Abstract
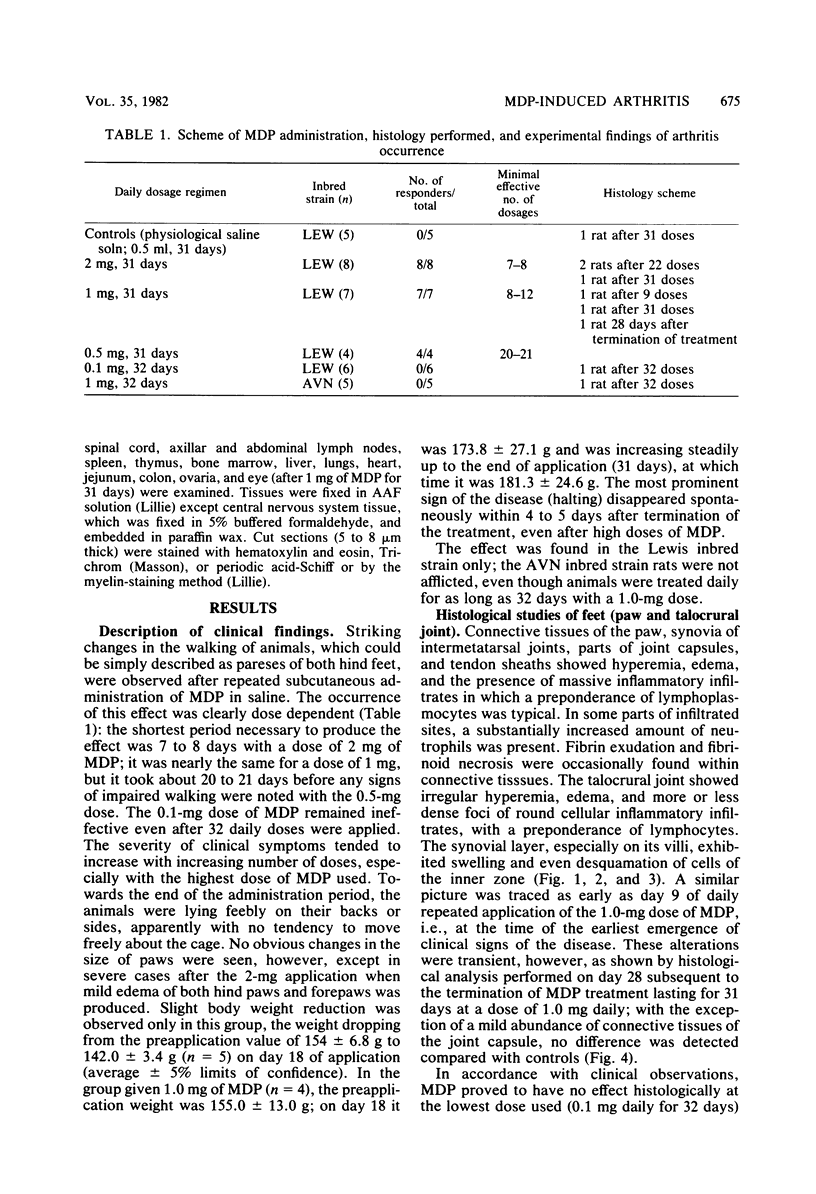
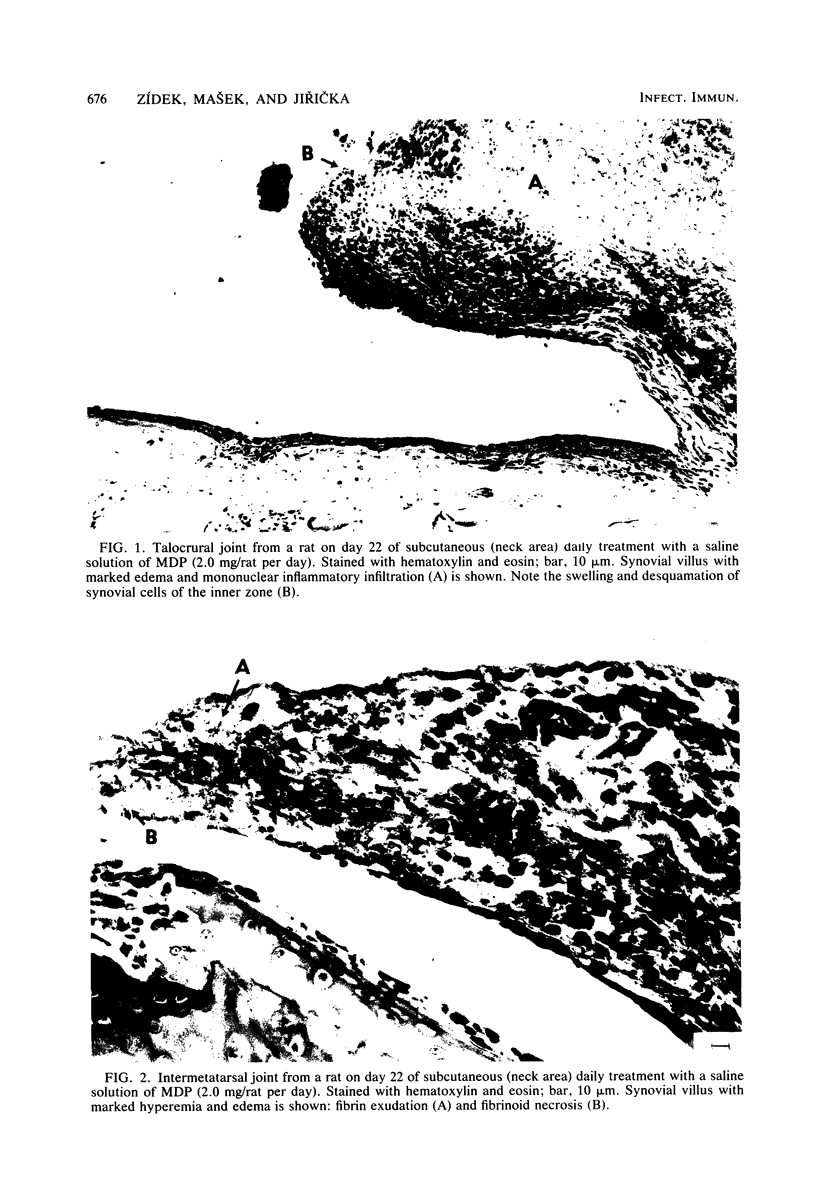
A synthetic muramyl dipeptide, N-acetylmuramyl-L-alanyl-D-isoglutamine, dissolved in saline only and applied subcutaneously to rats of the Lewis inbred strain, produced arthritis, clinically manifest by hind feet paresis but without apparent paw swelling in most cases. Histologically, the disease was characterized by edema and hyperemia of connective tissues, joint synovias, and tendon sheaths, with massive accumulation of inflammatory cell infiltrates composed mainly of lymphoplasmocytes and partly of neutrophil leukocytes. Fibrin exudation and fibrinoid necrosis in connective tissues were observed in the most severe cases. Synovial layers of the talocrural joint, especially on their villi, exhibited marked swelling or cell desquamation of the inner zone. Clinical symptoms of the disease disappeared spontaneously within 5 days after cessation of the treatment; also, histological examinations showed that the effects were reversible. Our results prove that (i) muramyl dipeptide is the principal substance involved in the production of arthritis, (ii) there is no necessity for the presence of additional mycobacterial cell wall components, and (iii) the involvement of the oil moiety is not requisite for the production of arthritis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ellouz F., Adam A., Ciorbaru R., Lederer E. Minimal structural requirements for adjuvant activity of bacterial peptidoglycan derivatives. Biochem Biophys Res Commun. 1974 Aug 19;59(4):1317–1325. doi: 10.1016/0006-291x(74)90458-6. [DOI] [PubMed] [Google Scholar]
- Koga T., Maeda K., Onoue K., Kato K., Kotani S. Chemical structure required for immunoadjuvant and arthritogenic activities of cell wall peptidoglycans. Mol Immunol. 1979 Mar;16(3):153–162. doi: 10.1016/0161-5890(79)90140-8. [DOI] [PubMed] [Google Scholar]
- Kohashi O., Pearson C. M., Tamaoki N., Tanaka A., Shimamura K., Ozawa A., Kotani S., Saito M., Hioki K. Role of thymus for N-acetyl muramyl-L-alanyl-D-isoglutamine-induced polyarthritis and granuloma formation in euthymic and athymic nude rats or in neonatally thymectomized rats. Infect Immun. 1981 Feb;31(2):758–766. doi: 10.1128/iai.31.2.758-766.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohashi O., Pearson C. M., Watanabe Y., Kotani S., Koga T. Structural requirements for arthritogenicity of peptidoglycans from Staphylococcus aureus and Lactobacillus plant arum and analogous synthetic compounds. J Immunol. 1976 Jun;116(6):1635–1639. [PubMed] [Google Scholar]
- Kohashi O., Tanaka A., Kotani S., Shiba T., Kusumoto S., Yokogawa K., Kawata S., Ozawa A. Arthritis-inducing ability of a synthetic adjuvant, N-acetylmuramyl peptides, and bacterial disaccharide peptides related to different oil vehicles and their composition. Infect Immun. 1980 Jul;29(1):70–75. doi: 10.1128/iai.29.1.70-75.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani S., Watanabe Y., Kinoshita F., Shimono T., Morisaki I. Immunoadjuvant activities of synthetic N-acetyl-muramyl-peptides or -amino acids. Biken J. 1975 Jun;18(2):105–111. [PubMed] [Google Scholar]
- Nagao S., Tanaka A. Muramyl dipeptide-induced adjuvant arthritis. Infect Immun. 1980 May;28(2):624–626. doi: 10.1128/iai.28.2.624-626.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEARSON C. M. Development of arthritis, periarthritis and periostitis in rats given adjuvants. Proc Soc Exp Biol Med. 1956 Jan;91(1):95–101. doi: 10.3181/00379727-91-22179. [DOI] [PubMed] [Google Scholar]
- PEARSON C. M., WOOD F. D. Studies of arthritis and other lesions induced in rats by the injection of mycobacterial adjuvant. VII. Pathologic details of the arthritis and spondylitis. Am J Pathol. 1963 Jan;42:73–95. [PMC free article] [PubMed] [Google Scholar]
- Parant M., Parant F., Chedid L., Yapo A., Petit J. F., Lederer E. Fate of the synthetic immunoadjuvant, muramyl dipeptide (14C-labelled) in the mouse. Int J Immunopharmacol. 1979;1(1):35–41. doi: 10.1016/0192-0561(79)90028-6. [DOI] [PubMed] [Google Scholar]
- Stewart-Tull D. E., Shimono T., Kotani S., Kato M., Ogawa Y., Yamamura Y., Koga T., Pearson C. M. The adjuvant activity of a non-toxic, water-soluble glycopeptide present in large quantities in the culture filtrate of Mycobacterium tuberculosis strain DT. Immunology. 1975 Jul;29(1):1–15. [PMC free article] [PubMed] [Google Scholar]
- Tanaka A., Nagao S., Nagao R., Kotani S., Shiba T., Kusumoto S. Stimulation of the reticuloendothelial system of mice by muramyl dipeptide. Infect Immun. 1979 May;24(2):302–307. doi: 10.1128/iai.24.2.302-307.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl S. M., Wahl L. M., McCarthy J. B., Chedid L., Mergenhagen S. E. Macrophage activation by mycobacterial water soluble compounds and synthetic muramyl dipeptide. J Immunol. 1979 Jun;122(6):2226–2231. [PubMed] [Google Scholar]
- Zídek Z., Perlík F. Genetic control of adjuvant-induced arthritis in rats. J Pharm Pharmacol. 1971 May;23(5):389–390. doi: 10.1111/j.2042-7158.1971.tb09938.x. [DOI] [PubMed] [Google Scholar]






