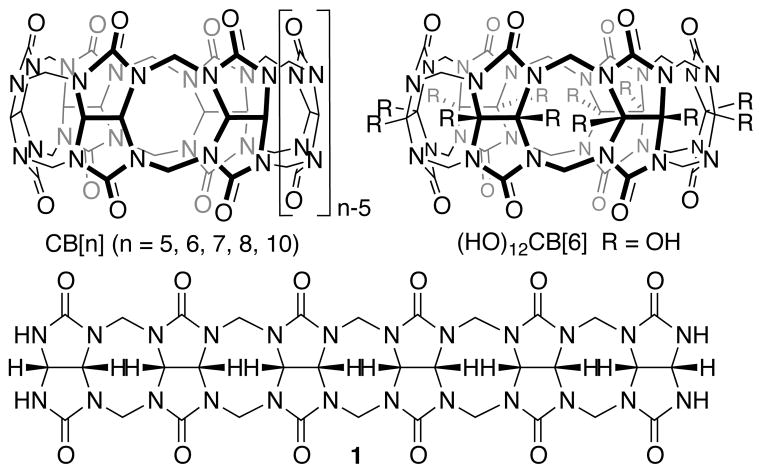
The cucurbit[n]uril (CB[n]; n = 5, 6, 7, 8, 10) family of molecular containers1,2 is formed by the condensation reaction of glycoluril and formaldehyde under strongly acidic conditions.3,4,5 The defining structural features of the CB[n] compounds (Chart 1) are their two symmetry equivalent ureidyl C=O portals and their hydrophobic cavity which imparts the ability to bind to hydrophobic and cationic species in water by a combination of ion-dipole interactions and the hydrophobic effect.6 CB[n] compounds have emerged as a truly privileged class of receptors for molecular recognition in water as a result of their high affinity (Ka routinely 106 – 1012 M−1), highly selective, and stimuli responsive binding processes.6–8 Accordingly, CB[n] compounds have been used in a wide range of applications including drug delivery,9,10,11 molecular machines,12 supramolecular polymers,13 sensing ensembles,14 and biomimetic systems.15 Despite the wide range of applications that are enabled by CB[n] molecular containers there is an ongoing need for versatile synthetic approaches toward functionalized CB[n] compounds that retain their recognition properties and can be incorporated into more complex functional systems. Until quite recently, the only route to functionalized CB[n] compounds involved the direct (per)hydroxylation of CB[n] introduced by Kim.16,17 Kim and co-workers have used these functionalized CB[n] compounds in a variety of applications including nanocapsules for drug delivery, membrane protein fishing, and hydrogels for cellular engineering.10,18,19 Recently, Scherman and co-workers tamed the (per)hydroxylation reaction which allowed them to isolate (HO)1CB[6] and perform subsequent functionalization reactions to deliver a self-complexing CB[6] derivative.20
Chart 1.
Structure of CB[n], (HO)12CB[6], and hexamer 1.
Our group has been investigating the mechanism of CB[n] formation21–24 as a route to prepare CB[n]-type receptors with exciting structures and functions. For example, we have used this mechanistic knowledge to prepare CB[n]-type receptors that display homotropic allostery, chiral recognition, and are useful in drug delivery applications.11,25 In 2011, we reported the templated synthesis of methylene bridged glycoluril hexamer 1 and its transformation into monofunctionalized CB[6] derivatives by macrocyclization reactions with substituted phthalaldehydes.24 Very recently, we reported the synthesis of a clickable CB[6] derivative, its transformation into a CB[6] derivative functionalized with an ammonium ion tail, and its self-assembly into a cyclic [c2] daisy chain assembly.26 Despite the recent advances in the synthesis of monofunctionalized CB[6] derivatives,20,24,26 there is still an unmet need for versatile synthetic routes toward CB[7] derivatives,16,18,27 and especially monofunctionalized CB[7] derivatives that are amenable to further functionalization reactions. For example, CB[7] is nicely soluble in water whereas CB[6] and the CB[6] derivatives prepared by us possess only modest solubility in the absence of guest. In addition, the more spacious cavity of CB[7] and its derivatives are able to bind to a wider variety of chemically and biologically interesting guests. Finally, the Ka values achieved with CB[7] are unsurpassed. In this paper we present a building block approach21,22,27,28,29,30 toward monofunctionalized CB[7] derivatives and report on their self-assembly behavior.
Results and Discussion
This results and discussion section is organized as follows. First, we discuss the preparation CB[7] derivatives Me2CB[7], CyCB[7], and MePhCB[7] by a building block approach using glycoluril hexamer 1 and glycoluril bis(cyclic ethers) 2. Then, we describe their basic properties (e.g. x-ray crystallography, host-guest binding, aqueous solubility, and drug solubilization). Next, we synthesize monofunctionalized CB[7] derivatives that contain reactive alkylchloride and azide functional groups. Finally, we describe the self-assembly of a CB[7] derivative to yield a cyclic tetrameric assembly in water.
Synthesis of Me2CB[7] and CyCB[7]
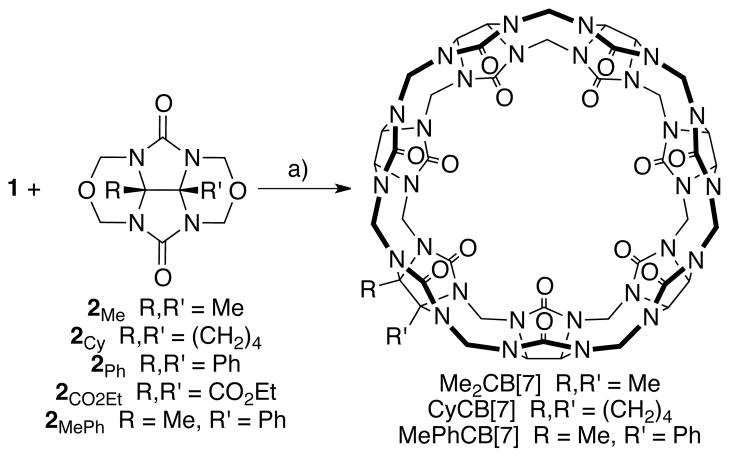
Given the ready access to gram scale quantities of glycoluril hexamer 1 and glycoluril bis (cyclic ethers) 2 we decided to investigate their transformation into CB[7] derivatives (Scheme 1). After some experimentation, we performed the reaction between 1 and 2Me with added KI in 9 M H2SO4 at 110 °C for 30 minutes.31 Analysis of the crude reaction mixture using p-xylenediammonium ion (3) as 1H NMR probe24 showed the presence of a 55:45 ratio of CB[6] and Me2CB[7]. As expected, the major competing reaction in this hexamer plus monomer building block approach to CB[7] derivatives is the unimolecular cyclization of hexamer to give CB[6]. Purification of the mixture was achieved by the addition of aqueous KI to an aqueous solution of the crude reaction mixture which results in precipitation of most of the CB[6] byproduct followed by final purification by treatment with activated carbon to yield Me2CB[7] (380 mg) in 31% yield. A similar reaction was performed between 1 and 2Cy which delivered CyCB[7] in 18% yield. We find that Me2CB[7] (≥ 264 mM) and CyCB[7] (≥ 181 mM) are significantly more soluble in water than CB[7] itself (20–30 mM)1 which suggests that they might find utility in applications where highly soluble compounds are needed (e.g. supramolecular polymers or drug solubilization).32 When we performed related reactions with 2Ph or 2CO2Et we did not observe the formation of any CB[7] derivatives but rather observed the formation of CB[6] by unimolecular cyclization of 1. From our previous work21,33 we know that 2Ph and 2CO2Et are less reactive than alkylated glycolurils 2Me or 2Cy which makes them less able to undergo bimolecular reaction with 1 to give CB[7] derivatives. In contrast, however, a similar reaction between 1 and 2MePh resulted in a mixture of CB[6] and MePhCB[7] in a 61:30 ratio based on analysis of the crude 1H NMR in the presence of 3. Pure MePhCB[7] could only be obtained in a meager 3% yield after Dowex™ ion exchange chromatography.
Scheme 1.
Synthesis of Me2CB[7], CyCB[7], and MePhCB[7]. Conditions: a) 9M H2SO4, 110 °C, KI, 30 min.
X-ray Crystal Structure of Me2CB[7]
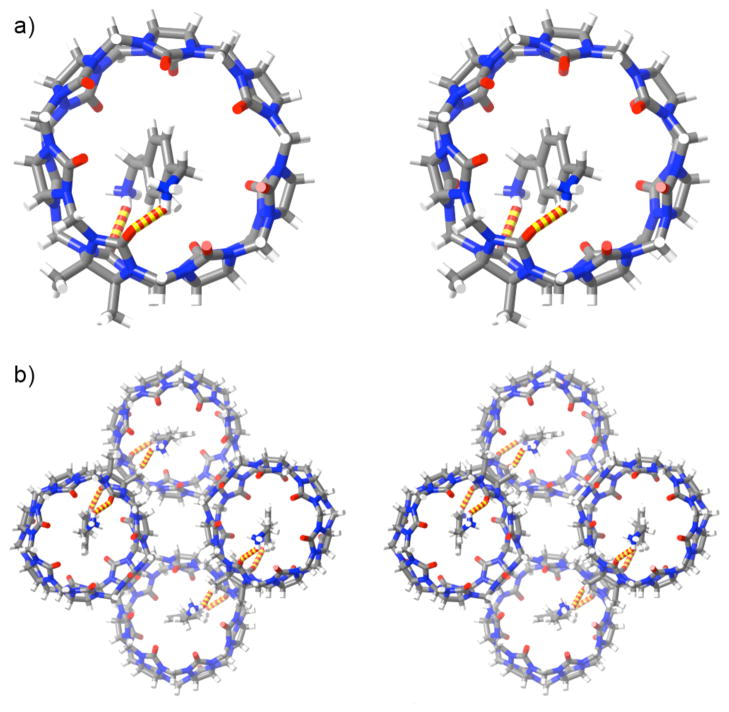
We were fortunate to obtain single crystals of Me2CB[7] as its Me2CB[7]•3 complex and to solve its structure by X-ray crystallography. Figure 1a shows a cross eyed stereoview of the structure of the one of the Me2CB[7]•3 complexes in the crystal. In contrast to CB[6] derivatives which display an ellipsoidal deformation along their equator upon functionalization,24,29 the CB[7] derivative Me2CB[7] appears structurally similar to CB[7] itself.4 For example, the distance between the ureidyl C=O O-atoms of a single glycoluril unit average 6.05 Å (range 5.87 – 6.154 Å) which is comparable to that observed for CB[7] (6.05 Å; range 5.913 – 6.114) itself. Similarly, the dimensions along the equator of Me2CB[7] are comparable to that of CB[7] as well. For example, the distance between the opposing methine C-atoms on each fourth glycoluril on Me2CB[7] averages 11.398 Å (range 11.247 – 11.516 Å) whereas CB[7] averages 11.404 Å (range 11.173 – 11.591 Å). One structural parameter that is rather different for CB[7] and Me2CB[7] is the average distance between ureidyl C=O O-atoms on every fourth glycoluril at one portal. For Me2CB[7] the distances average 8.188 Å (range 7.197 – 9.026 Å; standard deviation = 0.644 Å) whereas for CB[7] the distances average 8.139 Å (range 7.553 – 8.718 Å, standard deviation = 0.364 Å) for CB[7] itself. The glycolurils appear to pivot such that one O-atoms moves inward and one moves outward which results in the ureidyl C=O portals undergoing an ellipsoidal deformation. Overall, the molecular structures of the cavity of CB[7] and Me2CB[7] are similar. Figure 1b shows a cross eyed stereoview of the basic packing of individual complexes of Me2CB[7]•3 into a square array parallel to the xy-plane within the crystal. The Me groups of two adjacent Me2CB[7]•3 complexes orient themselves toward each others ureidyl C=O portals. The iodide counterions (not depicted) are found at the corners of the square array and extend in columns along the z-axis.
Figure 1.
Cross-eyed stereoviews of: a) the x-ray crystal structure of Me2CB[7]•3, and b) a portion of the crystal lattice showing the three dimensional packing motif. Color code: C, gray; H, white; N, blue; O, red; H-bonds, red-yellow striped.
Molecular Recognition Properties of Me2CB[7] and CyCB[7]
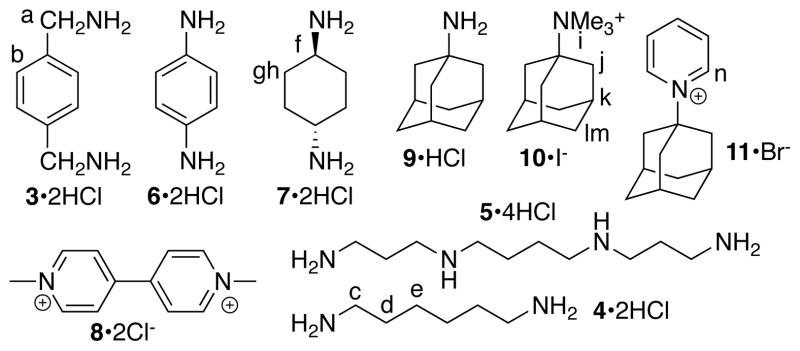
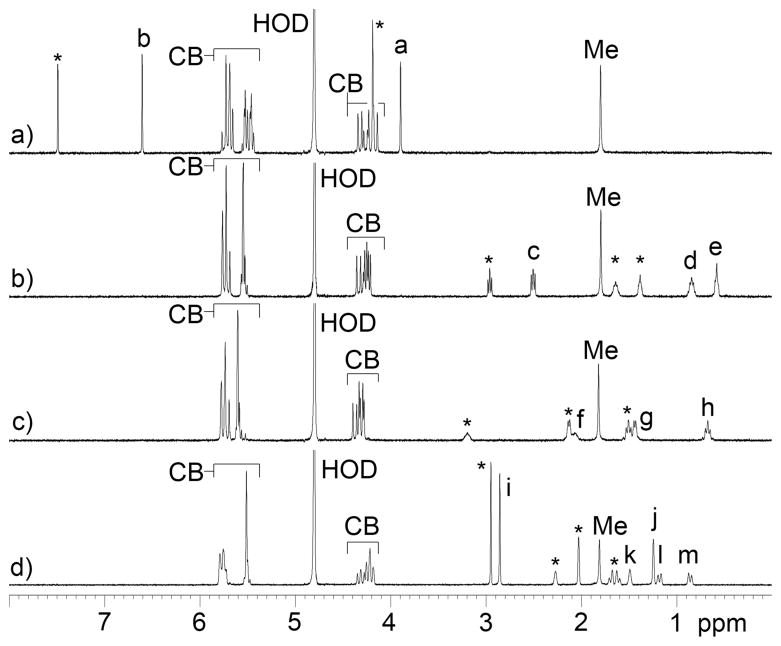
After establishing the basic structural features of Me2CB[7] we decided to investigate its molecular recognition properties. For this purpose, we used guests (3 – 11) shown in Chart 2 which increase in size from hexanediamine 4 to adamantane derivatives 9 – 11. Guests 3 – 11 are well known to form complexes with unsubstitut-ed CB[7] with binding constants up to 4.2 × 1012 M−1 for CB[7]•9.7 Figure 2 shows the 1H NMR spectra recorded for Me2CB[7]•3, Me2CB[7]•4, Me2CB[7]•7, and Me2CB[7]•10. The guest resonances observed in 1H NMR spectra are nearly identical in chemical shift to those measured for the corresponding CB[7] complexes (Supporting Information) which indicates that the magnetic environment inside the cavity of CB[7] and Me2CB[7] are quite similar. The resonances observed in the 1H NMR spectra that correspond to the H-atoms of C2v-symmetric Me2CB[7] reflect its lower symmetry. For example, three doublets of relative integral two and one doublet of relative integral one are observed for the upfield shifted H-atoms of the diastereotopic CH2-groups of the Me2CB[7]•3 complex (Figure 2a). The main reason for synthesizing CB[7] derivatives is to be able to encorporate them into more complex systems while maintaining their recognition preoprties. Therefore, we viewed it as critical to verify that the high binding constants observed for CB[7] are maintained for CB[7] derivatives like Me2CB[7]. For this purpose, we used the complexes CB[7]•11 and Me2CB[7]•11 because the H-atoms adjacent to the pyridinium N-atom are located at the ureidyl C=O portal in the complexes. Given that the presence of the two Me groups induce a change in the O•••O distance in the substituted glycoluril (vide supra) we thought that these protons might exhibit different chemical shifts in the CB[7]•11 and Me2CB[7]•11 complexes. Experimentally, we find that Hn resonates at 8.95 ppm for CB[7]•11 and 8.96 ppm for Me2CB[7]•11 (Supporting Information). We used a 1H NMR competition experiment (Supporting Information) to determine that the Ka value for Me2CB[7]•11 is 3.2 × 1012 M−1 relative to the known Ka value for CB[7]•11 (1.98 × 1012 M−1).7 Accordingly, it seems reasonable to expect that CB[7] derivatives like Me2CB[7] will function well as CB[7] surrogates in more complicated systems.
Chart 2.
Structure of guests used in this study.
Figure 2.
1H NMR spectra (400 MHz, D2O, RT) recorded for mixtures of: a) Me2CB[7] and 3 (2 equiv.), b) Me2CB[7] and 4 (2 equiv.), c) Me2CB[7] and 7 (2 equiv.), d) Me2CB[7] and 10 (2 equiv.). Resonances marked with * arise from free guest.
Stability of Me2CB[7]
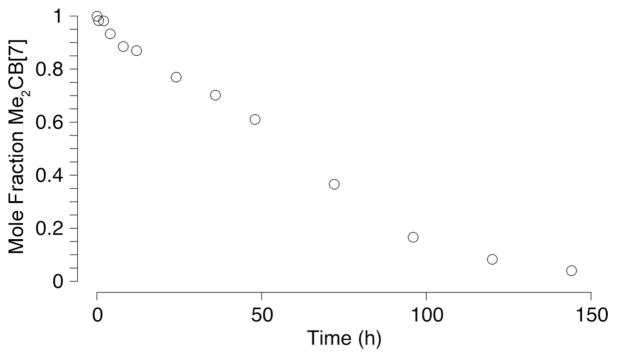
Experiments performed by Day and coworkers established that CB[5], CB[6], and CB[7] are quite stable under the hot acidic conditions (conc. HCl, 100 °C, 24 h) used in their formation.5 Accordingly, we wondered whether the Me substituents on the convex face of Me2CB[7] might stabilize cationic intermediates accessible under acidic conditions and thereby accelerate decomposition reactions of Me2CB[7]. Figure 3 shows a plot of the mole fraction of Me2CB[7] versus time for a solution of Me2CB[7] heated at 110 °C in 9M H2SO4. Over the course of 6 days, Me2CB[7] completely decomposes. The 1H NMR spectrum of the crude reaction mixture using 3 as probe after 6 days shows the presence of macrocycles with CB[6] sized cavities (≈ 24% of the crude mixture). Electrospray mass spectrometry of the crude reaction mixture allows us to identify the presence of comparable amounts of CB[6] and Me2CB[6]. Given that few applications require such highly acidic and high temperature conditions, we believe that dialkylated CB[7] derivatives will be sufficiently stable in most situations.
Figure 3.
Plot of the mole fraction of Me2CB[7] upon heating at 110 °C in 9M H2SO4 as a function of time.
Drug Solubilization Using Me2CB[7]
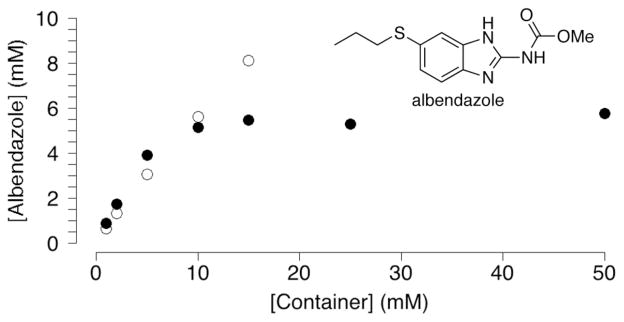
One of the emerging application areas of CB[n] molecular containers involves their use as solubilizing excipients for insoluble pharmaceutical agents.11,34 Given the high solubility of Me2CB[7] in water we decided to test its ability to solubilize albendazole and camptothecin which have previously been solubilized by unsubstituted CB[7].35 Figure 4 shows the phase solubility diagrams constructed for albendazole with either Me2CB[7] or CB[7]. To construct the phase solubility diagrams36 we stirred a solution of a known concentration of host with an excess of insoluble drug overnight, filtered the solution to remove excess insoluble drug, and measured the concentration of soluble drug by 1H NMR using CH3SO3- as a non-binding internal standard of known concentration. The phase solubility diagrams for albendazole and Me2CB[7] or CB[7] are quite similar at low concentrations of container (up to 10 mM). This result can be explained by the fact that the linear region of phase solubility diagrams for 1:1 host:guest complexes obey equation 1 where Ka is the host•guest binding constant (M−1) and s0 is the intrinsic solubility of guest (drug). Given that s0 for albendazole is the same regardless of which host is used and that the Ka values for the CB[7]•albendazole and Me2CB[7]•albendazole are expected to be very similar, then the initial slopes should also be very similar according to equation 1. Somewhat surprisingly, at higher concentrations of Me2CB[7] (e.g. 25 – 50 mM), the concentration of albendazole in solution reaches a plateau of 5.8 mM which is lower than that achieved with 15 mM CB[7] (8.1 mM). The plateau in the phase solubility diagram for Me2CB[7]•albendazole indicates that the complex possesses only moderate solubility in water (≈ 5.8 mM). Why does Me2CB[7] – which is far more water soluble CB[7] – perform less well in the solubilization of albendazole? We believe that the introduction of the Me groups on the convex face of uncomplexed Me2CB[7] dramatically increases its solubility relative to CB[7] because they prevent the CH•••O interactions between the methine C-H groups on the convex face of one container with the ureidyl C=O portals of another container in the solid state which has been implicated as a controlling factor in the solubility trends of CB[n] compounds.37 Within the Me2CB[7]•albendazole and CB[7]•albendazole complexes the drug fills the cavity and protrudes through the ureidyl C=O portals. Accordingly, the Me groups cannot enhance the solubility of Me2CB[7]•albendazole in the same way as they do Me2CB[7]. On the contrary, the presence of the hydrophobic Me groups decrease the solubility of Me2CB[7]•albendazole relative to CB[7]•albendazole. A similar trend was noted for the solubilization of camptothecin by CB[7] and Me2CB[7] (Supporting Information). These result suggest that a major consideration in the design of CB[n] derivatives for use as solubilizing excipients is the incorporation of groups designed to enhance the solubility of both the container and its container•drug complexes.
Figure 4.
Phase solubility diagram constructed (D2O, DCl, pH 2.0, room temperature) for the solubilization of albendazole in the presence of CB[7] (o) or Me2CB[7] (•).
| (1) |
Synthesis of glycoluril derivative 12
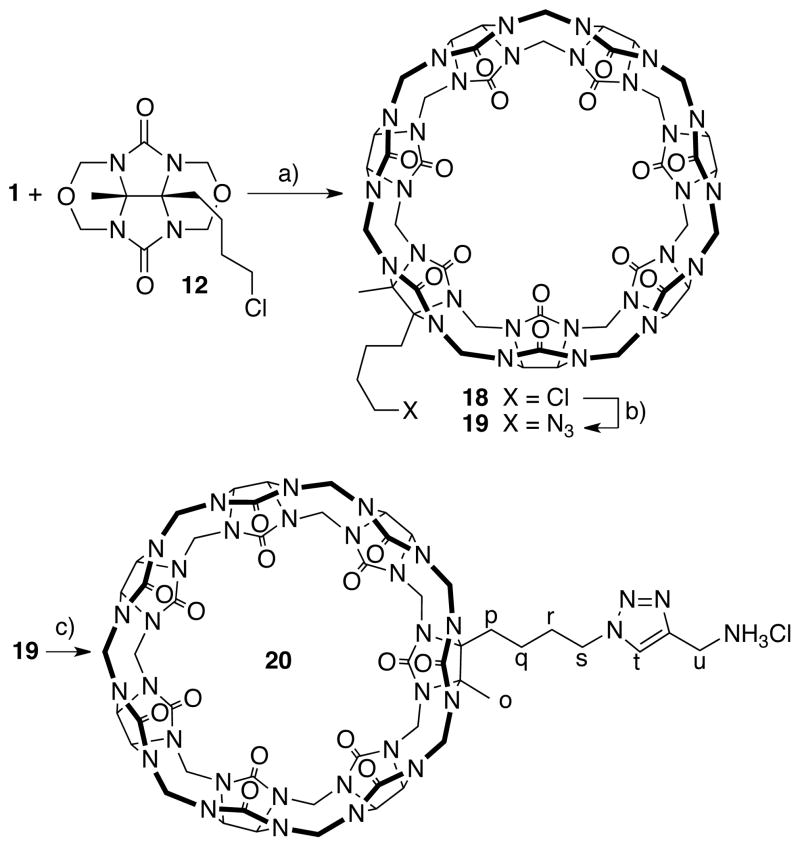
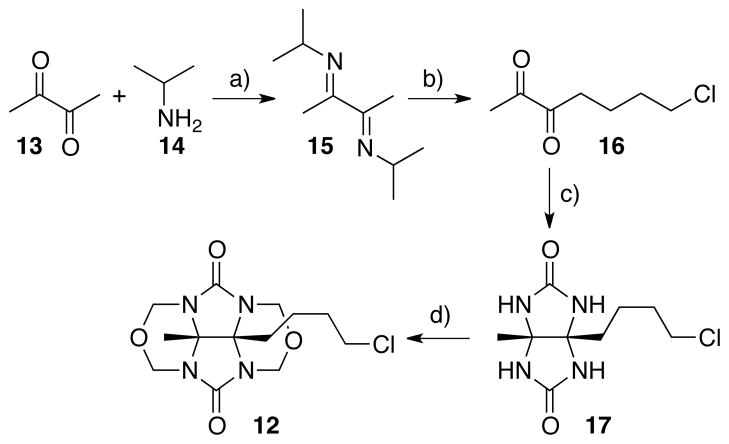
The preparation of glycoluril derivative 1230 bearing a primary alkyl chloride is shown in Scheme 3. First, we react butanedione 13 with isopropylamine 14 in Et2O with TiCl4 to deliver the known diimine 15.38 Next, we deprotonated 15 with LDA in THF followed by subsequent alkylation with 3-iodo-1-chloropropane to yield 16 in 69% yield after hydrolytic workup.39 Compound 16 was transformed into glycoluril 17 by reaction with urea in HCl at room temperature in 35% yield. Finally, treatment of 17 with formalin in HCl gives 12 in 68% yield.
Scheme 3.
Synthesis of CB[7] derivatives 18 – 20. Conditions: a) 9M H2SO4, KI, 110 °C, 16%, b) NaN3, 80 °C, 81%, c) 21, Pericàs’ catalyst, H2O, 50 °C, 95%.
Synthesis of Monofunctionalized CB[7] Derivatives 18 – 20
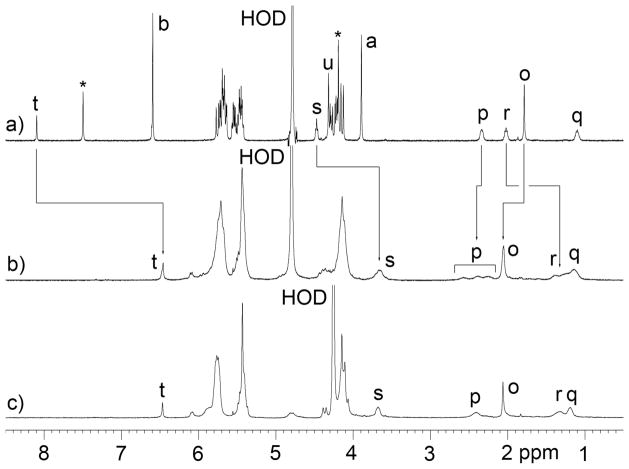
With access to gram scale quantities of 12 we decided to synthesize monofunctionalized CB[7] derivative 18 (Scheme 3). The reaction between 1 and 12 was conducted in 9M H2SO4 at 110 °C in the presence of KI as developed for the synthesis of Me2CB[7], CyCB[7], and MePhCB[7] described above. The 1H NMR spectrum of the crude reaction mixture obtained using 3 as probe allowed us to estimate that 18 comprised 66% of the crude material. Purification by Dowex™ ion exchange chromatography allowed us to isolate 210 mg 18 in pure form in 16% yield. Clearly, the purification process is far from ideal and we are working to improve the process. We found that 18 can be transformed into monofunctionalized CB[7] derivative 19 which contains a reactive azide functional group in 81% yield by simply heating with NaN3 in H2O at 80 °C for 2 days. Azide 19 reacts with propargyl ammonium chloride (21) in the presence of Pericàs’ catalyst40 in H2O at 50 °C to give 20 in 95% yield. The 1H NMR spectrum of the purified sample of 20 in the presence of 3 – which is a strong binder for CB[7] sized cavities7 and thereby disrupts any potential self-assembly processes of 20 – is shown in Figure 5a.
Figure 5.
1H NMR spectra (400 MHz, D2O) recorded for mixtures of: a) 20 and 3 (1.4 equiv.), b) 20 (3.3 mM) at 20 °C, and c) 20 (3.3 mM) at 80 °C.
Self-Assembly of 20 to Yield Cyclic Tetramer 204
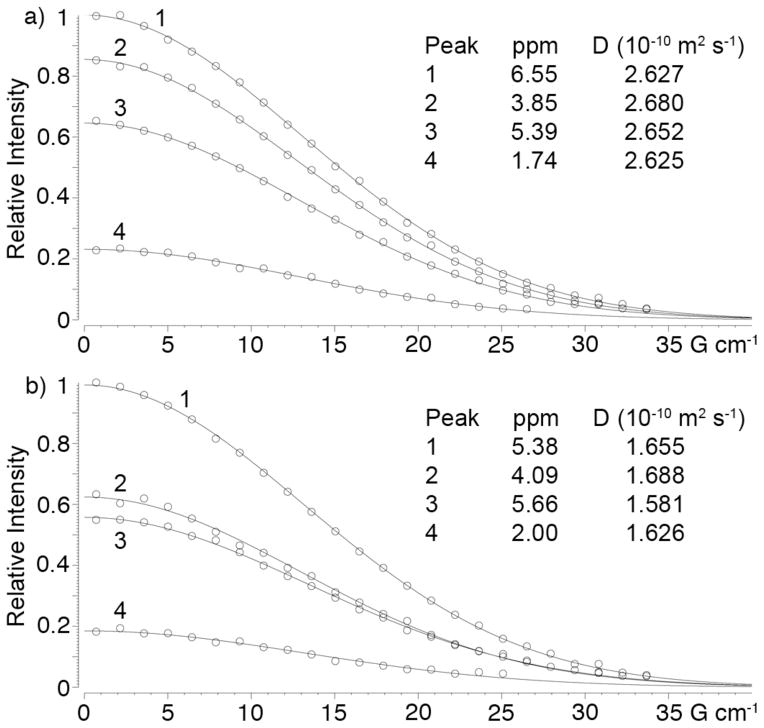
We anticipated that 20 which contains both a CB[7] sized cavity and a covalently attached triazolyl ammonium ion – which is a good guest for CB[7] sized cavities – would undergo self assembly processes in water. A priori it was hard to predict whether supramolecular polymerization processes or formation of discrete cyclic assemblies would predominate.41 Figure 5b shows the 1H NMR spectrum recorded for a 3.3 mM solution of 20 in D2O at room temperature. A single relatively sharp triazole C-H resonance is observed at 6.45 ppm which suggested the formation of a well defined assembly. On the other hand, the upfield region of the spectrum between 2.70 and 1.00 ppm corresponding to the (CH2)4 linker between the CB[7] moiety and the triazole binding unit are broadened and the presence of several groups of resonances suggested the presence of several different assemblies. Figure 5c shows the 1H NMR spectrum recorded at 80 °C which shows that these different groups of resonances coalesce and sharpen into four distinct resonances corresponding to each of the four CH2-groups of the linking chain. The fact that the resonances for Ht and Hu are still strongly upfield shifted in the 1H NMR spectrum recorded at 80°C (Figure 5b versus 5c) suggests that the assembly 20n persists at high temperature. To gain insight into the degree of oligomerization (n) of the self-assembled species (20n) formed in D2O we performed diffusion ordered spectroscopy (DOSY)42 for the monomeric complex 20•3 and for the self-assembled species 20n as shown in Figure 6. The diffusion coefficients measured using four different resonances for 20•3 and 20n averaged 2.646 ± 0.026 × 10−10 m2 s−1 and 1.638 ± 0.045 × 10−10 m2 s−1, respectively (Figure 6). The ratio of diffusion constants for 20•3 and 20n is 1.616. The Stokes-Einstein equation (eq. 2) shows the relationship between the diffusion coefficient (D) and the hydrodynamic radius (rs) in a medium of viscosity η where kB is Boltzmann’s constant and T is temperature.42 If we assume that 20•3 and 20n are roughly spherical then the ratio of the measured diffusion coefficients can be converted into a ratio of molecular weights which gives the oligomerization number. For a trimeric, tetrameric, or pentameric assembly theory predicts the ratio D(20•3)/D(20n) = 1.442 (n = 3), 1.587 (n = 4), and 1.709 (n = 5). In this manner, the measured ratio of diffusion coefficients of 1.616 strongly suggests the formation of the cyclic tetrameric assembly 204.
Figure 6.
DOSY spectra recorded (600 MHz, D2O, RT) for: a) 20•3, and b) cyclic tetramer 204.
| (2) |
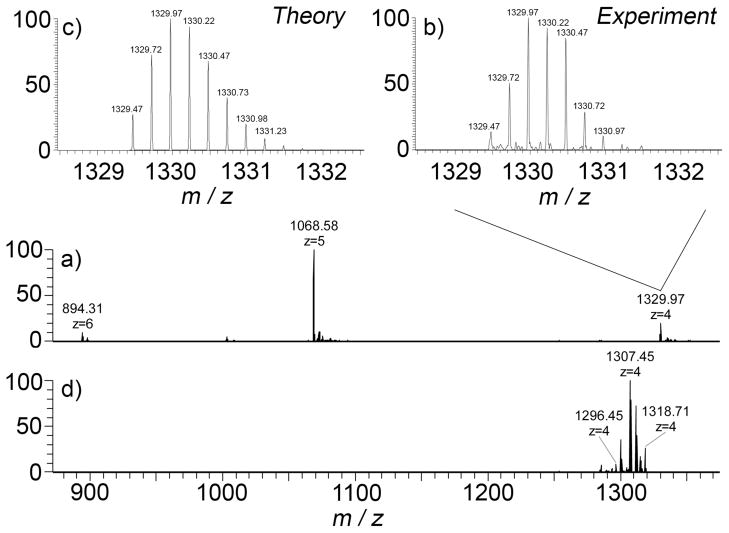
To provide additional evidence for the formation of the cyclic tetrameric assembly 204 we performed electrospray ionization mass spectrometry of 100 μM solutions in H2O and 100,000 resolution on an LTQ-Orbitrap instrument (ThermoFisher, San Jose, CA). Figure 7a shows the mass spectrum obtained which shows a 4+ ion at m/z = 1329.97, a 5+ ion at m/z = 1068.68, and a 6+ at m/z = 894.31. The 4+ ion corresponds to the 2044+ assembly. Figure 7b shows the expansion of the region of the 2044+ ion and Figure 7c shows the theoretical ion distribution for molecular formula C200H228N128O56. The excellent match between Figure 7b and 7c provides strong evidence for the description of this structure as the cyclic tetramer 2044+. Other ions of significant intensity in Figure 7a were observed at m/z = 1068.68 which corresponds to [204•Na]5+ and m/z = 894.31 which corresponds corresponds to [204•Na2]6+. To gain further insight into this system, we isolated ion 2044+ and performed collisional induced dissociation experiments (Figure 7d). We observed the cleavage of covalent bonds rather than the expected dissociation of non-covalent aggregate 2044+ into smaller non-covalent aggregate ions (e.g. monomers, dimers, or trimers). Overall, the electrospray mass spectrometric investigations strongly support that 20 self-assembles to give a highly stable cyclic tetrameric aggregate 2044+.
Figure 7.
Electrospray ionization mass spectra recorded for: a) a solution of 14 (100 μM, H2O), b) expansion of the 144+ ion region, and c) theoretical distribution obtained for the molecular formula C200H228N128O564+, and d) mass spectrum obtained upon collisional induced dissociation.
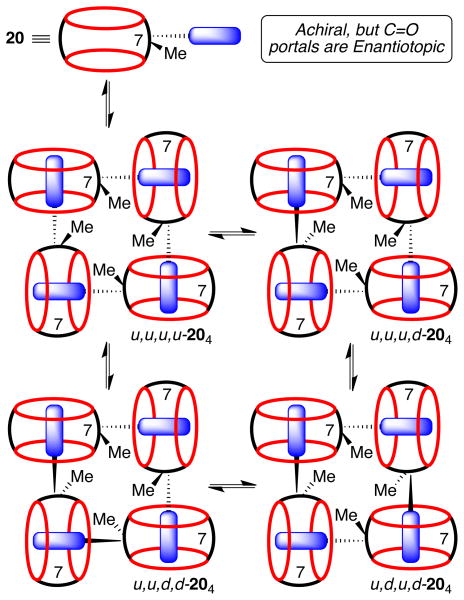
Enumeration of the Different Diastereomers of 204
Although compound 1 is achiral due to the presence of a mirror plane that runs through the equator of the molecule, complexation events that desymmetrize the cavity result in the formation of complexes that are enantiomers and the two C=O portals can be described as enantiotopic. Accordingly, when four molecules of 1 self-assemble to form 14, there are several diastereomers that can form (Scheme 4). For example, all four Me groups can be pointing up (u,u,u,u-14), three up and one down (u,u,u,d-14), and two different combinations of two up and two down (u,u,d,d-14 and u,d,u,d-14).43 The various diastereomers are able to interconvert with one another by a sequence of dissociation of one of the CB[7]•triazolyl ammonium complexes to give a linear tetramer, followed by rotation of the CB[7] group and recomplexation of the triazolyl ammonium through the opposite ureidyl C=O portal of the CB[7] moiety. In this way, the averaging of the signals observed in the high temperature 1H NMR spectrum shown in Figure 5c is readily understandable.
Scheme 4.
Self-assembly of CB[7] derivative 20 to give the cyclic tetramer 204 as a mixture of diastereomers.
Conclusion
In summary, we have shown that CB[7] derivatives are accessible by a building block approach that involves the condensation of glycoluril hexamer 1 with glycoluril bis(cyclic ethers) 2Me, 2Cy, 2MePh, and 12. Just like CB[7] itself, the high water solubility of Me2CB[7] and its outstanding host•guest recognition properties make it well suited as a solubilization agent for poorly soluble pharmaceutical agents. Compound 18 which bears a reactive primary alkylchloride group is the first monofunctionalized CB[7] derivative to be reported. Compound 18 undergoes further functionalization reactions to yield 19 and subsequently 20 by click chemistry. We find that 20 undergoes a self-assembly process in water to yield cyclic tetramer 14 as established by NMR (VT and DOSY) and mass spectrometric measurements.
We believe that the implications of this research go well beyond the system specific details described above. For example, unfunctionalized CB[7] has been the most widely applied CB[n] compound because of its good solubility in water and its exceptional binding affinity and selectivity toward its guests in water. This paper provides two water soluble monofunctionalized CB[7] derivatives that are amenable to further functionalization reactions by SN2 and click chemistry which promises to allow the homogenous covalent attachment of CB[7] to solid phases, macromolecules like proteins and polymers, and incorporation into other complex and functional (bio)molecular systems. When that occurs, the continued impact of the CB[n] family of molecular containers on the chemical sciences will be significant.
Experimental
Compound 18
A mixture of 1 (1.00 g, 1.03 mmol) and KI (0.230 g, 1.35 mmol) were dissolved in 9M aqueous H2SO4 (5 mL) and then treated with 12 (0.510 g, 1.55 mmol). The flask was then sealed with a rubber septum and heated at 110 °C for 30 min. The reaction solution was poured into MeOH (40 mL) which resulted in a gray precipitate. The mixture was centrifuged at 7200 rpm for 5 min. The supernatant was decanted and the precipitate was washed with MeOH (40 mL × 3) and centrifuged at 7200 rpm for 5 min. The precipitate was dried under high vacuum to give a crude, gray powder (1.46 g). The crude solid was dissolved in 88% formic acid/0.4 M HCl (1:1, v:v, 10 mL). The solution containing the crude solid was loaded onto a column (3 cm diameter × 20 cm long) containing Dowex 50WX2 ion-exchange resin pretreated with 88% formic acid/0.4 M HCl (1:1, v:v). The column was eluted with 88% formic acid/0.4 M HCl (1:1, v:v, 400 mL), and then 88% formic acid/0.6 M HCl (1:1, v:v, 400 mL), and then 88% formic acid/0.8 M HCl (1:1, v:v, 400 mL). The fraction purity was assessed by 1H NMR using 3 as a probe. The appropriate fractions were combined and solvent was removed by rotary evaporation and dried under high vacuum. The yellow solid was then washed with MeOH (40 mL) and centrifuged at 7200 rpm for 5 min. The supernatant was decanted and the precipitate was dried under high vacuum to give 18 as a white powder (0.21 g, 0.16 mmol, 16%). M.p. > 300 °C. IR (KBr, cm−1): 3001w, 2917w, 1729s, 1479s, 1423m, 1376m, 1321s, 1290m, 1235s, 1193s, 968m, 828m, 807s. 1H NMR (500 MHz, D2O, >1 equiv. 3): 7.51 (s, unbound 3), 6.62 (s, 4H), 5.80-5.65 (m, 14H), 5.60-5.40 (m, 12H), 4.40-4.20 (m, 10H), 4.21 (s, unbound 3), 4.16 (d, J = 15.4, 4H), 3.92 (s, 4H), 3.64 (t, J = 6.3, 2H), 2.40-2.30 (m, 2H), 1.90 (s, 3H), 1.95-185 (m, 2H), 1.40-1.30 (m, 2H). 13C NMR (125 MHz, D2O, dioxane as internal reference, >1 equiv. 3): 156.8, 156.7, 156.5, 156.5, 156.5, 156.2, 133.7, 133.6, 129.6, 128.0, 80.5, 78.8, 71.7, 71.6, 71.5, 71.4, 71.3, 71.2, 71.2, 71.1, 70.3, 53.2, 53.1, 52.6, 52.6, 52.3, 49.3, 48.9, 44.8, 42.7, 42.4, 31.2, 27.4, 19.6, 14.7 (only 35 of the 39 resonances expected were observed). HR-MS: m/z 702.2476 ([18•3]2+, calcd. for C47H51ClN28O14•C8H14N22+, 702.2493).
Compound 19
A mixture of 18 (100 mg, 0.079 mmol) and NaN3 (39 mg, 0.60 mmol) were dissolved in H2O (2 mL). The mixture was heated at 80 °C for 2 days. The reaction solution was poured into MeOH (3 mL) which resulted in a gray precipitate. The mixture was centrifuged at 7200 rpm for 5 min. The supernatant was decanted and the precipitate was washed with MeOH (5 mL × 3) and centrifuged at 7200 rpm for 5 min. The precipitate was dried under high vacuum to give 19 as a white powder (81 mg, 0.064 mmol, 81%). M.p. > 300 °C. IR (KBr, cm−1): 3442m, 3001w, 2923w, 2099w, 2034m, 1729s, 1476s, 1420m, 1378m, 1322m, 1236s, 1192m, 971m. 1H NMR (400 MHz, D2O, >1 equiv. 3): 7.47 (s, unbound 3), 6.62 (s, 4H), 5.80-5.60 (m, 14H), 5.60-5.40 (m, 12H), 4.35 (d, J = 16.0, 2H), 4.29 (d, J = 15.6, 4H), 4.23 (d, J = 15.6, 2H), 4.22 (d, J = 15.6, 2H), 4.15 (d, J = 15.6, 4H), 4.13 (s, unbound 3), 3.91 (s, 4H), 3.36 (t, J = 6.2, 2H), 2.40-2.30 (m, 2H), 1.89 (s, 3H), 1.75-1.65 (m, 2H), 1.35-1.25 (m, 2H). 13C NMR (125 MHz, D2O, dioxane as internal reference, >1 equiv. 3): 156.7, 156.6, 156.5, 156.5, 156.4, 156.2, 134.3, 134.3, 128.3, 127.8, 80.4, 78.8, 71.7, 71.6, 71.6, 71.5, 71.4, 71.3, 71.2, 71.2, 71.0, 70.3, 53.1, 53.0, 52.6, 52.5, 52.3, 50.6, 49.2, 48.8, 43.9, 42.6, 27.6, 19.6, 14.7 (only 35 of the 39 resonances expected were observed). HR-MS: m/z 705.7703 ([19•3]2+, calcd. for C47H51N31O14•C8H14N22+, 705.7694).
Compound 20
A mixture of 19 (50 mg, 0.039 mmol) and propargyl amine hydrochloride (21, 36 mg, 0.39 mmol), and Pericàs’ catalyst (0.0039 mmol, 2.4 mg)40 were dissolved in H2O (2 mL). The mixture was heated at 50 °C for 1 day. The reaction solution was poured into MeOH (3 mL) which resulted in a gray precipitate. The mixture was centrifuged at 7200 rpm for 5 min. The supernatant was decanted and the precipitate was washed with MeOH (5 mL × 3) and centrifuged at 7200 rpm for 5 min. The precipitate was dried under high vacuum to give 20 as a white powder (50 mg, 0.037 mmol, 95%). M.p. > 300 °C. IR (KBr, cm−1): 3442m, 2994w, 2916w, 1729s, 1473s, 1422m, 1378m, 1322m, 1235s, 1194m, 970m. 1H NMR (500 MHz, D2O, >1 equiv. 3): 8.10 (s, 1H), 7.50 (s, unbound 3), 6.61 (s, 4H), 5.80-5.60 (m, 14H), 5.60-5.40 (m, 12H), 4.48 (t, J = 6.3, 2H), 4.32 (s, 2H), 4.35-4.20 (m, 10H), 4.20 (s, unbound 3), 4.15 (d, J = 15.4, 4H), 3.89 (s, 4H), 2.40-2.30 (m, 2H), 2.10-2.00 (m, 2H), 1.79 (s, 3H), 1.15-1.05 (m, 2H). 13C NMR (125 MHz, D2O, dioxane as internal reference, >1 equiv. 3): 156.7, 156.7, 156.5, 156.5, 156.5, 156.1, 133.7, 133.6, 129.6, 128.0, 125.4, 80.4, 78.7, 71.7, 71.6, 71.5, 71.4, 71.3, 71.2, 71.2, 71.1, 53.2, 53.1, 52.6, 52.6, 52.3, 49.9, 49.2, 48.8, 42.7, 42.4, 34.0, 28.8, 27.4, 19.1, 14.7 (only 36 of the 42 resonances expected were observed). HR-MS: m/z 733.2910 ([20•3]2+, calcd. for C50H56N32O14•C8H14N22+, 733.2905).
Supplementary Material
Scheme 2.
Synthesis of glycoluril derivative 12. Conditions: a) Et2O, TiCl4, b) LDA, THF, Cl(CH2)3I, 69%, c) HCl, urea, 35%, d) HCl, formalin, 68%.
Acknowledgments
We thank Dr. Derick Lucas for initial experiments directed toward the preparation of Me2CB[7]. We thank the National Science Foundation (CHE-1110911 to L.I.) and the National Institutes of Health (GM021248 to C.F.) for financial support. This paper is dedicated to Professor François Diederich – inspiring mentor and outstanding scientist – on the occasion of his 60th birthday.
Footnotes
Supporting Information. Synthetic procedures, characterization data and 1H and 13C NMR spectra for all new compounds, 1H NMR spectra for host-guest complexes, details of the x-ray structure of Me2CB[7]•3, 1H NMR spectra used for Krel calculation, ES-MS data for the decomposition reaction of Me2CB[7], and MMFF minimzed models of 204. This material is available free of charge via Internet at http://pubs.acs.org.
References
- 1.Lee JW, Samal S, Selvapalam N, Kim HJ, Kim K. Acc Chem Res. 2003;36:621–630. doi: 10.1021/ar020254k. [DOI] [PubMed] [Google Scholar]
- 2.Lagona J, Mukhopadhyay P, Chakrabarti S, Isaacs L. Angew Chem, Int Ed. 2005;44:4844–4870. doi: 10.1002/anie.200460675. [DOI] [PubMed] [Google Scholar]; Nau WM, Florea M, Assaf KI. Isr J Chem. 2011;51:559–577. [Google Scholar]; Masson E, Ling X, Joseph R, Kyeremeh-Mensah L, Lu X. RSC Adv. 2012;2:1213–1247. [Google Scholar]
- 3.Freeman WA, Mock WL, Shih NY. J Am Chem Soc. 1981;103:7367–7368. [Google Scholar]; Day AI, Blanch RJ, Arnold AP, Lorenzo S, Lewis GR, Dance I. Angew Chem, Int Ed. 2002;41:275–277. doi: 10.1002/1521-3773(20020118)41:2<275::aid-anie275>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]; Liu S, Zavalij PY, Isaacs L. J Am Chem Soc. 2005;127:16798–16799. doi: 10.1021/ja056287n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim J, Jung IS, Kim SY, Lee E, Kang JK, Sakamoto S, Yamaguchi K, Kim K. J Am Chem Soc. 2000;122:540–541. [Google Scholar]
- 5.Day AI, Arnold AP, Blanch RJ, Snushall B. J Org Chem. 2001;66:8094–8100. doi: 10.1021/jo015897c. [DOI] [PubMed] [Google Scholar]
- 6.Mock WL, Shih NY. J Org Chem. 1986;51:4440–4446. [Google Scholar]
- 7.Liu S, Ruspic C, Mukhopadhyay P, Chakrabarti S, Zavalij PY, Isaacs L. J Am Chem Soc. 2005;127:15959–15967. doi: 10.1021/ja055013x. [DOI] [PubMed] [Google Scholar]
- 8.Rekharsky MV, Mori T, Yang C, Ko YH, Selvapalam N, Kim H, Sobransingh D, Kaifer AE, Liu S, Isaacs L, Chen W, Moghaddam S, Gilson MK, Kim K, Inoue Y. Proc Natl Acad Sci U S A. 2007;104:20737–20742. doi: 10.1073/pnas.0706407105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uzunova VD, Cullinane C, Brix K, Nau WM, Day AI. Org Biomol Chem. 2010;8:2037–2042. doi: 10.1039/b925555a. [DOI] [PubMed] [Google Scholar]; McInnes FJ, Anthony NG, Kennedy AR, Wheate NJ. Org Biomol Chem. 2010;8:765–773. doi: 10.1039/b918372h. [DOI] [PubMed] [Google Scholar]; Angelos S, Khashab NM, Yang YW, Trabolsi A, Khatib HA, Stoddart JF, Zink JI. J Am Chem Soc. 2009;131:12912–12914. doi: 10.1021/ja9010157. [DOI] [PubMed] [Google Scholar]; Zhang J, Coulston RJ, Jones ST, Geng J, Scherman OA, Abell C. Science. 2012;335:690–694. doi: 10.1126/science.1215416. [DOI] [PubMed] [Google Scholar]
- 10.Kim E, Kim D, Jung H, Lee J, Paul S, Selvapalam N, Yang Y, Lim N, Park CG, Kim K. Angew Chem, Int Ed. 2010;49:4405–4408. doi: 10.1002/anie.201000818. [DOI] [PubMed] [Google Scholar]
- 11.Ma D, Hettiarachchi G, Nguyen D, Zhang B, Wittenberg JB, Zavalij PY, Briken V, Isaacs L. Nat Chem. 2012;4:503–510. doi: 10.1038/nchem.1326. [DOI] [PubMed] [Google Scholar]
- 12.Jeon WS, Ziganshina AY, Lee JW, Ko YH, Kang JK, Lee C, Kim K. Angew Chem, Int Ed. 2003;42:4097–4100. doi: 10.1002/anie.200351925. [DOI] [PubMed] [Google Scholar]; Ko YH, Kim E, Hwang I, Kim K. Chem Commun. 2007:1305–1315. doi: 10.1039/b615103e. [DOI] [PubMed] [Google Scholar]; Jeon WS, Kim E, Ko YH, Hwang I, Lee JW, Kim SY, Kim HJ, Kim K. Angew Chem, Int Ed. 2005;44:87–91. doi: 10.1002/anie.200461806. [DOI] [PubMed] [Google Scholar]
- 13.Appel EA, Biedermann F, Rauwald U, Jones ST, Zayed JM, Scherman OA. J Am Chem Soc. 2010;132:14251–14260. doi: 10.1021/ja106362w. [DOI] [PubMed] [Google Scholar]; Wang W, Kaifer AE. Angew Chem, Int Ed. 2006;45:7042–7046. doi: 10.1002/anie.200602220. [DOI] [PubMed] [Google Scholar]; Kim K, Kim D, Lee JW, Ko YH, Kim K. Chem Commun. 2004:848–849. doi: 10.1039/b400783b. [DOI] [PubMed] [Google Scholar]; Liu Y, Yu Y, Gao J, Wang Z. Angew Chem, Int Ed. 2010;49:6576–6579. doi: 10.1002/anie.201002415. [DOI] [PubMed] [Google Scholar]
- 14.Ghale G, Ramalingam V, Urbach AR, Nau WM. J Am Chem Soc. 2011;133:7528–7535. doi: 10.1021/ja2013467. [DOI] [PubMed] [Google Scholar]; Biedermann F, Rauwald U, Cziferszky M, Williams KA, Gann LD, Guo BY, Urbach AR, Bielawski CW, Scherman OA. Chem Eur J. 2010;16:13716–13722. doi: 10.1002/chem.201002274. [DOI] [PubMed] [Google Scholar]; Baumes LA, Sogo MB, Montes-Navajes P, Corma A, Garcia H. Chem Eur J. 2010;16:4489–4495. doi: 10.1002/chem.200903127. [DOI] [PubMed] [Google Scholar]; Wu J, Isaacs L. Chem Eur J. 2009;15:11675–11680. doi: 10.1002/chem.200901522. [DOI] [PubMed] [Google Scholar]
- 15.Liu S, Zavalij PY, Lam YF, Isaacs L. J Am Chem Soc. 2007;129:11232–11241. doi: 10.1021/ja073320s. [DOI] [PubMed] [Google Scholar]; Ghosh S, Isaacs L. J Am Chem Soc. 2010;132:4445–4454. doi: 10.1021/ja910915k. [DOI] [PubMed] [Google Scholar]; Kim C, Agasti SS, Zhu Z, Isaacs L, Rotello VM. Nat Chem. 2010;2:962–966. doi: 10.1038/nchem.858. [DOI] [PMC free article] [PubMed] [Google Scholar]; Nguyen HD, Dang DT, van Dongen JLJ, Brunsveld L. Angew Chem, Int Ed. 2010;49:895–898. doi: 10.1002/anie.200904413. [DOI] [PubMed] [Google Scholar]; Chinai JM, Taylor AB, Ryno LM, Hargreaves ND, Morris CA, Hart PJ, Urbach AR. J Am Chem Soc. 2011;133:8810–8813. doi: 10.1021/ja201581x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jon SY, Selvapalam N, Oh DH, Kang JK, Kim SY, Jeon YJ, Lee JW, Kim K. J Am Chem Soc. 2003;125:10186–10187. doi: 10.1021/ja036536c. [DOI] [PubMed] [Google Scholar]
- 17.Kim K, Selvapalam N, Ko YH, Park KM, Kim D, Kim J. Chem Soc Rev. 2007;36:267–279. doi: 10.1039/b603088m. [DOI] [PubMed] [Google Scholar]
- 18.Lee DW, Park KM, Banerjee M, Ha SH, Lee T, Suh K, Paul S, Jung H, Kim J, Selvapalam N, Ryu SH, Kim K. Nat Chem. 2011;3:154–159. doi: 10.1038/nchem.928. [DOI] [PubMed] [Google Scholar]
- 19.Park KM, Yang J-A, Jung H, Yeom J, Park JS, Park K-H, Hoffman AS, KHS, Kim K. ACS Nano. 2012;6:2960–2968. doi: 10.1021/nn204123p. [DOI] [PubMed] [Google Scholar]
- 20.Zhao N, Lloyd GO, Scherman OA. Chem Commun. 2012;48:3070–3072. doi: 10.1039/c2cc17433b. [DOI] [PubMed] [Google Scholar]
- 21.Chakraborty A, Wu A, Witt D, Lagona J, Fettinger JC, Isaacs L. J Am Chem Soc. 2002;124:8297–8306. doi: 10.1021/ja025876f. [DOI] [PubMed] [Google Scholar]
- 22.Huang WH, Zavalij PY, Isaacs L. J Am Chem Soc. 2008;130:8446–8454. doi: 10.1021/ja8013693. [DOI] [PubMed] [Google Scholar]
- 23.Ma D, Gargulakova Z, Zavalij PY, Sindelar V, Isaacs L. J Org Chem. 2010;75:2934–2941. doi: 10.1021/jo100186q. [DOI] [PubMed] [Google Scholar]
- 24.Lucas D, Minami T, Iannuzzi G, Cao L, Wittenberg JB, Anzenbacher PJ, Isaacs L. J Am Chem Soc. 2011;133:17966–17976. doi: 10.1021/ja208229d. [DOI] [PubMed] [Google Scholar]
- 25.Huang WH, Liu S, Zavalij PY, Isaacs L. J Am Chem Soc. 2006;128:14744–14745. doi: 10.1021/ja064776x. [DOI] [PubMed] [Google Scholar]; Huang WH, Zavalij PY, Isaacs L. Angew Chem, Int Ed. 2007;46:7425–7427. doi: 10.1002/anie.200702189. [DOI] [PubMed] [Google Scholar]; Hettiarachchi G, Nguyen D, Wu J, Lucas D, Ma D, Isaacs L, Briken V. PLoS One. 2010;5:e10514. doi: 10.1371/journal.pone.0010514. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ma D, Zavalij PY, Isaacs L. J Org Chem. 2010;75:4786–4795. doi: 10.1021/jo100760g. [DOI] [PubMed] [Google Scholar]
- 26.Cao L, Isaacs L. Org Lett. 2012;14:3072–3075. doi: 10.1021/ol3011425. [DOI] [PubMed] [Google Scholar]
- 27.Wu F, Wu LH, Zhang YQ, Xue SF, Tao Z, Day AI. J Org Chem. 2012;77:606–611. doi: 10.1021/jo2021778. [DOI] [PubMed] [Google Scholar]
- 28.Day AI, Arnold AP, Blanch RJ. Molecules. 2003;8:74–84. [Google Scholar]; Lagona J, Fettinger JC, Isaacs L. Org Lett. 2003;5:3745–3747. doi: 10.1021/ol035468w. [DOI] [PubMed] [Google Scholar]; Wittenberg JB, Costales MG, Zavalij PY, Isaacs L. Chem Commun. 2011;47:9420–9422. doi: 10.1039/c1cc13358f. [DOI] [PubMed] [Google Scholar]
- 29.Zhao Y, Xue S, Zhu Q, Tao Z, Zhang J, Wei Z, Long L, Hu M, Xiao H, Day AI. Chin Sci Bull. 2004;49:1111–1116. [Google Scholar]
- 30.Day AI, Arnold AP, Blanch RJ. WO 2005/026168 A1. Method for Preparing Cucurbiturils. 2005 (Unisearch Limited)
- 31.We choose to use 9M H2SO4 because this was the solvent used in the high yielding synthesis of CB[7] reported by Nau and to use KI because metal ions are known from the work of Day to influence the distribution of CB[n] obtained. Analysis of the crude reaction mixtures between 1 and 12 conducted in 9M H2SO4 in the absence of KI contain less 18 (CB[6]: 70%, 18: 25%), as do reactions conducted in conc. HCl in the presence of KI (CB[6]: 70%, 18: 30%) or the absence of KI (CB[6]: 59%, 18: 39%) as shown in the Supporting Information. See: Marquez C, Huang F, Nau WM. IEEE Trans Nanobiosci. 2004;3:39–45. doi: 10.1109/tnb.2004.824269.Day AI, Blanch RJ, Coe A, Arnold AP. J Inclusion Phenom Macrocyclic Chem. 2002;43:247–250.
- 32.Stella VJ, Rajewski RA. Pharm Res. 1997;14:556–567. doi: 10.1023/a:1012136608249. [DOI] [PubMed] [Google Scholar]; De Greef TFA, Smulders MMJ, Wolffs M, Schenning APHJ, Sijbesma RP, Meijer EW. Chem Rev. 2009;109:5687. doi: 10.1021/cr900181u. [DOI] [PubMed] [Google Scholar]
- 33.Witt D, Lagona J, Damkaci F, Fettinger JC, Isaacs L. Org Lett. 2000;2:755–758. doi: 10.1021/ol991382k. [DOI] [PubMed] [Google Scholar]; Wu A, Chakraborty A, Witt D, Lagona J, Damkaci F, Ofori MA, Chiles JK, Fettinger JC, Isaacs L. J Org Chem. 2002;67:5817–5830. doi: 10.1021/jo0258958. [DOI] [PubMed] [Google Scholar]
- 34.Walker S, Oun R, McInnes FJ, Wheate NJ. Isr J Chem. 2011;51:616–624. [Google Scholar]; Ghosh I, Nau WM. Adv Drug Delivery Rev. 2012;64:764–783. doi: 10.1016/j.addr.2012.01.015. [DOI] [PubMed] [Google Scholar]; Day AI, Collins JG. Supramol Chem Mol Nanomater. 2012;3:983–1000. [Google Scholar]
- 35.Zhao Y, Buck DP, Morris DL, Pourgholami MH, Day AI, Collins JG. Org Biomol Chem. 2008;6:4509–4515. doi: 10.1039/b813759e. [DOI] [PubMed] [Google Scholar]; Koner AL, Ghosh I, Saleh Ni, Nau WM. Can J Chem. 2011;89:139–147. [Google Scholar]; Dong N, Xue SF, Zhu QJ, Tao Z, Zhao Y, Yang LX. Supramol Chem. 2008;20:659–665. [Google Scholar]
- 36.Connors KA. Binding Constants. John Wiley & Sons; New York: 1987. [Google Scholar]
- 37.Bardelang D, Udachin KA, Leek DM, Margeson JC, Chan G, Ratcliffe CI, Ripmeester JA. Cryst Growth Des. 2011;11:5598–5614. [Google Scholar]
- 38.Kimpe ND, D’Hondt L, Stanoeva E. Tetrahedron Lett. 1991;32:3879–3882. [Google Scholar]
- 39.Kimpe ND, Stevens C. Tetrahedron. 1995;51:2387–2402. [Google Scholar]
- 40.Ozcubukcu S, Okzal E, Jimeno C, Pericàs M. Org Lett. 2009;11:6480–6483. doi: 10.1021/ol9018776. [DOI] [PubMed] [Google Scholar]
- 41.Park KM, Kim SY, Heo J, Whang D, Sakamoto S, Yamaguchi K, Kim K. J Am Chem Soc. 2002;124:2140–2147. doi: 10.1021/ja011654q. [DOI] [PubMed] [Google Scholar]; Ko YH, Kim K, Kang JK, Chun H, Lee JW, Sakamoto S, Yamaguchi K, Fettinger JC, Kim K. J Am Chem Soc. 2004;126:1932–1933. doi: 10.1021/ja031567t. [DOI] [PubMed] [Google Scholar]; Da Silva JP, Jayaraj N, Jockusch S, Turro N, Ramamurthy V. Org Lett. 2011;13:2410–2413. doi: 10.1021/ol200647j. [DOI] [PubMed] [Google Scholar]
- 42.Cohen Y, Avram L, Frisch L. Angew Chem Int Ed. 2005;44:520–554. doi: 10.1002/anie.200300637. [DOI] [PubMed] [Google Scholar]
- 43.Assemblies u,u,u,u-14 and u,u,u,d-14 are chiral with enantiomers d,d,d,d-14 and d,d,d,u-14. Assemblies u,u,d,d-14 and u,d,u,d-14 are achiral due the presence of an inversion center and an S4 axis, respectively.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.