Abstract
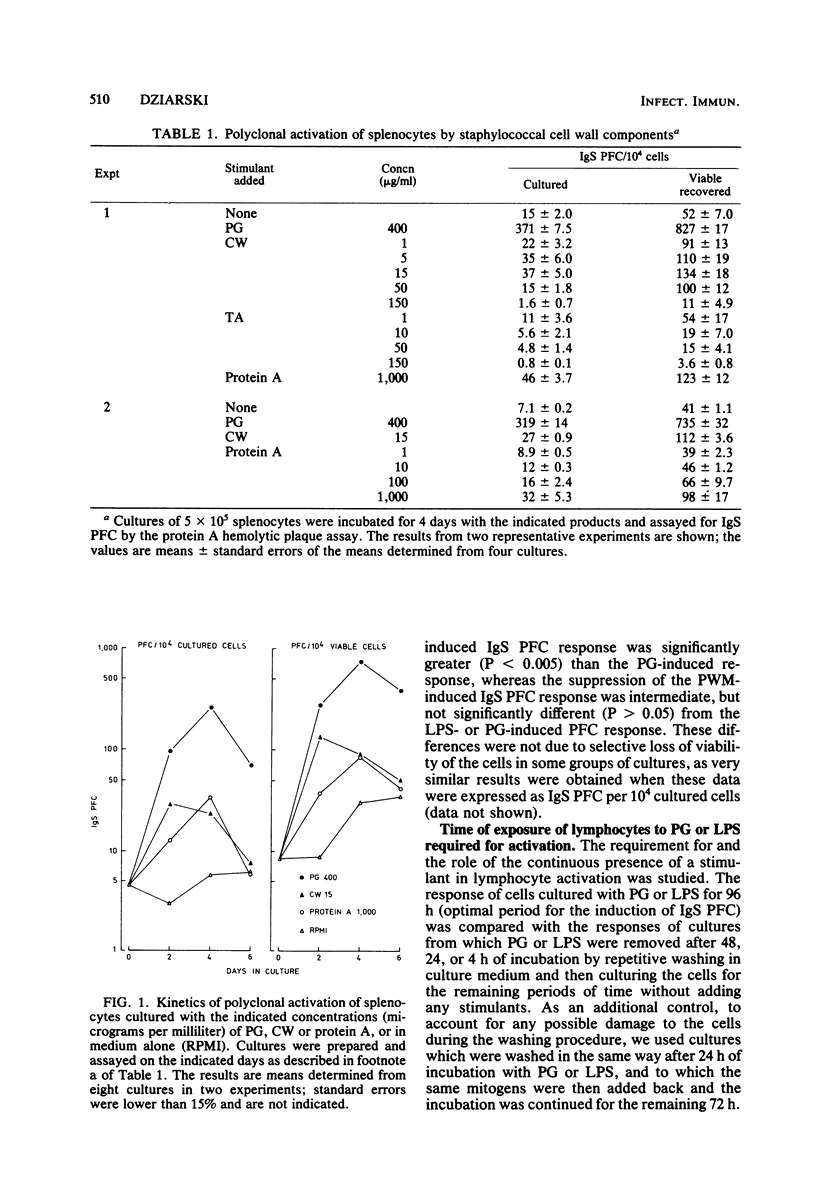
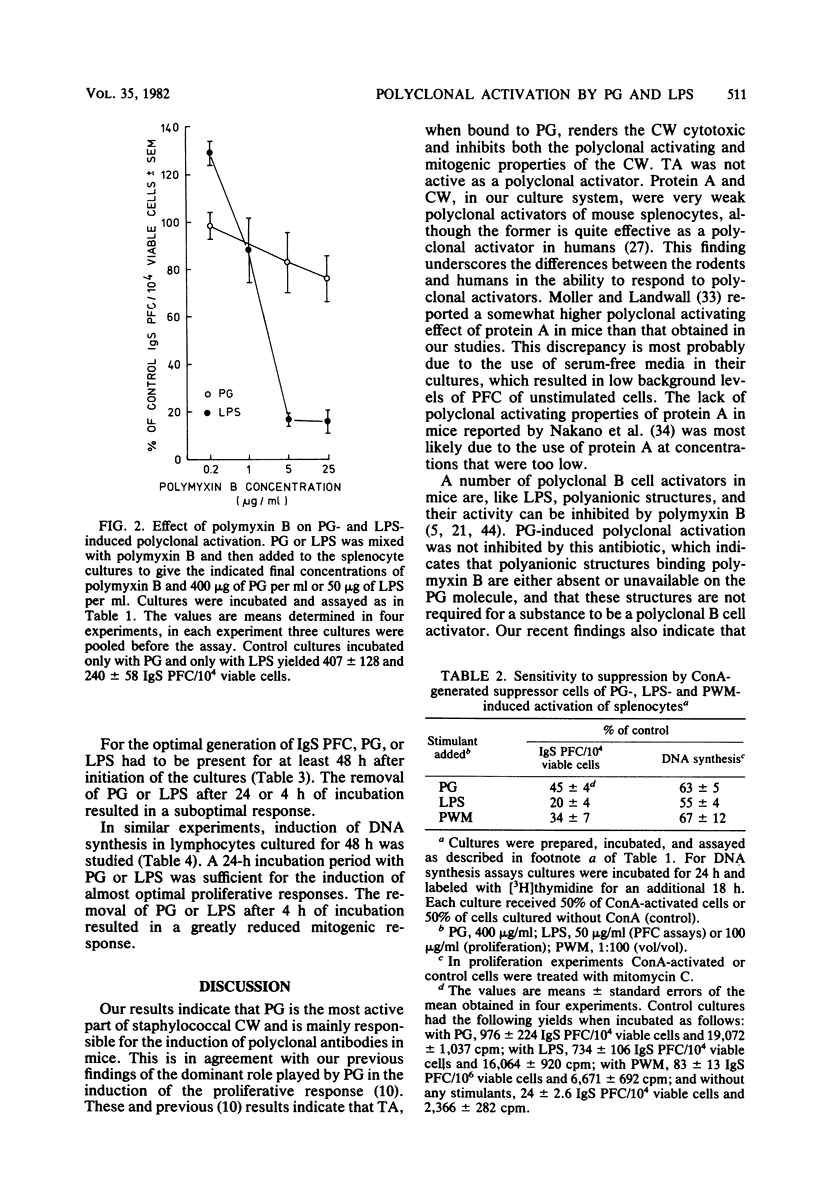
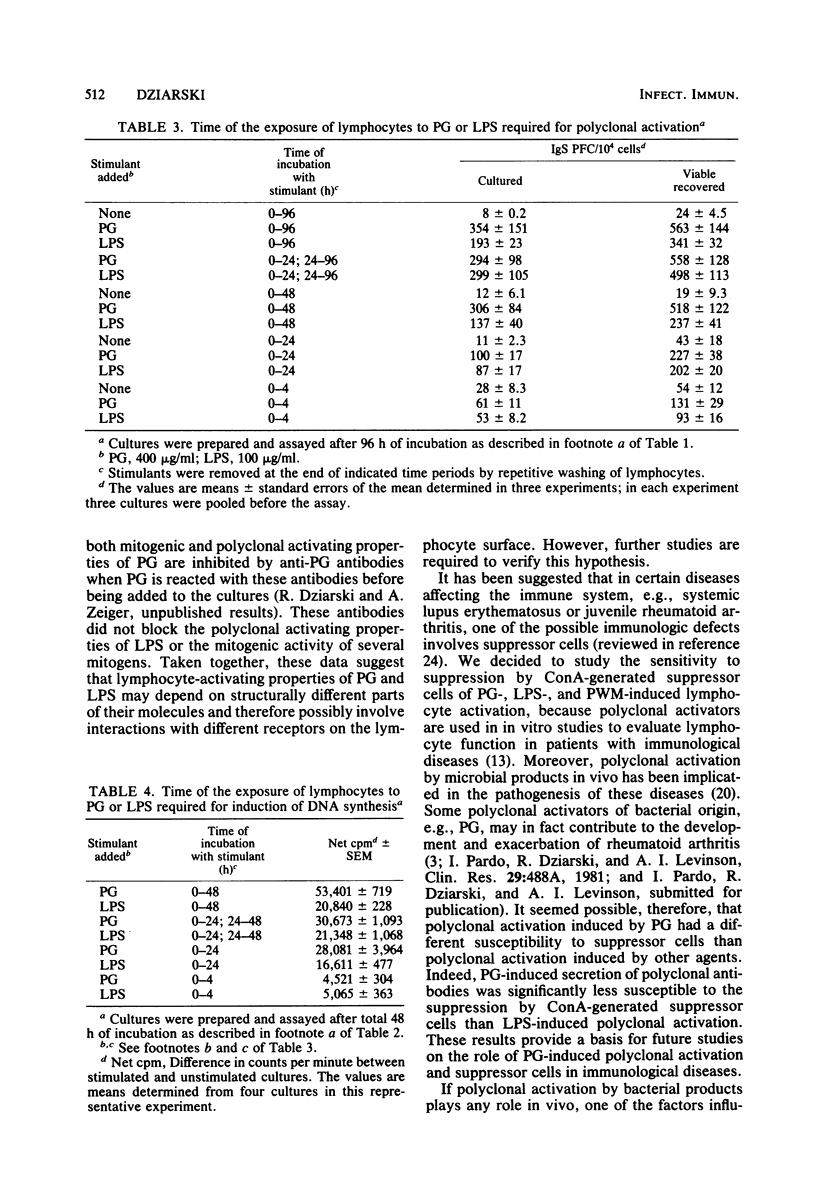
Peptidoglycan (PG) and lipopolysaccharide (LPS) are T cell-independent B cell mitogens and polyclonal activators in mice. The mechanism of in vitro proliferation and polyclonal activation of mouse splenocytes induced by PG from Staphylococcus aureus and LPS from Escherichia coli was further studied by using [3H]thymidine incorporation and protein A hemolytic plaque assays. Concanavalin A-generated suppressor cells suppressed both polyclonal and proliferative responses induced by PG, LPS, and pokeweed mitogen. The suppression of the proliferative responses was similar for all these mitogens, but was significantly less pronounced than the suppression of the polyclonal antibody response. Polyclonal activation induced by LPS was the most susceptible to suppression by concanavalin A-generated suppressor cells, and the suppression was significantly greater than in the PG-induced polyclonal response. Also, PG-induced polyclonal activation was not susceptible to inhibition by polymyxin B, which is an inhibitor of other B cell mitogens and polyclonal activators. For optimal generation of immunoglobulin-secreting cells, PG of LPS had to be present for at least 48 h after the initiation of the cultures. Removal of the mitogens after 4 or 24 h of incubation resulted in a suboptimal response. For effective induction of the proliferative response, the mitogens had to be present in cultures for over 24 h. Polyclonal-activating properties of staphylococcal cell wall components were also compared. PG was by far the most potent inducer of polyclonal antibodies. Teichoic acid was not active as a polyclonal activator, whereas purified cell wall and protein A were very weak inducers of polyclonal antibodies. These studies demonstrate that PG, in addition to LPS, can be a useful probe for studies on polyclonal activation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Lernhardt W., Melchers F. The purified protein derivative of turberculin, a B-cell mitogen that distinguishes in its action resting, small B cells from activated B-cell blasts. J Exp Med. 1979 Dec 1;150(6):1339–1350. doi: 10.1084/jem.150.6.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banck G., Forsgren A. Many bacterial species are mitogenic for human blood B lymphocytes. Scand J Immunol. 1978;8(4):347–354. doi: 10.1111/j.1365-3083.1978.tb00528.x. [DOI] [PubMed] [Google Scholar]
- Bennett J. C. The infectious etiology of rheumatoid arthritis. New considerations. Arthritis Rheum. 1978 Jun;21(5):531–538. doi: 10.1002/art.1780210507. [DOI] [PubMed] [Google Scholar]
- Bona C. Modulation of immune responses by Nocardia immunostimulants. Prog Allergy. 1979;26:97–136. [PubMed] [Google Scholar]
- Butler T., Smith E., Hammarström L., Möller G. Polymyxins as inhibitors of polyclonal B-cell activators in murine lymphocyte cultures. Infect Immun. 1977 May;16(2):449–455. doi: 10.1128/iai.16.2.449-455.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. J., Rodriguez G. E., Kind P. D., Campbell P. A. Listeria cell wall fraction: a B cell mitogen. J Immunol. 1975 Mar;114(3):1132–1134. [PubMed] [Google Scholar]
- Damais C., Bona C., Chedid L., Fleck J., Nauciel C., Martin J. P. Mitogenic effect of bacterial peptidoglycans possessing adjuvant activity. J Immunol. 1975 Jul;115(1):268–271. [PubMed] [Google Scholar]
- Dziarski R., Dziarski A., Levinson A. I. Mitogenic responsiveness of mouse, rat and human lymphocytes to Staphylococcus aureus cell wall, teichoic acid, and peptidoglycan. Int Arch Allergy Appl Immunol. 1980;63(4):383–395. doi: 10.1159/000232654. [DOI] [PubMed] [Google Scholar]
- Dziarski R., Dziarski A. Mitogenic activity of staphylococcal peptidoglycan. Infect Immun. 1979 Mar;23(3):706–710. doi: 10.1128/iai.23.3.706-710.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziarski R. Polyclonal activation of immunoglobulin secretion in B lymphocytes induced by staphylococcal peptidoglycan. J Immunol. 1980 Dec;125(6):2478–2483. [PubMed] [Google Scholar]
- Engel D., Clagett J., Page R., Williams B. Mitogenic activity of Actinomyces viscosus. I. Effects on murine B and T lymphocytes, and partial characterization. J Immunol. 1977 Apr;118(4):1466–1471. [PubMed] [Google Scholar]
- Forsgren A., Svedjelund A., Wigzell H. Lymphocyte stimulation by protein A of Staphylococcus aureus. Eur J Immunol. 1976 Mar;6(3):207–213. doi: 10.1002/eji.1830060312. [DOI] [PubMed] [Google Scholar]
- Ginsburg I., Sela M. N. The role of leukocytes and their hydrolases in the persistence, degradation, and transport of bacterial constituents in tissues: relation to chronic inflammatory processes in staphylococcal, streptococcal, and mycobacterial infections and in chronic periodontal disease. CRC Crit Rev Microbiol. 1976 Mar;4(3):249–322. doi: 10.3109/10408417609106944. [DOI] [PubMed] [Google Scholar]
- Goodman G. W., Sultzer B. M. Endotoxin protein is a mitogen and polyclonal activator of human B lymphocytes. J Exp Med. 1979 Mar 1;149(3):713–723. doi: 10.1084/jem.149.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronowicz E., Coutinho A. Functional analysis of B cell heterogeneity. Transplant Rev. 1975;24:3–40. doi: 10.1111/j.1600-065x.1975.tb00164.x. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Fauci A. S. Activation of human B lymphocytes. V. Kinetics and mechanisms of suppression of plaque-forming cell responses by concanavalin A-generated suppressor cells. J Immunol. 1978 Mar;120(3):700–708. [PubMed] [Google Scholar]
- Ivanyi L. Stimulation of human B lymphocytes by Listeria cell wall fraction. Clin Exp Immunol. 1978 Nov;34(2):193–198. [PMC free article] [PubMed] [Google Scholar]
- Izui S., McConahey P. J., Dixon F. J. Increased spontaneous polyclonal activation of B lymphocytes in mice with spontaneous autoimmune disease. J Immunol. 1978 Dec;121(6):2213–2219. [PubMed] [Google Scholar]
- Jacobs D. M., Morrison D. C. Inhibition of the mitogenic response to lipopolysaccharide (LPS) in mouse spleen cells by polymyxin B. J Immunol. 1977 Jan;118(1):21–27. [PubMed] [Google Scholar]
- Kapp J. A., Pierce C. W., Theze J., Benacerraf B. Modulation of immune responses by suppressor T cells. Fed Proc. 1978 Aug;37(10):2361–2364. [PubMed] [Google Scholar]
- Keightley R. G., Cooper M. D., Lawton A. R. The T cell dependence of B cell differentiation induced by pokeweed mitogen. J Immunol. 1976 Nov;117(5 Pt 1):1538–1544. [PubMed] [Google Scholar]
- Krakauer R. S., Clough J. D. Suppressor factors: potential for immunotherapy. Immunopharmacology. 1980 Jun;2(3):271–284. doi: 10.1016/0162-3109(80)90056-9. [DOI] [PubMed] [Google Scholar]
- Krakauer R. S., Waldmann T. A., Strober W. Loss of suppressor T cells in adult NZB/NZW mice. J Exp Med. 1976 Sep 1;144(3):662–673. doi: 10.1084/jem.144.3.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunori T., Ringdén O., Möller E. Optimal conditions for polyclonal antibody secretion and DNA synthesis in human blood and spleen lymphocytes by lipopolysaccharide. Scand J Immunol. 1978;8(5):451–458. doi: 10.1111/j.1365-3083.1978.tb00541.x. [DOI] [PubMed] [Google Scholar]
- Lipsky P. E. Staphylococcal protein A, a T cell-regulated polyclonal activator of human B cells. J Immunol. 1980 Jul;125(1):155–162. [PubMed] [Google Scholar]
- Melchers F., Braun V., Galanos C. The lipoprotein of the outer membrane of Escherichia coli: a B-lymphocyte mitogen. J Exp Med. 1975 Aug 1;142(2):473–482. doi: 10.1084/jem.142.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. A., Gartner S., Kaplan H. S. Stimulation of mitogenic responses in human peripheral blood lymphocytes by lipopolysaccharide: serum and T helper cell requirements. J Immunol. 1978 Dec;121(6):2160–2164. [PubMed] [Google Scholar]
- Möller G., Landwall P. The polyclonal B-cell-activating property of protein A is not due to its interaction with the FC part of immunoglobulin receptors. Scand J Immunol. 1977;6(4):357–366. doi: 10.1111/j.1365-3083.1977.tb00405.x. [DOI] [PubMed] [Google Scholar]
- Möller G. One non-specific signal triggers b lymphocytes. Transplant Rev. 1975;23:126–137. [PubMed] [Google Scholar]
- Nakano M., Toyoda H., Tanabe M. J., Matsumoto T., Masuda S. Polyclonal antibody production in murine spleen cells induced by Staphylococcus. Microbiol Immunol. 1980;24(10):981–994. doi: 10.1111/j.1348-0421.1980.tb02903.x. [DOI] [PubMed] [Google Scholar]
- OPPENHEIM J. J., PERRY S. EFFECTS OF ENDOTOXINS ON CULTURED LEUKOCYTES. Proc Soc Exp Biol Med. 1965 Apr;118:1014–1019. doi: 10.3181/00379727-118-30033. [DOI] [PubMed] [Google Scholar]
- Peavy D. L., Adler W. H., Smith R. T. The mitogenic effects of endotoxin and staphylococcal enterotoxin B on mouse spleen cells and human peripheral lymphocytes. J Immunol. 1970 Dec;105(6):1453–1458. [PubMed] [Google Scholar]
- Primi D., Hammarström L., Möller G., Smith C. I., Uhr J. Con-A-activated T cells secrete factors with polyclonal B-cell-activating properties. Scand J Immunol. 1979;9(5):467–475. doi: 10.1111/j.1365-3083.1979.tb03069.x. [DOI] [PubMed] [Google Scholar]
- Ringdén O., Rynnel-Dagö B., Waterfield E. M., Möller E., Möller G. Polyclonal antibody secretion in human lymphocytes induced by killed staphylococcal bacteria and by lipopolysaccharide. Scand J Immunol. 1977;6(11):1159–1169. doi: 10.1111/j.1365-3083.1977.tb00355.x. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Lipsky P. E. Monocyte dependence of pokeweed mitogen-induced differentiation of immunoglobulin-secreting cells from human peripheral blood mononuclear cells. J Immunol. 1979 Mar;122(3):926–931. [PubMed] [Google Scholar]
- Rynnel-Dagö B. Polyclonal activation to immunoglobulin secretion in human adenoid lymphocytes induced by bacteria from nasopharynx in vitro. Clin Exp Immunol. 1978 Dec;34(3):402–410. [PMC free article] [PubMed] [Google Scholar]
- Sakane T., Green I. Protein A from Staphylococcus aureus-a mitogen for human T lymphocytes and B lymphocytes but not L lymphocytes. J Immunol. 1978 Jan;120(1):302–311. [PubMed] [Google Scholar]
- Smith C. I., Hammarström L., Bird A. G., Kunori T., Gustafsson B., Holme T. Lipopolysaccharide and lipid A-induced human blood lymphocyte activation as detected by a protein A plaque assay. Eur J Immunol. 1979 Aug;9(8):619–625. doi: 10.1002/eji.1830090809. [DOI] [PubMed] [Google Scholar]
- Smith C. I., Hammarström L. Sodium polyanethole sulfonate: a new macrophage-dependent polymyxin-inhibitable, polyclonal B cell activator. J Immunol. 1978 Sep;121(3):823–828. [PubMed] [Google Scholar]
- Tribble J. L., Bolen J. B. Delayed hypersensitivity to Staphylococcus aureus in mice: in vitro responses to isolated Staphylococcal antigens. Immunology. 1979 Dec;38(4):819–825. [PMC free article] [PubMed] [Google Scholar]


