Abstract
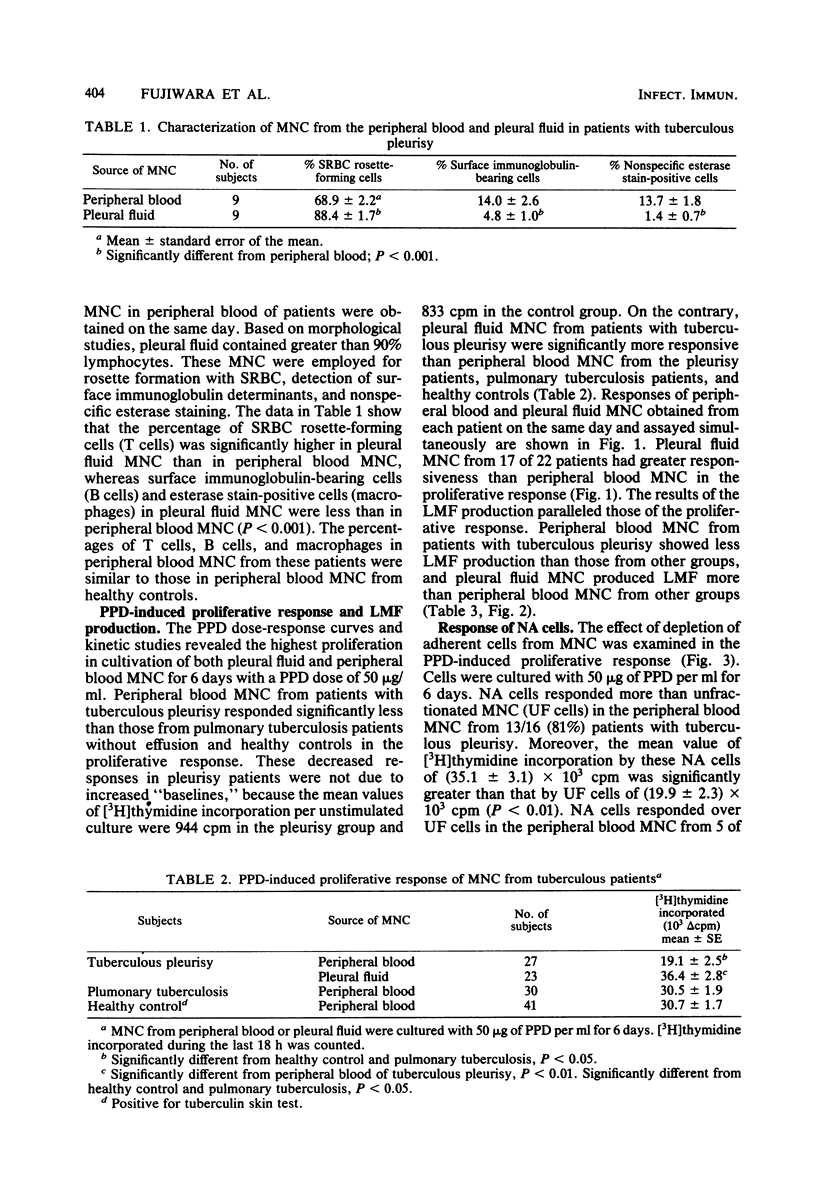
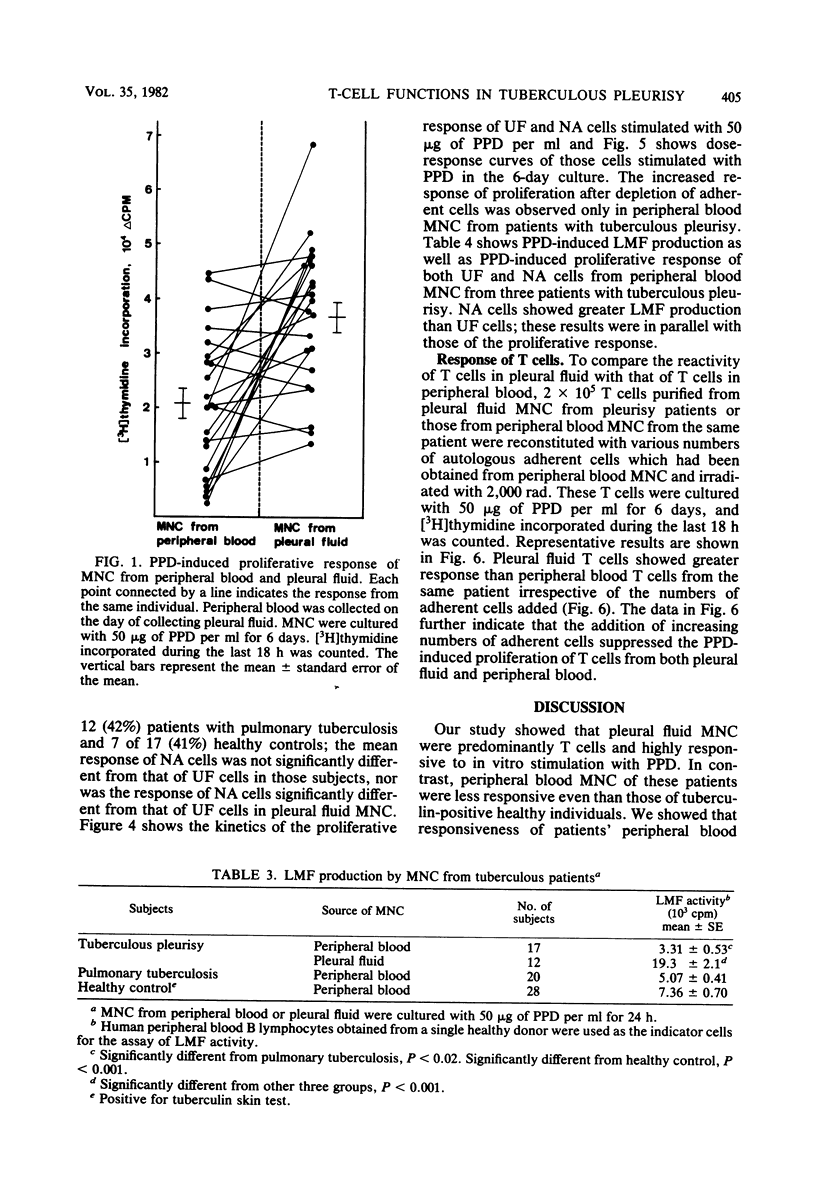
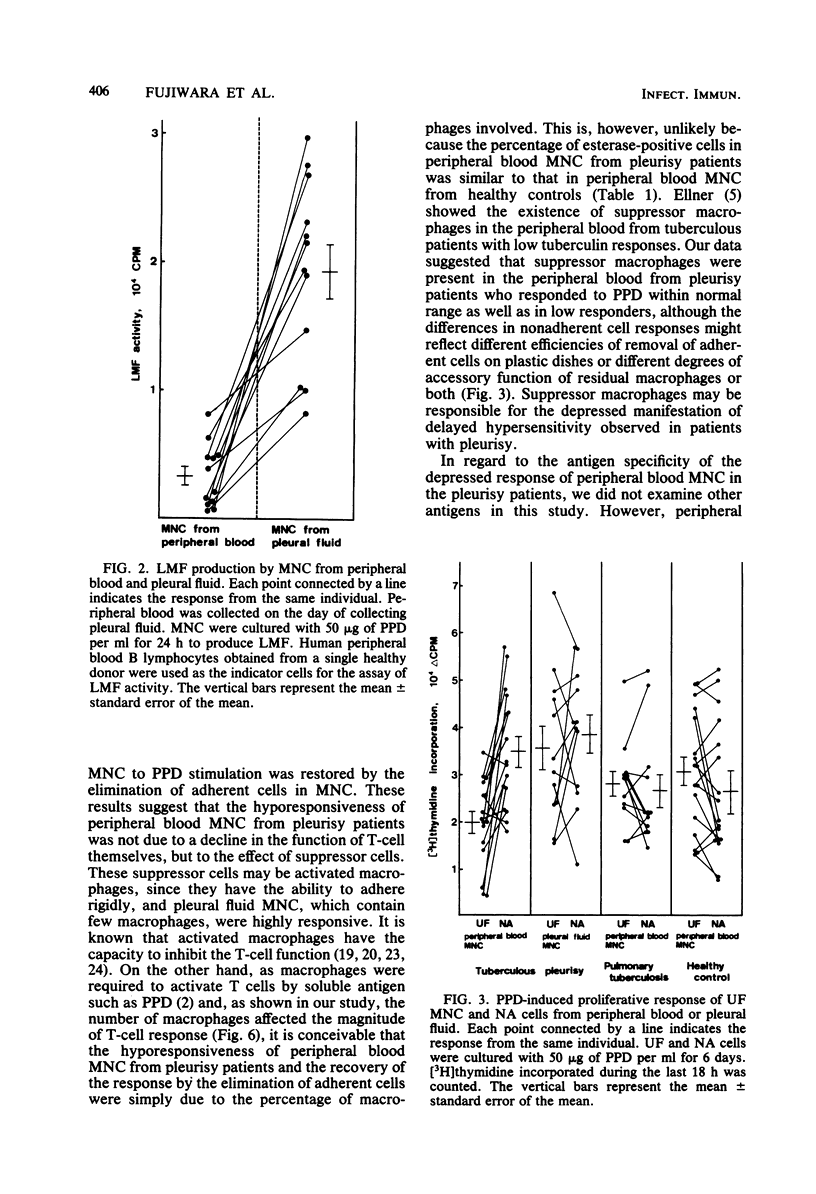
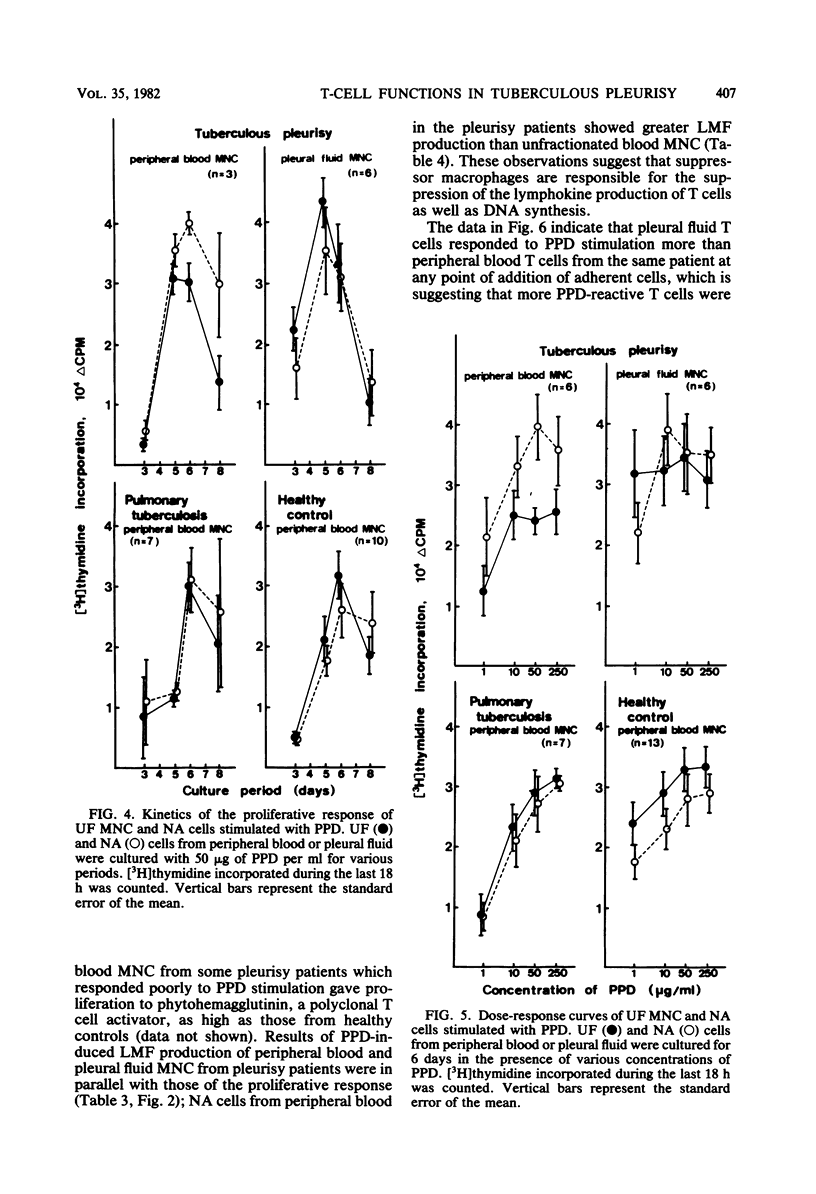
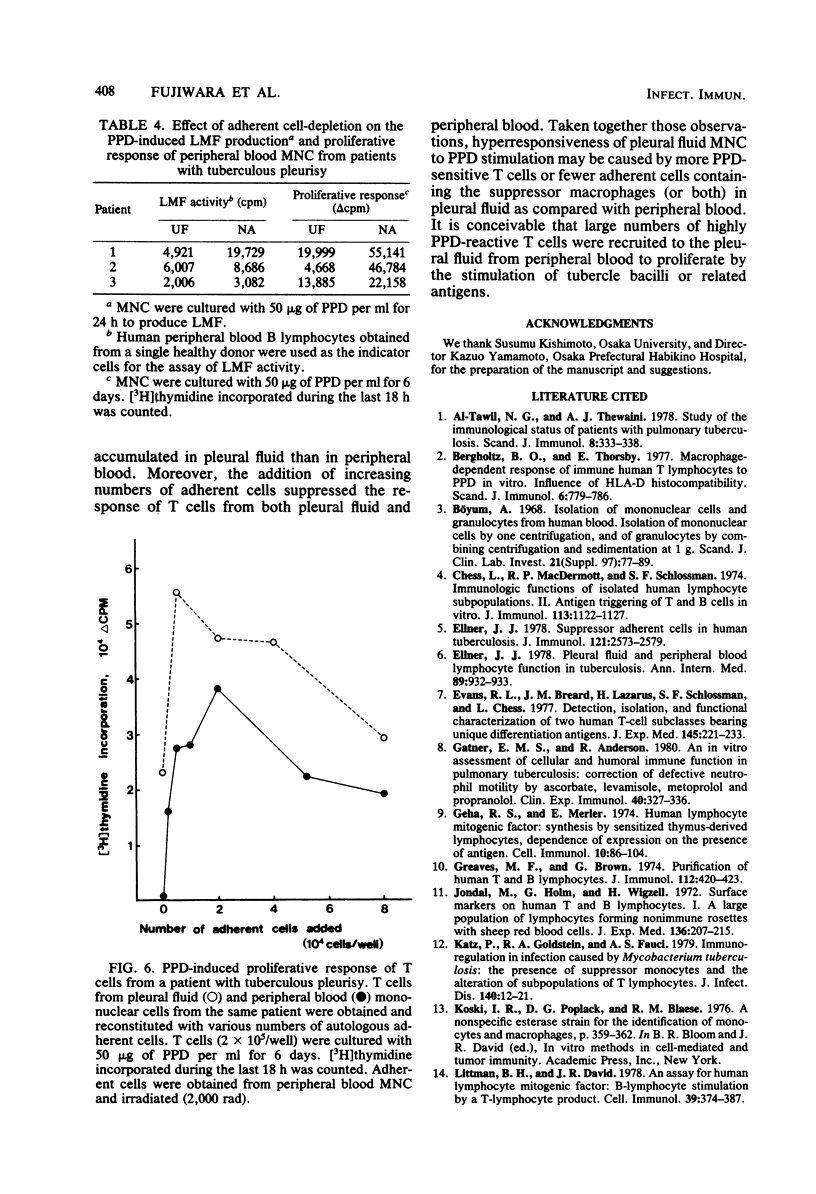
Mononuclear cells in pleural fluid from patients with tuberculous pleurisy were predominantly T cells. Responsiveness of pleural fluid T cells to purified protein derivative of tuberculin were studied by the assay of cell proliferation and production of lymphocyte mitogenic factor by the stimulation with purified protein derivative. Peripheral blood lymphocytes were also studied from patients and tuberculin-positive healthy controls. The order of responsiveness was as follows: pleural fluid lymphocytes greater than peripheral blood lymphocytes of patients without effusion = peripheral blood lymphocytes of healthy controls greater than peripheral blood lymphocytes of patients with effusion. The poor response of peripheral blood lymphocytes from pleurisy patients were recovered by the elimination of adherent cells in peripheral blood lymphocytes to the level of the response of peripheral blood lymphocytes from healthy controls. T cells purified from pleural fluid mononuclear cells responded more than those from peripheral blood. These results suggested that in the pleurisy patients purified protein derivative-reactive T cells in peripheral blood did not decrease in activity, but were depressed by suppressor cells, and further suggested that highly purified protein derivative-reactive T cells were accumulated in the pleural fluid.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Tawil N. G., Thewaini A. J. Study of the immunological status of patients with pulmonary tuberculosis. Scand J Immunol. 1978;8(4):333–338. doi: 10.1111/j.1365-3083.1978.tb00526.x. [DOI] [PubMed] [Google Scholar]
- Bergholtz B. O., Thorsby E. Macrophage-dependent response of immune human T lymphocytes to PPD in vitro. Influence of HLA-D histocompatibility. Scand J Immunol. 1977;6(8):779–786. doi: 10.1111/j.1365-3083.1977.tb02151.x. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Chess L., MacDermott R. P., Schlossman S. F. Immunologic functions of isolated human lymphocyte subpopulations. II. Antigen triggering of T and B cells in vitro. J Immunol. 1974 Oct;113(4):1122–1127. [PubMed] [Google Scholar]
- Ellner J. J. Pleural fluid and peripheral blood lymphocyte function in tuberculosis. Ann Intern Med. 1978 Dec;89(6):932–933. doi: 10.7326/0003-4819-89-6-932. [DOI] [PubMed] [Google Scholar]
- Ellner J. J. Suppressor adherent cells in human tuberculosis. J Immunol. 1978 Dec;121(6):2573–2579. [PubMed] [Google Scholar]
- Evans R. L., Breard J. M., Lazarus H., Schlossman S. F., Chess L. Detection, isolation, and functional characterization of two human T-cell subclasses bearing unique differentiation antigens. J Exp Med. 1977 Jan 1;145(1):221–233. doi: 10.1084/jem.145.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatner E. M., Anderson R. An in vitro assessment of cellular and humoral immune function in pulmonary tuberculosis: correction of defective neutrophil motility by ascorbate, levamisole, metoprolol and propranolol. Clin Exp Immunol. 1980 May;40(2):327–335. [PMC free article] [PubMed] [Google Scholar]
- Geha R. S., Merler E. Human lymphocyte mitogenic factor: synthesis by sensitized thymus-derived lymphocytes, dependence of expression on the presence of antigen. Cell Immunol. 1974 Jan;10(1):86–104. doi: 10.1016/0008-8749(74)90154-3. [DOI] [PubMed] [Google Scholar]
- Greaves M. F., Brown G. Purification of human T and B lymphocytes. J Immunol. 1974 Jan;112(1):420–423. [PubMed] [Google Scholar]
- Jondal M., Holm G., Wigzell H. Surface markers on human T and B lymphocytes. I. A large population of lymphocytes forming nonimmune rosettes with sheep red blood cells. J Exp Med. 1972 Aug 1;136(2):207–215. doi: 10.1084/jem.136.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz P., Goldstein R. A., Fauci A. S. Immunoregulation in infection caused by Mycobacterium tuberculosis: the presence of suppressor monocytes and the alteration of subpopulations of T lymphocytes. J Infect Dis. 1979 Jul;140(1):12–21. doi: 10.1093/infdis/140.1.12. [DOI] [PubMed] [Google Scholar]
- Littman B. H., David J. R. An assay for human lymphocyte mitogenic factor: B-lymphocyte stimulation by a T-lymphocyte product. Cell Immunol. 1978 Sep;39(2):374–387. doi: 10.1016/0008-8749(78)90113-2. [DOI] [PubMed] [Google Scholar]
- Moisan T., Chandrasekhar A. J., Robinson J., McKenna J., Marti G. Distribution of lymphocyte subpopulations in patients with exudative pleural effusions. Am Rev Respir Dis. 1978 Mar;117(3):507–511. doi: 10.1164/arrd.1978.117.3.507. [DOI] [PubMed] [Google Scholar]
- Pettersson T., Klockars M., Hellström P. E., Riska H., Wangel A. T and B lymphocytes in pleural effusions. Chest. 1978 Jan;73(1):49–51. doi: 10.1378/chest.73.1.49. [DOI] [PubMed] [Google Scholar]
- Rieger M., Trnka L., Skvor J. Immunoprofile studies in patients with pulmonary tuberculosis. IV. Tuberculin skin reaction and test of inhibition of blood leukcoyte migration. Scand J Respir Dis. 1979 Dec;60(6):355–357. [PubMed] [Google Scholar]
- Rieger M., Trnka L., Skvor J., Mison P. Immunoprofile studies in patients with pulmonary tuberculosis. III. Study of haemolytic complement in serum and phagocytic activity of blood neutrophils. Scand J Respir Dis. 1979 Aug;60(4):172–175. [PubMed] [Google Scholar]
- Riglar C., Cheers C. Macrophage activation during experimental murine brucellosis. II. Inhibition of in vitro lymphocyte proliferation by brucella-activated macrophages. Cell Immunol. 1980 Jan;49(1):154–167. doi: 10.1016/0008-8749(80)90065-9. [DOI] [PubMed] [Google Scholar]
- Rinehart J. J., Orser M., Kaplan M. E. Human monocyte and macrophage modulation of lymphocyte proliferation. Cell Immunol. 1979 Apr;44(1):131–143. doi: 10.1016/0008-8749(79)90034-0. [DOI] [PubMed] [Google Scholar]
- Skvor J., Trnka L. Immunoprofile studies in patients with pulmonary tuberculosis. I. Correlation of pretherapy cellular tests with characteristics of the disease. Scand J Respir Dis. 1979 Aug;60(4):161–167. [PubMed] [Google Scholar]
- Skvor J., Trnka L., Kugukovová Z. Immunoprofile studies in patients with pulmonary tuberculosis. II. Correlation of levels of different classes of immunoglobulins and specific antibodies with the extent of tuberculosis. Scand J Respir Dis. 1979 Aug;60(4):168–171. [PubMed] [Google Scholar]
- Suzuki K., Tomasi T. B., Jr The regulatory effect of macrophages on the antigen-induced lymph node cell proliferative response. Cell Immunol. 1980 Feb;49(2):317–328. doi: 10.1016/0008-8749(80)90033-7. [DOI] [PubMed] [Google Scholar]
- Wing E. J., Remington J. S. Studies on the regulation of lymphocyte reactivity by normal and activated macrophages. Cell Immunol. 1977 Apr;30(1):108–121. doi: 10.1016/0008-8749(77)90052-1. [DOI] [PubMed] [Google Scholar]


